Summary
Background
Patients undergoing major surgery frequently experience major uncontrolled bleeding. The aim of this systematic review and meta‐analysis was to evaluate the clinical efficacy of using viscoelastic haemostatic assays to manage peri‐operative bleeding in elective surgery.
Methods
We searched PubMed/MEDLINE and Embase databases for randomised controlled trials according to pre‐determined criteria. The primary outcomes were blood product requirements; duration of stay in the operating theatre or ICU; and surgical reintervention rate.
Results
We included 20 randomised controlled trials. The overall risk of bias was low to moderate. Twelve studies used thromboelastography‐based transfusion algorithms, while eight used thromboelastometry. Viscoelastic haemostatic assay‐guided therapy was associated with a statistically significant reduction in transfusion of red blood cells (standardised mean difference (95%CI) 0.16 (‐0.29 to 0.02)), platelets (standardised mean difference (95%CI) ‐0.33 (‐0.56 to ‐0.10)) and fresh frozen plasma (standardised mean difference (95%CI) ‐0.64 (‐1.01 to ‐0.28)). There was no evidence of an effect of viscoelastic haemostatic assay‐guided therapy on surgical reintervention (relative risk (95%CI) 1.09 (0.70–1.69)). Viscoelastic haemostatic assay‐guided therapy was associated with lower blood loss and shorter ICU duration of stay. There was no evidence of any effect on total duration of stay and all‐cause mortality.
Conclusions
Viscoelastic haemostatic assay‐guided therapy may reduce peri‐operative blood product transfusion requirements and blood loss during major elective surgery, with no discernible effect on patient‐centred outcomes. The overall quality of evidence was modest.
Keywords: blood coagulation, thromboelastography, transfusion, viscoelastic haemostatic assays
Introduction
Patients undergoing major surgery frequently experience major or uncontrolled bleeding [1]. Current best practice guidelines support monitoring coagulopathy and administering goal‐directed therapy in patients at high risk of major bleeding (e.g. major traumatic haemorrhage, patients with liver disease, cardiac surgery, liver transplant surgery and postpartum haemorrhage [2, 3, 4, 5, 6]).
Management of peri‐operative bleeding includes empirical transfusion of blood products which can be monitored with conventional laboratory coagulation assays or by using viscoelastic haemostatic assays (VHA) [7]. Examples of VHAs include thromboelastography (TEG® haemostasis analyser, Haemonetics Corporation, Boston, MA, USA); rotational thromboelastometry (ROTEM® analyzer, Werfen Instrumentation Laboratory, S.p.A., Milan, Italy); the Sonoclot® system (Sienco Inc., Boulder, CO, USA); and the Quantra® system (HemoSonics, Durham, NC, USA) [8, 9, 10]. Viscoelastic haemostatic assays use whole blood with different activators to examine clot initiation, formation and possibly clot lysis stages [11]. The need for rapid coagulation assessment is critical to guide specific therapy in patients experiencing major bleeding, and VHAs potentially provide a real‐time assessment of haemostasis to allow for more targeted intervention [12]. Viscoelastic haemostatic assay assessment of fibrinolysis may also be useful in other settings, such as liver disease [13].
The use of coagulation‐guided algorithms with peri‐operative monitoring of bleeding has the potential to inform specific therapeutic interventions leading to optimised haemostatic management which may reduce the requirement for allogeneic blood product transfusion and improve clinical outcomes. Guidelines recommend application of transfusion algorithms with predefined intervention triggers according to the type of VHA being used [2, 14, 15, 16]; however, only the European Society of Cardiology provides a grade 1A recommendation for this approach [17]. Previous work has shown that VHA‐guided therapy may reduce transfusion requirements with possible improvements in clinical outcomes [18]. However, these studies included heterogeneous patient groups which can limit meta‐analysis due to differences in monitoring and management (e.g. elective vs. emergency surgery/major trauma) [19]. Therefore, the aim of this systematic review was to summarise the published evidence on the use of VHA‐guided therapy, focusing only on patients undergoing major elective surgery.
Methods
This review was conducted and reported according to PRISMA guidelines [20]. A systematic literature search was undertaken in PubMed/MEDLINE and Embase databases from inception to 28 February 2023 and updated on 5 July 2024. A detailed search strategy is provided in online Supporting Information Appendix S1. Inclusion criteria were prospective randomised controlled trials that evaluated VHA‐guided therapy vs. usual care controls in adult patients undergoing major elective surgery. Only articles available in English were included. All identified articles were screened using predefined inclusion and exclusion criteria by two independent researchers and approved by all authors.
Data were extracted using pre‐determined criteria by two independent researchers. In case of disagreement, the authors were consulted and all authors reviewed and agreed on the final extracted data. The primary outcomes were the use of blood products (red blood cells (RBCs); platelets and fresh frozen plasma (FFP)); duration of stay in ICU and/or in the operating theatre; and surgical reintervention rate. Secondary outcomes included cryoprecipitate transfusion; use of fibrinogen concentrate; blood loss (volume of blood loss over a defined period of time); other peri‐operative complications (e.g. infection, myocardial infarctions, cerebral vascular accident, venous thromboembolism); all‐cause mortality; and total hospital duration of stay. The quality of evidence was assessed through a risk of bias analysis of all included studies, performed by two independent authors, using the Cochrane Risk of Bias 2 (RoB2) tool [21].
