Abstract
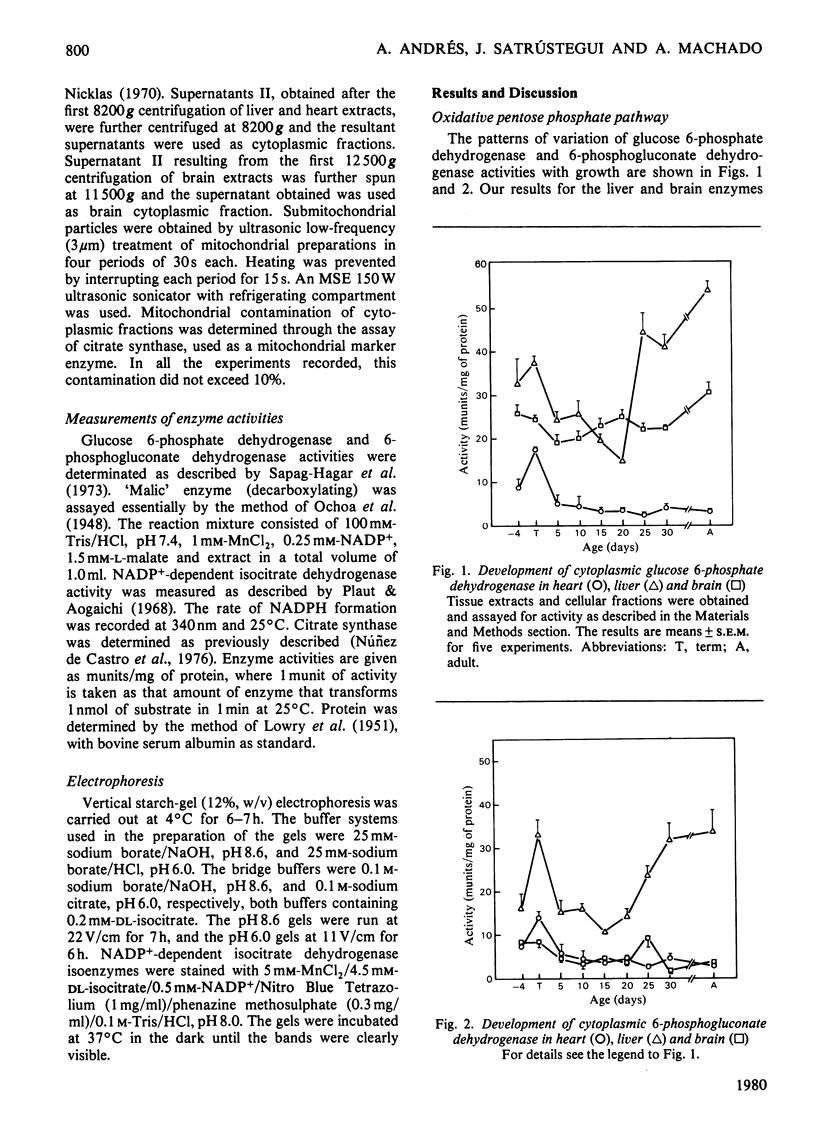
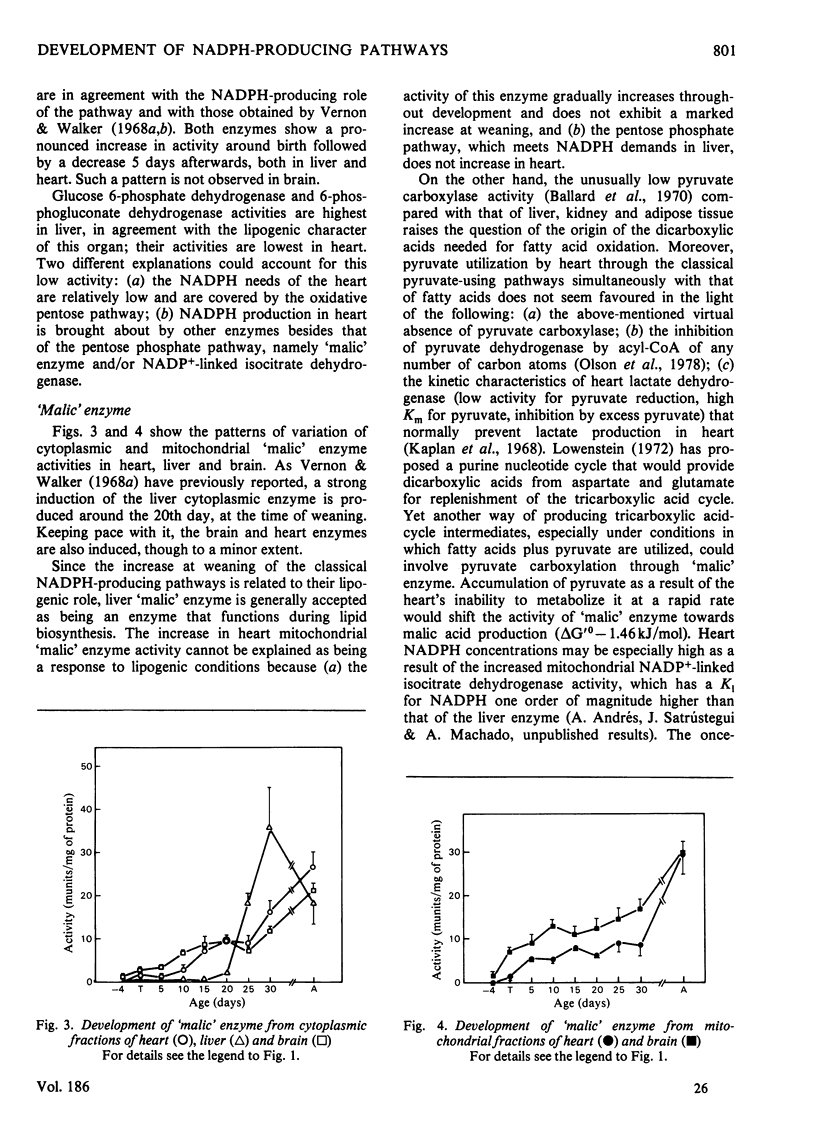
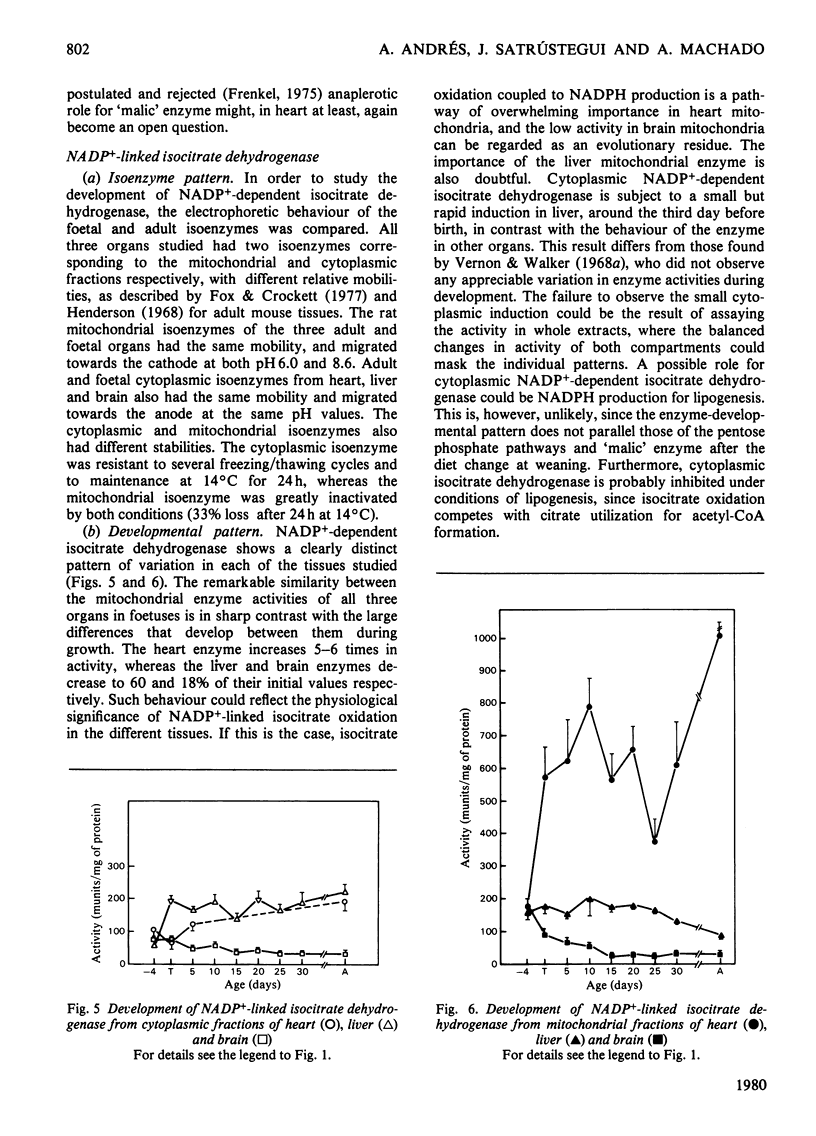
The behaviours of the principal NADPH-producing enzymes (glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, cytoplasmic and mitochondrial 'malic' enzyme and NAPD+-dependent isocitrate dehydrogenase) were studied during the development of rat heart and compared with those in brain and liver. 1. The enzymes belonging to the pentose phosphate pathway exhibit lower activities in heart than in other tissues throughout development. 2. The pattern of induction of heart cytoplasmic and mitochondrial 'malic' enzymes does not parallel that found in liver. Heart mitochondrial enzyme is slowly induced from birth onwards. 3. NADP+-dependent isocitrate dehydrogenase has similar activities in all tissues in 18-day foetuses. 4. Heart mitochondrial NADP+-dependent isocitrate dehydrogenase is greatly induced in the adult, where it attains a 10-fold higher activity than in liver. 5. The physiological functions of mitochondrial 'malic' enzyme and NADP+-dependent isocitrate dehydrogenase are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Hanson R. W., Reshef L. Immunochemical studies with soluble and mitochondrial pyruvate carboxylase activities from rat tissues. Biochem J. 1970 Oct;119(4):735–742. doi: 10.1042/bj1190735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chico E., Satrustegui J., Machado A. Constancy of the NADP-linked isocitrate dehydrogenase/transhydrogenase ratios of mitochondria of different mammalian tissues. Biochimie. 1977;59(11-12):933–934. doi: 10.1016/s0300-9084(78)80709-3. [DOI] [PubMed] [Google Scholar]
