Abstract
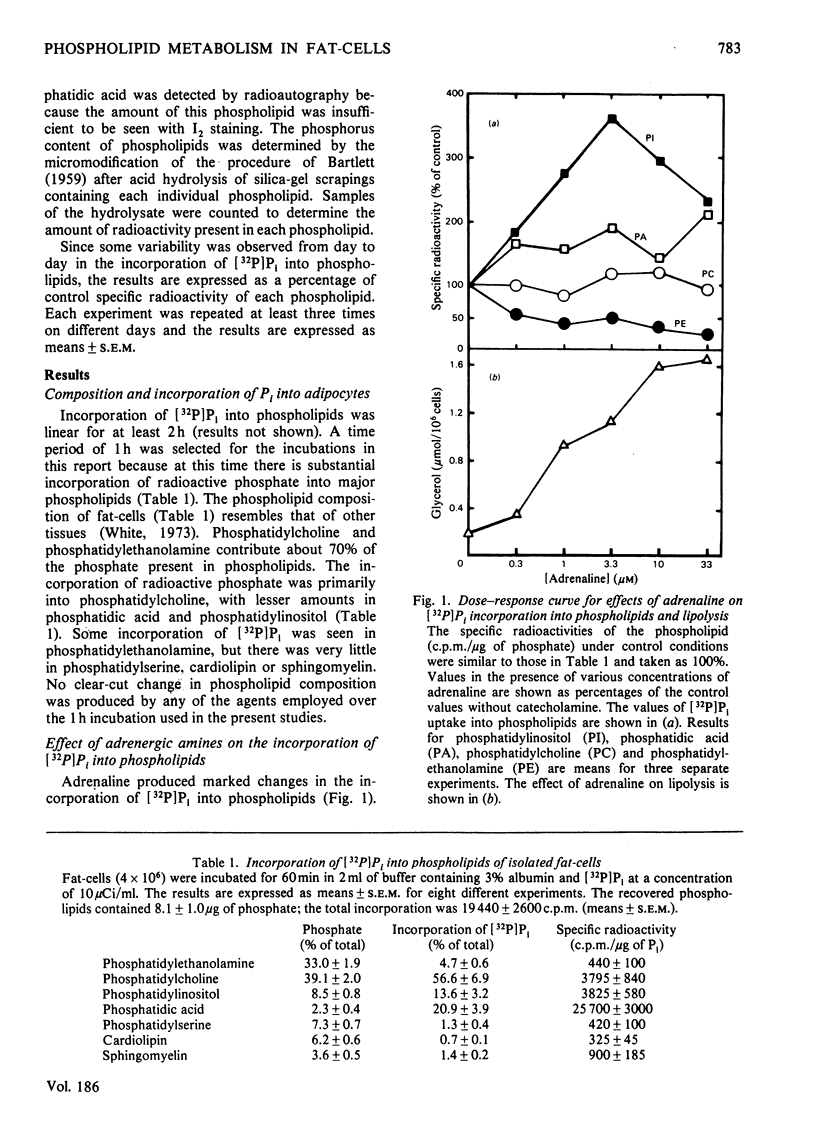
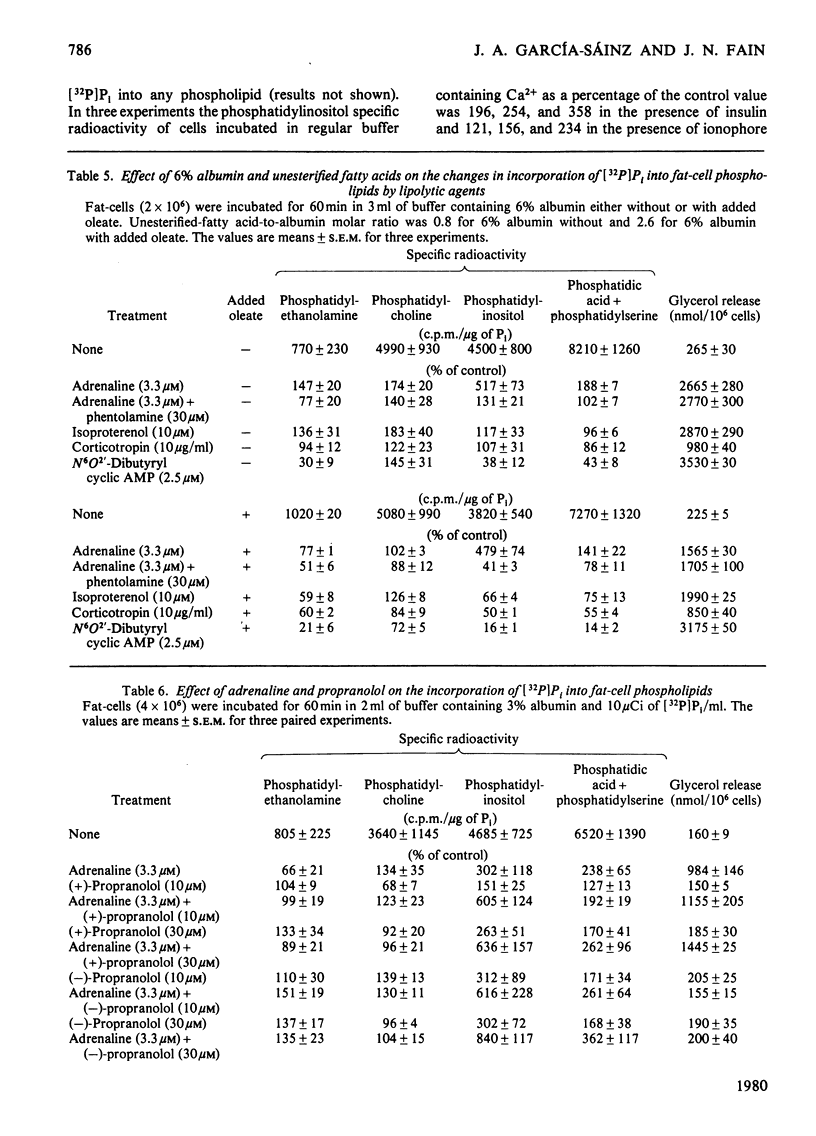
The incorporation of [32P]Pi into phosphatidylinositol by rat fat-cells was markedly increased in the presence of adrenaline. Phosphatidic acid labelling was also increased, but to a lesser extent. These effects are due to α1-adrenergic stimulation since they were unaffected by propranolol, blocked by α-blockers in the potency order prazosin«phentolamine<yohimbine and mimicked by methoxamine. The α-adrenergic stimulation of phosphatidylinositol labelling did not require extracellular Ca2+, which supports the hypothesis that an increased turnover of phosphatidylinositol is involved in α-adrenergic activation of Ca2+ entry. Insulin and the ionophore A23187 gave a small increase in 32P labelling of phosphatidylinositol in Ca2+-free medium containing 1mm-EGTA. The increases due to insulin or ionophore A23187 were abolished if 2.5mm-Ca2+ was added to medium containing EGTA. However, the increases in labelling of phosphatidylinositol due to α-adrenergic amines were still evident in medium containing EGTA and Ca2+. Lipolytic agents such as corticotropin, dibutyryl cyclic AMP, adrenaline in the presence of phentolamine and isoproterenol decreased [32P]Pi incorporation into phosphatidylinositol, phosphatidylethanolamine and phosphatidic acid. This inhibitory effect may be secondary to accumulation of intracellular unesterified fatty acids, since it was decreased by incubating fewer cells in medium with 6 rather than 3% albumin and was restored by the addition of oleate to the medium. The incorporation of [32P]Pi into phosphatidylcholine was unaffected by lipolytic agents. The data suggest that there is an inhibition of the synthesis of certain phospholipids in the presence of lipolytic agents, which may be secondary to intracellular accumulation of unesterified fatty acids.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Michell R. H. Enhanced synthesis de novo of phosphatidylinositol in lymphocytes treated with cationic amphiphilic drugs. Biochem J. 1975 Jun;148(3):471–478. doi: 10.1042/bj1480471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel A., Desai K. S., Halperin M. L. Reduction in adipocyte ATP by lipolytic agents: relation to intracellular free fatty acid accumulation. J Lipid Res. 1971 Mar;12(2):203–213. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Berridge M. J., Fain J. N. Inhibition of phosphatidylinositol synthesis and the inactivation of calcium entry after prolonged exposure of the blowfly salivary gland to 5-hydroxytryptamine. Biochem J. 1979 Jan 15;178(1):59–69. doi: 10.1042/bj1780059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelsen S., Pettinger W. A. A functional basis for classification of alpha-adrenergic receptors. Life Sci. 1977 Sep 1;21(5):595–606. doi: 10.1016/0024-3205(77)90066-2. [DOI] [PubMed] [Google Scholar]
- Brindley D. N., Bowley M. Drugs affecting the synthesis of glycerides and phospholipids in rat liver. The effects of clofibrate, halofenate, fenfluramine, amphetamine, cinchocaine, chlorpromazine, demethylimipramine, mepyramine and some of their derivatives. Biochem J. 1975 Jun;148(3):461–469. doi: 10.1042/bj1480461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Torrontegui G., Berthet J. The action of insulin on the incorporation of [32P]phosphate in the phospholipids of rat adipose tissue. Biochim Biophys Acta. 1966 Jun 1;116(3):477–481. doi: 10.1016/0005-2760(66)90117-2. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between hormonal activation of phosphatidylinositol hydrolysis, fluid secretion and calcium flux in the blowfly salivary gland. Biochem J. 1979 Jan 15;178(1):45–58. doi: 10.1042/bj1780045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser T. R. Is insulin's second messenger calcium? Proc R Soc Med. 1975 Dec;68(12):785–791. doi: 10.1177/003591577506801226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Strittmatter W. J., Axelrod J. beta-Adrenergic receptor agonists increase phospholipid methylation, membrane fluidity, and beta-adrenergic receptor-adenylate cyclase coupling. Proc Natl Acad Sci U S A. 1979 Jan;76(1):368–372. doi: 10.1073/pnas.76.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. B., De Lean A., Wood C. L., Schocken D. D., Lefkowitz R. J. Alpha-adrenergic receptor subtypes: quantitative assessment by ligand binding. Life Sci. 1979 May 7;24(19):1739–1745. doi: 10.1016/0024-3205(79)90061-4. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. Stimulus-response coupling at alpha-adrenergic receptors. Biochem Soc Trans. 1978;6(3):673–688. doi: 10.1042/bst0060673. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Larner J. Effects of insulin, methoxamine, and calcium on glycogen synthase in rat adipocytes. Mol Pharmacol. 1978 Nov;14(6):1079–1091. [PubMed] [Google Scholar]