All analyses were performed using the DerSimonian‐Laird random‐effects model, regardless of the heterogeneity between studies. Heterogeneity between studies was assessed based on the significance of the between‐study heterogeneity and the size of the I2 value. Substantial heterogeneity was assumed if I2 > 50%. The studies typically reported the results for the continuous outcomes in different ways. For example, some studies reported values in units for the transfusion‐related outcomes, while others reported values in millilitres. Due to the difficultly in converting between units, the analysis for continuous outcomes was performed by calculating the standardised mean difference (SMD) between groups, rather than the raw difference. The SMD is calculated by taking the difference in the group means and dividing it by the standard deviation of the groups. For example, for the outcome evaluating RBC units, an SMD of ‐1 means that on average one fewer unit of RBCs was used. For categorical outcomes the pooled treatment differences between the groups were expressed as relative risk (RR). Results are reported as SMD or RR with 95%CI. The two‐sided level of statistical significance was 0.05. Statistical analyses were performed using Stata version 15.1 (STATACorp LP, College Station, TX, USA).
Results
The search identified 658 unique articles which went through the full screening process (Fig. 1). After a full text review, 20 randomised controlled trials [12, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40], which enrolled 2405 participants, were included in this systematic review. Details of all included studies can be found in online Supporting information Table S1. The population size varied from 26 patients to 224 patients and only three types of surgery were included: cardiac (n = 12); liver (n = 7); and orthopaedic (n = 1). Twelve studies applied a fully TEG‐based transfusion algorithm, with 10 studies [12, 22, 23, 25, 30, 32, 34, 37, 39, 40] using the TEG haemostasis analyser (Haemonetics Corporation) and two studies [33, 36] using MonoTEM‐A® (FramarHemologix, Rome, Italy). The remaining eight studies used a thromboelastometry‐based transfusion algorithm (ROTEM® devices, Werfen Instrumentation Laboratory) [24, 26, 27, 28, 29, 31, 35, 38]. No studies using any other VHA device met the inclusion criteria. Compared with our previous meta‐analysis, which included nine studies in two elective surgery settings [18], this updated meta‐analysis includes 12 additional studies (eight using ROTEM assays and four using TEG assays). Patient characteristics of all included studies are shown in online Supporting Information Table S1 and the transfusion algorithms used are summarised in online Supporting Information Table S2.
Figure 1.
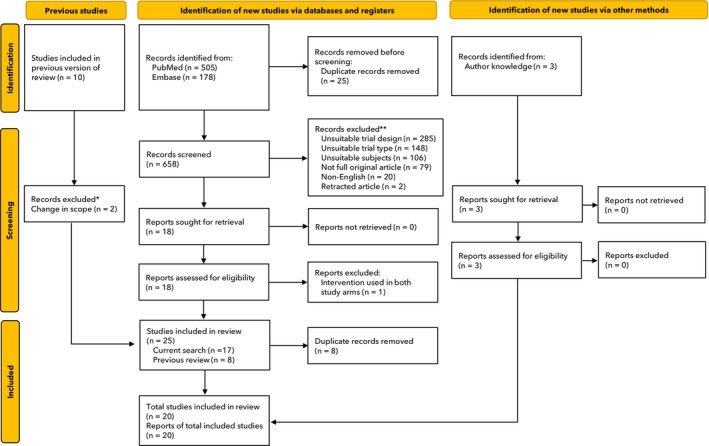
Study flow diagram. *The previous review included non‐elective surgery which were excluded from this analysis; **Trials that included non‐elective surgery, did not use a viscoelastic device and/or were not conducted in the peri‐operative setting.
The overall risk of bias in the included studies ranged from low to unclear (Fig. 2). Six studies were judged to be at low risk of bias [12, 24, 25, 31, 33, 40], with one study judged high risk because of four patients being moved from the algorithm group to the control group due to unavailability of study personnel on the day [30]. The remainder of the studies had some concerns, usually due to a lack of specification of the randomisation method and allocation blinding. Bias also occurred due to difficulties of blinding an intervention involving interpreting a device output and therefore treating clinicians or outcome assessors may have been aware of the allocated intervention.
Figure 2.