- Clark J. B., Nicklas W. J. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970 Sep 25;245(18):4724–4731. [PubMed] [Google Scholar]
- Fox D. J., Crockett R. V. Characterization of mitochondrial NADP-dependent isocitrate dehydrogenase from normal and genetically obese (ob/ob) mice. Biochem Med. 1977 Oct;18(2):192–205. doi: 10.1016/0006-2944(77)90090-4. [DOI] [PubMed] [Google Scholar]
- Frenkel R. Regulation and physiological functions of malic enzymes. Curr Top Cell Regul. 1975;9:157–181. doi: 10.1016/b978-0-12-152809-6.50012-3. [DOI] [PubMed] [Google Scholar]
- Guerra F. C. Rapid isolation techniques for mitochondria: technique for rat liver mitochondria. Methods Enzymol. 1974;31:299–305. doi: 10.1016/0076-6879(74)31031-2. [DOI] [PubMed] [Google Scholar]
- Henderson N. S. Intracellular location and genetic control of isozymes of NADP-dependent isocitrate dehydrogenase and malate dehydrogenase. Ann N Y Acad Sci. 1968 Jun 14;151(1):429–440. doi: 10.1111/j.1749-6632.1968.tb11906.x. [DOI] [PubMed] [Google Scholar]
- Kaplan N. O., Everse J., Admiraal J. Significance of substrate inhibition of dehydrogenases. Ann N Y Acad Sci. 1968 Jun 14;151(1):400–412. doi: 10.1111/j.1749-6632.1968.tb11903.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nuñez de castro I., Arias de Saavedra J. M., Machado A., Mayor F. Regulation of the level of yeasts citrate synthase by oxygen availability. Mol Cell Biochem. 1976 Sep 30;12(3):161–169. doi: 10.1007/BF01741714. [DOI] [PubMed] [Google Scholar]
- Olson M. S., Dennis S. C., DeBuysere M. S., Padma A. The regulation of pyruvate dehydrogenase in the isolated perfused rat heart. J Biol Chem. 1978 Oct 25;253(20):7369–7375. [PubMed] [Google Scholar]