- Malbon C. C., Moreno F. J., Cabelli R. J., Fain J. N. Fat cell adenylate cyclase and beta-adrenergic receptors in altered thyroid states. J Biol Chem. 1978 Feb 10;253(3):671–678. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Perry M. C., Hales C. N. Factors affecting the permeability of isolated fat-cells from the rat to [42K] potassium and [36Cl] chloride ions. Biochem J. 1970 Apr;117(3):615–621. doi: 10.1042/bj1170615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Smith T. L., Eichberg J., Hauser G. Postsynaptic localization of the alpha receptor-mediated stimulation of phosphatidylinositol turnover in pineal gland. Life Sci. 1979 Jun 4;24(23):2179–2184. doi: 10.1016/0024-3205(79)90116-4. [DOI] [PubMed] [Google Scholar]
- Sribney M., Knowles C. L., Lyman E. M. Regulation of phosphatidylcholine synthesis in rat liver endoplasmic reticulum. Biochem J. 1976 Jun 15;156(3):507–514. doi: 10.1042/bj1560507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. M., Hales C. N. Effect of adrenaline on 32 P incorporation into rat fat-cell phospholipids. Biochem J. 1972 Jul;128(3):531–541. doi: 10.1042/bj1280531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. M., Hales C. N. The effect of insulin on 32Pi incorporation into rat fat cell phospholipids. Biochim Biophys Acta. 1974 Jan 23;337(1):41–49. doi: 10.1016/0005-2760(74)90038-1. [DOI] [PubMed] [Google Scholar]
- Stein J. M. The effect of adrenaline and of alpha- and beta-adrenergic blocking agents on ATP concentration and on incorporation of 32Pi into ATP in rat fat cells. Biochem Pharmacol. 1975 Sep 15;24(18):1659–1662. doi: 10.1016/0006-2952(75)90002-7. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Charness M. E., Robertson D., Snyder S. H. Prazosin: differential affinities for two populations of alpha-noradrenergic receptor binding sites. Eur J Pharmacol. 1978 Jul 1;50(1):87–89. doi: 10.1016/0014-2999(78)90258-3. [DOI] [PubMed] [Google Scholar]
- Wood C. L., Arnett C. D., Clarke W. R., Tsai B. S., Lefkowitz R. J. Subclassification of alpha-adrenergic receptors by direct binding studies. Biochem Pharmacol. 1979 Apr 15;28(8):1277–1282. doi: 10.1016/0006-2952(79)90424-6. [DOI] [PubMed] [Google Scholar]