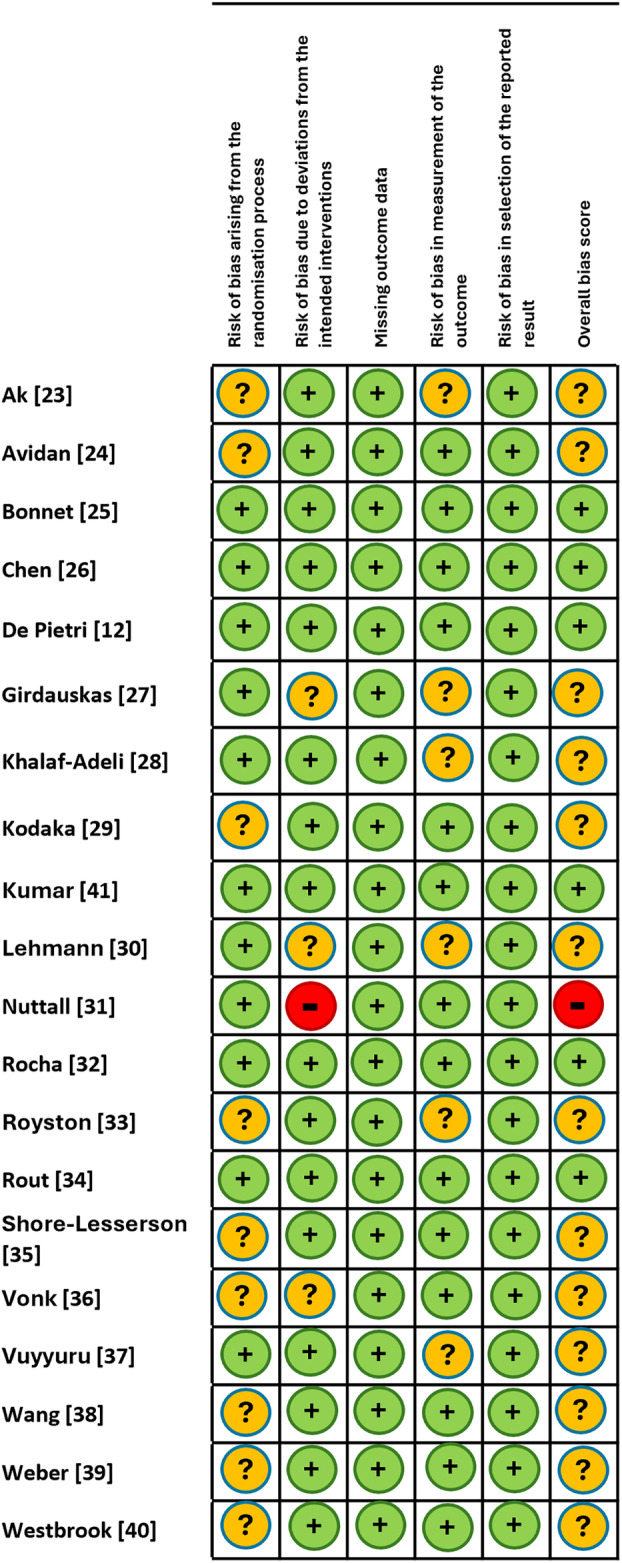
Risk of bias in included studies. Green circle, low risk of bias; yellow circle, unclear risk of bias; red circle, high risk of bias.
The pooled results for key primary and secondary outcomes are summarised in Table 1 and all analysed outcomes are in online Supporting Information Table S3. The effects of VHA‐guided therapy on transfusion‐related and clinical outcomes are shown in Fig. 3 and online Supporting Information Figure S1, respectively. The largest contributions of the new included studies when compared with our previous review [18] were on the effects of VHA‐guided therapy on transfusion of platelets (39.7% weighting, three new studies), RBC (38.8% weighting, four new studies) and FFP (32.4% weighting, two new studies). Viscoelastic haemostatic assay‐guided therapy was associated with less RBC transfusion (SMD (95%CI) ‐0.16 (‐0.29 to ‐0.02), I2 = 0%, p = 0.02), less platelet transfusion (SMD (95%CI) ‐0.33 (‐0.56 to ‐0.10), I2 = 41%, p = 0.004) and less FFP transfusion (SMD (95%CI) ‐0.64 (‐1.01 to ‐0.28), I2 = 78%, p = 0.001) when compared with the control group. There was no evidence of an effect of VHA‐guided therapy on surgical reintervention rates (RR (95%CI) 1.09 (0.70–1.69), p = 0.71). Differences in transfusion requirements according to the type of VHA used are shown in Table 1 and online Supporting Information Table S3.
Table 1.
Summary of key primary and secondary outcomes.