- Plaut G. W., Aogaichi T. Purification and properties of diphosphopyridine nuleotide-linked isocitrate dehydrogenase of mammalian liver. J Biol Chem. 1968 Nov 10;243(21):5572–5583. [PubMed] [Google Scholar]
- Sapag-Hagar M., Lagunas R., Sols A. Apparent unbalance between the activities of 6-phosphogluconate and glucose-6-phosphate dehydrogenases in rat liver. Biochem Biophys Res Commun. 1973 Jan 4;50(1):179–185. doi: 10.1016/0006-291x(73)91080-2. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Estabrook R. W. Mitochondrial malic enzyme: the source of reduced nicotinamide adenine dinucleotide phosphate for steroid hydroxylation in bovine adrenal cortex mitochondria. Arch Biochem Biophys. 1969 Jan;129(1):384–395. doi: 10.1016/0003-9861(69)90190-8. [DOI] [PubMed] [Google Scholar]
- Stein A. M., Stein J. H., Kirkman S. K. Diphosphopyridine nucleotide specific isocitric dehydrogenase of mammalian mitochondria. I. On the roles of pyridine nucleotide transhydrogenase and the isocitric dehydrogenases in the respiration of mitochondria of normal and neoplastic tissues. Biochemistry. 1967 May;6(5):1370–1379. doi: 10.1021/bi00857a020. [DOI] [PubMed] [Google Scholar]
- Vernon R. G., Walker D. G. Adaptive behaviour of some enzymes involved in glucose utilization and formation in rat liver during the weaning period. Biochem J. 1968 Jan;106(2):331–338. doi: 10.1042/bj1060331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Walker D. G. Changes in activity of some enzymes involved in glucose utilization and formation in developing rat liver. Biochem J. 1968 Jan;106(2):321–329. doi: 10.1042/bj1060321. [DOI] [PMC free article] [PubMed] [Google Scholar]