| Outcome | Number of studies | Number of participants | SMD (95%CI) | I2 | p value for overall effect | p value for comparison |
|---|---|---|---|---|---|---|
| Red blood cell transfusion (all) | 9 | 424 | ‐0.16 (‐0.29 to ‐0.20) | 0% | 0.02 | 0.04 |
| TEG | 5 | 281 | ‐0.05 (‐0.22 to 0.11) | 0% | 0.54 | |
| ROTEM | 5 | 143 | ‐0.36 (‐0.59 to ‐0.13) | 0% | 0.002 | |
| Platelet transfusion (all) | 7 | 263 | ‐0.33 (‐0.56 to ‐0.10) | 41% | 0.004 | 0.02 |
| TEG | 4 | 146 | ‐0.52 (‐0.75 to ‐0.28) | 0% | < 0.001 | |
| ROTEM | 3 | 117 | ‐0.10 (‐0.36 to 0.16) | 0% | 0.44 | |
| Fresh frozen plasma transfusion (all) | 8 | 299 | ‐0.64 (‐1.01 to ‐0.28) | 78% | < 0.001 | 0.88 |
| TEG | 4 | 146 | ‐0.63 (‐0.90 to ‐0.35) | 21% | < 0.001 | |
| ROTEM | 4 | 153 | ‐0.61 (‐0.13 to 0.11) | 89% | 0.10 | |
| Blood loss (all) | 6 | 316 | ‐0.28 (‐0.43 to ‐0.12) | 0% | < 0.001 | 0.56 |
| TEG | 5 | 280 | ‐0.26 (‐0.43 to ‐0.09) | 0% | < 0.002 | |
| ROTEM | 1 | 36 | ‐0.41 (‐0.87 to 0.06) | ‐ | 0.09 | |
| ICU duration of stay (all) | 5 | 275 | ‐0.18 (‐0.36 to ‐0.01) | 0% | 0.04 | 0.58 |
| TEG | 4 | 225 | ‐0.21 (‐0.40 to ‐0.01) | 0% | 0.04 | |
| ROTEM | 1 | 50 | ‐0.08 (‐0.48 to 0.31) | 0.68 |
SMD, standardised mean difference; TEG, thromboelastography; ROTEM, rotational thromboelastometry.
Figure 3.
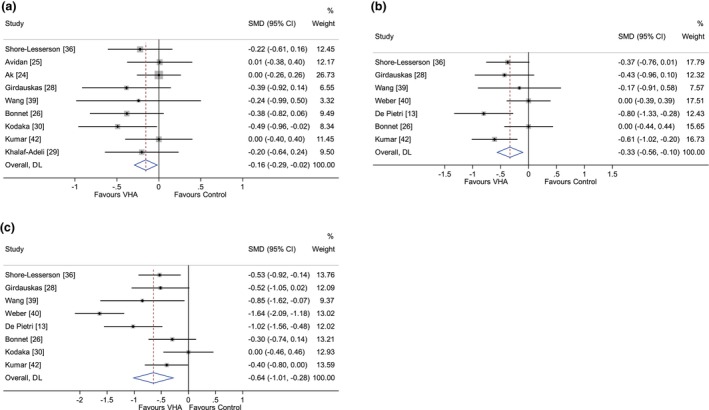
Primary transfusion outcomes for (a) red blood cell; (b) platelet; and (c) fresh frozen plasma transfusion. DL, DerSimonian‐Laird method; SMD, standardised mean difference; VHA, viscoelastic haemostatic assay.
New data were provided for ICU duration of stay (19.0% weighting, one study) and for rates of surgical reintervention (15.1% weighting, one study). Overall, VHA‐guided therapy was associated with a shorter operating theatre duration of stay (SMD (95%CI) ‐0.75 (‐1.27 to ‐0.23), p = 0.005) and ICU duration of stay (SMD (95%CI) ‐0.18 (‐1.36 to ‐0.01), I2 = 0%, p = 0.04), although only one study measured operating theatre duration of stay [12] (online Supporting Information Figures S1 and S2). Viscoelastic haemostatic assay‐guided therapy was associated with less cryoprecipitate transfusion, lower blood loss and fewer peri‐operative complications (SMD (95%CI) 0.46 (0.30–0.69), p < 0.001) but these estimates were only available in studies using thromboelastography. There was no evidence of an effect on all‐cause mortality (RR (95%CI) 0.95 [0.71–1.26], I2 = 0%, p = 0.71).
One study accounted for nearly one‐third of the total meta‐analysis study population and had the highest risk of bias [30]. We performed a sensitivity analysis which did not include this study and the overall trends did not differ from what was observed in our primary analysis (online Supporting Information Figures S4 and S5). A post‐hoc sensitivity analysis was performed with only the six studies at overall low risk of bias [12, 24, 25, 31, 33, 40] and this showed that VHA‐guided therapy was associated with less FFP and fibrinogen concentrate transfusion (data not shown). Funnels plots showed no evidence of publication bias (online Supporting information Figures S6 and S7). A second set of post‐hoc analyses were performed for the continuous outcomes, excluding studies where the assumption made during the conversion from median/IQR to mean/SD was unlikely to be valid (online Supporting Information Appendix S2). This sensitivity analysis also supported the findings of the main analysis and showed that VHA‐guided therapy is associated with less transfusion of RBCs, platelets and FFP and less fibrinogen concentrate administration.
Discussion
This systematic review and meta‐analysis suggests that VHA‐guided haemostatic therapy during elective surgery may reduce the transfusion of allogeneic blood products, including RBCs, platelets, FFP and cryoprecipitate. Viscoelastic haemostatic assay‐guided therapy may also result in lower blood loss and shorter duration of stay in the operating theatre and ICU. However, no differences were seen in rates of surgical reintervention, total duration of stay or mortality. The overall level of evidence in the included studies is modest due to risk of bias, mainly related to randomisation and blinding methods, and all included studies are single‐centre randomised controlled trials. These results align with other meta‐analyses showing a reduction in blood product transfusion with TEG‐guided therapy in patients with cirrhosis undergoing liver‐related procedures [13, 41]. Another systematic review and meta‐analysis in patients undergoing elective surgery found that TEG‐guided therapy was associated with reduced bleeding, fewer platelet and plasma transfusions and shorter total ICU and operating theatre duration of stay [18]. However, there was no evidence of an effect on RBC transfusion, mortality or surgical reintervention.
A search of the ClinicalTrials.gov registry revealed several ongoing studies (e.g. NCT05957822, non‐cardiac surgery; NCT05956769, hip arthroplasty; NCT05806346, cardiac surgery; NCT06328647, cardiac surgery; and NCT05698134, cirrhosis) across a range of clinical settings. Our current analysis evaluated multiple platforms including TEG, ROTEM and other VHA devices. Importantly, the total number of patients from whom data were obtained was also higher than reported previously. While the included studies continue to employ different VHA‐guided algorithms, contributing to data heterogeneity, the CIs for outcomes were either comparable or more precise. With the inclusion of new studies, the reduction of RBC transfusion was more pronounced and the findings reconfirmed reduction in platelet and FFP transfusion. Reducing exposure to allogeneic blood products is a clear goal of peri‐operative patient blood management guidelines [15, 16]. However, there were limited data provided on important patient‐centred outcomes in the new studies, highlighting a need for future studies to focus on these aspects. The new studies provided additional data on mortality, but the effect remains statistically non‐significant which may be due to lack of power. Ongoing studies across different clinical settings (as listed above) may provide additional data for future updates.
Nearly all the included studies were in patients undergoing cardiac or liver surgery, with only one study in orthopaedic surgery. Studies are still needed across a broader range of elective surgery settings where major bleeding occurs (e.g., vascular surgery). For instance, a recent study investigated targeted coagulation management using VHA (ROTEM) and 5% albumin as volume replacement therapy during lung transplantation [42]. We did not include this study due to the lack of comparable endpoints and the potential confounding effect introduced by the intervention with 5% albumin.
Mortality and surgical reintervention rates were low in both groups, most likely due to the focus on elective surgery patients and excluding patients in the trauma setting. In our analysis we chose to exclude trauma patients due to the inherent differences in patient characteristics and clinical management priorities compared with patients undergoing elective surgery. As data on mortality and surgical reintervention might be limited in the context of elective surgery, these were therefore considered secondary outcomes in our study. In one single‐centre study in patients experiencing major trauma, VHA‐guided therapy was associated with a survival advantage when compared with conventional coagulation tests [43]. However, the iTACTIC multicentre trial showed no difference in the proportion of patients who were alive and free of massive transfusion 24 h after injury, although VHA‐guided therapy was associated with a survival advantage in patients with traumatic brain injury [44]. Data from studies on VHA‐guided therapy in settings other than elective surgery and trauma are also very limited. For example, the OBS2 trial showed that infusion of fibrinogen concentrate triggered by Fibtem A5 ≤ 15 mm was not associated with less transfusion requirements in patients experiencing postpartum haemorrhage [45]. The ongoing OBS‐UK multicentre study is investigating clinical and cost‐effectiveness of a maternity quality improvement programme which includes point‐of‐care testing and a VHA‐guided transfusion protocol [46], and aims to provide new data on whether this bundle‐care programme can effectively reduce excess bleeding and the need for transfusion after childbirth.
The clinical outcomes reported across the studies included in this meta‐analysis were mostly short‐term and further evaluation of the longer‐term effects of reducing transfusion are needed. For example, the current ongoing IMOTEC study [32] contains a 1‐year follow‐up to assess the impact on long‐term outcomes, including cost‐effectiveness of VHA‐guided management and patient quality of life. The cost‐effectiveness of using VHAs should be evaluated if implementation in the hospital setting is being considered. Viscoelastic haemostatic assay‐guided therapy may reduce unnecessary blood product use, improving resource utilisation and potentially lowering the associated costs. These would need to be balanced against equipment and staff training costs. However, while the clinical benefits of VHA‐guided blood management are recognised, data regarding its cost‐effectiveness are limited to patients undergoing cardiac or liver transplant surgery [47].
Although the current emphasis of the use of VHA monitoring treatment algorithms has been on reducing bleeding, VHA testing also has potential utility in stratifying hypercoagulable patients undergoing cardiac surgery who are at risk of developing thrombotic complications [48]. This requires further investigation in patients undergoing non‐cardiac surgery.
The key strength of our review is the focus on randomised controlled trials and strict methodological processes which enabled us to draw meaningful conclusions about the overall effect of VHA use. Limitations of our review can be attributed to the clinical and methodological differences between the included studies, leading to heterogeneity. Different VHA algorithms were used in studies caused by the lack of standardisation. As a result, we combined data to include a large group of patients at risk of major bleeding that could help guide clinical decision‐making in terms of haemostasis monitoring, surgical reintervention and allogeneic transfusion in elective surgery. We excluded some studies due to their data not being suitable for converting into an appropriate form for analysis. Overall, we found a limited number of robust, multicentre international randomised controlled trials evaluating the effectiveness of VHA‐guided haemostatic therapy in patients undergoing elective surgery.
In conclusion, this updated meta‐analysis suggests that using VHA‐guided therapy may reduce peri‐operative transfusion requirements and blood loss in patients predominantly undergoing elective cardiac or liver surgery, with potential improvements in clinical outcomes. Prospective, multicentre studies in other patients at high risk of peri‐operative major bleeding, with standardised VHA protocols, are needed.
Supporting information
Appendix S1. Search strategy.
Appendix S2. Sensitivity analysis.
Figure S1. Clinical outcomes.
Figure S2. Transfusion outcomes.
Figure S3. Secondary clinical outcomes.
Figure S4. Sensitivity analysis of transfusion outcomes.
Figure S5. Sensitivity analysis of clinical outcomes.
Figure S6. Funnel plots for publication bias for red blood cell transfusion; platelet transfusion; fresh frozen plasma transfusion; fibrinogen concentrate administration; and cryoprecipitate transfusion.
Figure S7. Funnel plots for publication bias for blood loss; ICU duration of stay; total duration of stay; surgical reintervention; peri‐operative complications; and mortality.
Table S1. Characteristics of included studies.
Table S2. Algorithms of VHA‐guided therapy used in included studies.
Table S3. Pooled data for all primary and secondary outcomes.
Table S4. Summary of meta‐analysis results for continuous outcomes (after exclusions).
Acknowledgements
The study was prospectively registered with PROSPERO (CRD42022296171). Research and editing support were provided by Meridian Health Communications, funded by Haemonetics. JD and JH are employees of Haemonetics. JL has participated on data safety monitoring boards for Merck and Octapharma and steering committees for Takeda and Werfen. KT is participating in research studies funded by Octapharma and CellPhire. KZ has received honoraria for participation in advisory board meetings for Haemonetics and Vifor and received speaker fees from CSL Behring, Masimo, Pharmacosmos, Boston Scientific, Salus, iSEP, Edwards and GE Healthcare. He is the Principal Investigator of the EU‐Horizon 2020 project ENVISION (Intelligent plug‐and‐play digital tool for real‐time surveillance of COVID‐19 patients and smart decision‐making in intensive care units) and Horizon Europe 2021 project COVend (Biomarker and AI‐supported FX06 therapy to prevent progression from mild and moderate to severe stages of COVID‐19). KZ leads as CEO the Christoph Lohfert Foundation and the Health, Patient Safety and PBM Foundation.
1 Haemonetics SA, Signy, Switzerland
2 Departments of Anesthesiology, Critical Care and Surgery, Duke University School of Medicine, Durham, NC, USA
3 Department of Anesthesiology, University of Oklahoma, Health Sciences Center, Oklahoma City, OK, USA
4 Department of Anesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Goethe University, Frankfurt, Germany
5 Haemonetics Corp, Boston, MA, USA
References
- 1. Serraino GF, Murphy GJ. Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: updated systematic review and meta‐analysis. Br J Anaesth 2017; 118: 823–833. 10.1093/bja/aex100. [DOI] [PubMed] [Google Scholar]
- 2. American Society of Anesthesiologists Task Force on Perioperative Blood Management . Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology 2015; 122: 241–275. 10.1097/aln.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 3. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute‐on‐chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med 2020; 48: e173–e191. 10.1097/ccm.0000000000004192. [DOI] [PubMed] [Google Scholar]
- 4. Roberts LN, Lisman T, Stanworth S, Hernandez‐Gea V, Magnusson M, Tripodi A, Thachil J. Periprocedural management of abnormal coagulation parameters and thrombocytopenia in patients with cirrhosis: guidance from the SSC of the ISTH. J Thromb Haemost 2022; 20: 39–47. 10.1111/jth.15562. [DOI] [PubMed] [Google Scholar]
- 5. Escobar MF, Nassar AH, Theron G, et al. FIGO recommendations on the management of postpartum hemorrhage 2022. Int J Gynaecol Obstet 2022; 157(Suppl 1): 3–50. 10.1002/ijgo.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dias JD, Butwick AJ, Hartmann J, Waters JH. Viscoelastic haemostatic point‐of‐care assays in the management of postpartum haemorrhage: a narrative review. Anaesthesia 2022; 77: 700–711. 10.1111/anae.15662. [DOI] [PubMed] [Google Scholar]
- 7. Faraoni D, DiNardo JA. Viscoelastic hemostatic assays: update on technology and clinical applications. Am J Hematol 2021; 96: 1331–1337. 10.1002/ajh.26285. [DOI] [PubMed] [Google Scholar]
- 8. Dias JD, Norem K, Doorneweerd DD, Thurer RL, Popovsky MA, Omert LA. Use of thromboelastography (TEG) for detection of new oral anticoagulants. Arch Pathol Lab Med 2015; 139: 665–673. 10.5858/arpa.2014-0170-OA. [DOI] [PubMed] [Google Scholar]
- 9. Dias JD, Lopez‐Espina CG, Panigada M, Dalton HJ, Hartmann J, Achneck HE. Cartridge‐based thromboelastography can be used to monitor and quantify the activity of unfractionated and low‐molecular‐weight heparins. TH Open 2019; 3: e295–e305. 10.1055/s-0039-1696658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartmann J, Murphy M, Dias JD. Viscoelastic hemostatic assays: moving from the laboratory to the site of care‐a review of established and emerging technologies. Diagnostics (Basel) 2020; 10: 118. 10.3390/diagnostics10020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hartmann J, Hermelin D, Levy JH. Viscoelastic testing: an illustrated review of technology and clinical applications. Res Pract Thromb Haemost 2023; 7: 100031. 10.1016/j.rpth.2022.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography‐guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology 2016; 63: 566–573. 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]
- 13. Hartmann J, Dias JD, Pivalizza EG, Garcia‐Tsao G. Thromboelastography‐guided therapy enhances patient blood management in cirrhotic patients: a meta‐analysis based on randomized controlled trials. Semin Thromb Hemost 2023; 49: 162–172. 10.1055/s-0042-1753530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curry NS, Davenport R, Pavord S, et al. The use of viscoelastic haemostatic assays in the management of major bleeding. Br J Haematol 2018; 182: 789–806. 10.1111/bjh.15524. [DOI] [PubMed] [Google Scholar]
- 15. Kietaibl S, Ahmed A, Afshari A, et al. Management of severe peri‐operative bleeding: guidelines from the European Society of Anaesthesiology and Intensive Care: second update 2022. Eur J Anaesthesiol 2023; 40: 226–304. 10.1097/eja.0000000000001803. [DOI] [PubMed] [Google Scholar]
- 16. Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg 2017; 53: 79–111. 10.1093/ejcts/ezx325. [DOI] [PubMed] [Google Scholar]
- 17. Halvorsen S, Mehilli J, Cassese S, et al. 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non‐cardiac surgery. Eur Heart J 2022; 43: 3826–3924. 10.1093/eurheartj/ehac270. [DOI] [PubMed] [Google Scholar]
- 18. Dias JD, Sauaia A, Achneck HE, Hartmann J, Moore EE. Thromboelastography‐guided therapy improves patient blood management and certain clinical outcomes in elective cardiac and liver surgery and emergency resuscitation: a systematic review and analysis. J Thromb Haemost 2019; 17: 984–994. 10.1111/jth.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cochrane C, Chinna S, Um JY, Dias JD, Hartmann J, Bradley J, Brooks A. Site‐of‐care viscoelastic assay in major trauma improves outcomes and is cost neutral compared with standard coagulation tests. Diagnostics (Basel) 2020; 10: 486. 10.3390/diagnostics10070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ak K, Isbir CS, Tetik S, et al. Thromboelastography‐based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg 2009; 24: 404–410. 10.1111/j.1540-8191.2009.00840.x. [DOI] [PubMed] [Google Scholar]
- 23. Avidan MS, Alcock EL, Da Fonseca J, et al. Comparison of structured use of routine laboratory tests or near‐patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth 2004; 92: 178–186. 10.1093/bja/aeh037. [DOI] [PubMed] [Google Scholar]
- 24. Bonnet A, Gilquin N, Steer N, et al. The use of a thromboelastometry‐based algorithm reduces the need for blood product transfusion during orthotopic liver transplantation: a randomised controlled study. Eur J Anaesthesiol 2019; 36: 825–833. 10.1097/eja.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 25. Chen Z, Ma Y, Li Q, Deng Z, Zheng Q. The application of thromboelastography in risk stratification for selective thromboembolism prophylaxis after total joint arthroplasty in Chinese: a randomized controlled trial. Ann Palliat Med 2020; 9: 2498–2507. 10.21037/apm-19-385. [DOI] [PubMed] [Google Scholar]
- 26. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010; 140: 1117–1124.e2. 10.1016/j.jtcvs.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 27. Khalaf‐Adeli E, Pourfathollah AA, Noohi F, et al. Role of using a thromboelastometry‐based protocol for transfusion management in combined coronary artery bypass grafting and valve surgery: a randomized clinical trail. Indian J Hematol Blood Transfus 2021; 37: 422–429. 10.1007/s12288-020-01375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kodaka M, Ichikawa J, Ando K, Komori M. Thromboelastometry and a hemostasis management system are most beneficial for guiding hemostatic therapy in cardiac surgery patients with a EuroSCORE II of ≥1.83%: a randomized controlled two‐step trial. J Anesth 2020; 34: 666–674. 10.1007/s00540-020-02810-x. [DOI] [PubMed] [Google Scholar]
- 29. Lehmann F, Rau J, Malcolm B, et al. Why does a point of care guided transfusion algorithm not improve blood loss and transfusion practice in patients undergoing high‐risk cardiac surgery? A prospective randomized controlled pilot study. BMC Anesthesiol 2019; 19: 24. 10.1186/s12871-019-0689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nuttall GA, Oliver WC, Santrach PJ, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology 2001; 94: 773–781; discussion 5A‐6A. 10.1097/00000542-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 31. Rocha LL, Neto AS, Pessoa CMS, et al. Comparison of three transfusion protocols prior to central venous catheterization in patients with cirrhosis: a randomized controlled trial. J Thromb Haemost 2020; 18: 560–570. 10.1111/jth.14672. [DOI] [PubMed] [Google Scholar]
- 32. Royston D, von Kier S. Reduced haemostatic factor transfusion using heparinase‐modified thrombelastography during cardiopulmonary bypass. Br J Anaesth 2001; 86: 575–578. 10.1093/bja/86.4.575. [DOI] [PubMed] [Google Scholar]
- 33. Rout G, Shalimar , Gunjan D, et al. Thromboelastography‐guided blood product transfusion in cirrhosis patients with variceal bleeding: a randomized controlled trial. J Clin Gastroenterol 2020; 54: 255–262. 10.1097/mcg.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 34. Shore‐Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela‐Cantos F, Ergin MA. Thromboelastography‐guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 1999; 88: 312–319. 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 35. Vonk AB, Veerhoek D, van den Brom CE, van Barneveld LJ, Boer C. Individualized heparin and protamine management improves rotational thromboelastometric parameters and postoperative hemostasis in valve surgery. J Cardiothorac Vasc Anesth 2014; 28: 235–241. 10.1053/j.jvca.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 36. Vuyyuru SK, Singh AD, Gamanagatti SR, Rout G, Gunjan D, Shalimar . A randomized control trial of thromboelastography‐guided transfusion in cirrhosis for high‐risk invasive liver‐related procedures. Dig Dis Sci 2020; 65: 2104–2111. 10.1007/s10620-019-05939-2. [DOI] [PubMed] [Google Scholar]
- 37. Wang SC, Shieh JF, Chang KY, et al. Thromboelastography‐guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc 2010; 42: 2590–2593. 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 38. Weber CF, Görlinger K, Meininger D, et al. Point‐of‐care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012; 117: 531–547. 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 39. Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out‐performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ 2009; 18: 277–288. 10.1016/j.hlc.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 40. Kumar M, Ahmad J, Maiwall R, et al. Thromboelastography‐guided blood component use in patients with cirrhosis with nonvariceal bleeding: a randomized controlled trial. Hepatology 2020; 71: 235–246. 10.1002/hep.30794. [DOI] [PubMed] [Google Scholar]
- 41. Kovalic AJ, Khan MA, Malaver D, et al. Thromboelastography versus standard coagulation testing in the assessment and reversal of coagulopathy among cirrhotics: a systematic review and meta‐analysis. Eur J Gastroenterol Hepatol 2020; 32: 291–302. 10.1097/meg.0000000000001588. [DOI] [PubMed] [Google Scholar]
- 42. Vajter J, Vachtenheim J Jr, Prikrylova Z, et al. Effect of targeted coagulopathy management and 5% albumin as volume replacement therapy during lung transplantation on allograft function: a secondary analysis of a randomized clinical trial. BMC Pulm Med 2023; 23: 80. 10.1186/s12890-023-02372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gonzalez E, Moore EE, Moore HB, et al. Goal‐directed hemostatic resuscitation of trauma‐induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg 2016; 263: 1051–1059. 10.1097/sla.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baksaas‐Aasen K, Gall LS, Stensballe J, et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med 2021; 47: 49–59. 10.1007/s00134-020-06266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Collins PW, Cannings‐John R, Bruynseels D, et al. Viscoelastometric‐guided early fibrinogen concentrate replacement during postpartum haemorrhage: OBS2, a double‐blind randomized controlled trial. Br J Anaesth 2017; 119: 411–421. 10.1093/bja/aex181. [DOI] [PubMed] [Google Scholar]
- 46. Health Research Authority . Obstetric Bleeding Study UK (OBS UK). 2023. https://www.hra.nhs.uk/planning‐and‐improving‐research/application‐summaries/research‐summaries/obstetric‐bleeding‐study‐uk‐obs‐uk (accessed 12/09/2024).
- 47. National Institute for Health and Care Excellence . Detecting, managing and monitoring haemostasis: viscoelastometric point‐of‐care testing (ROTEM, TEG and Sonoclot systems). 2014. https://www.nice.org.uk/guidance/dg13 (accessed 12/09/2024).
- 48. Harahsheh Y, Ho KM. Use of viscoelastic tests to predict clinical thromboembolic events: a systematic review and meta‐analysis. Eur J Haematol 2018; 100: 113–123. 10.1111/ejh.12992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategy.
Appendix S2. Sensitivity analysis.
Figure S1. Clinical outcomes.
Figure S2. Transfusion outcomes.
Figure S3. Secondary clinical outcomes.
Figure S4. Sensitivity analysis of transfusion outcomes.
Figure S5. Sensitivity analysis of clinical outcomes.
Figure S6. Funnel plots for publication bias for red blood cell transfusion; platelet transfusion; fresh frozen plasma transfusion; fibrinogen concentrate administration; and cryoprecipitate transfusion.
Figure S7. Funnel plots for publication bias for blood loss; ICU duration of stay; total duration of stay; surgical reintervention; peri‐operative complications; and mortality.
Table S1. Characteristics of included studies.
Table S2. Algorithms of VHA‐guided therapy used in included studies.
Table S3. Pooled data for all primary and secondary outcomes.
Table S4. Summary of meta‐analysis results for continuous outcomes (after exclusions).


