Summary
Emerging research suggests that exposures to metals during pregnancy and consequent disruptions in gut microbiome (GM) are associated with depressive disorders in childhood. Akkermansia muciniphila, a GM bacteria, has been studied for its potential antidepressant effects. However, its role in influencing the association between prenatal metal exposures and depressive symptoms during childhood is unknown. Leveraging a well-characterized pediatric birth cohort and its microbiome substudy (n = 112), we investigated whether a certain subgroup of children at 9-11-year-of-age (characterized by a specific pattern of prenatal exposure to groups of metals or metal-clique) had worsened depressive symptoms and if the presence of A.muciniphila in GM modifies this association. A subgroup of children characterized by the prenatal metal-clique signature of zinc-chromium-cobalt had significantly increased depression scores; however, within that subgroup, children with A.muciniphila had much lower depression scores than those without A.muciniphila in the GM. Our analysis provides exploratory evidence hypothesizing A.muciniphila as an intervention attenuating the effect of prenatal metal-exposures-associated depressive disorders in late childhood.
Subject areas: public health, microbiome, female reproductive endocrinology, psychology
Graphical abstract
Highlights
-
•
Metal exposures during pregnancy may affect depression symptoms in childhood
-
•
Presence of Akkermansia muciniphila is associated with lower depression symptoms
-
•
Akkermansia muciniphila may modify metal-exposures-associated depressive symptoms
Public health; Microbiome; Female reproductive endocrinology; Psychology
Introduction
Depression is a global health burden, with 37% of adolescents experiencing elevated depressive symptoms worldwide between 2010 and 2020.1 Emerging research indicates that disruptions in gut microbiota may be a contributor to the development of depressive disorders.2 Likewise, beneficial or probiotic gut bacteria may help alleviate depressive symptoms. Akkermansia muciniphila is one such potentially beneficial bacteria that may be a promising avenue for preventive intervention of depression in childhood and adolescence.3
A. muciniphila is a commensal inhabitant of the human gastrointestinal tract throughout life.3,4 Extensive phylogenetic and metagenomic investigations have consistently identified A. muciniphila as one of the top twenty most prevalent species in the human gut.4,5,6,7,8,9 Within the first year after birth, A. muciniphila establishes stable colonization in the gut, reaching levels similar to those found in healthy adults, with abundance gradually declining in the elderly.3,4,10 A. muciniphila was detected in human milk, indicating its transfer from mothers to infants through breastfeeding and the presence in the gastrointestinal tract of newborn infants.3,4 During this early developmental stage, A. muciniphila exhibits successful colonization in the gastrointestinal tract due to its active acid resistance system and capacity to degrade human milk oligosaccharides in the stomach of newborn infants.3,11 A. muciniphila’s inhabitance of the human gut microbiome (GM) during critical stages of human development may lead to A. muciniphila playing an important role in neurodevelopment via the gut-brain axis, which facilitates bidirectional interactions between the brain and the gut.12 The GM, including A. muciniphila, has been significantly associated with neuropsychiatric disorders, notably depression and anxiety.13 A previous study found that A. muciniphila treatment significantly ameliorated depressive-like behavior in chronic restraint stress-exposed mice.14 Further, recent studies show that supplementation of A. muciniphila could relieve depression-like symptoms in recipient mice.15 These relationships may also exist in humans, though further epidemiologic investigation is needed.
Even at low levels, increased metal concentrations during childhood and adolescence were associated with increased depressive symptoms.16,17 Prenatal exposures to lead (Pb) and manganese (Mn) have also been associated with mid-childhood to adolescent internalizing symptoms.18 Exposure to multiple metals simultaneously may further strengthen this association. For instance, combined exposure to organochlorine and metal mixtures has been further associated with anxiety/depressive symptoms during adolescence.18 While this evidence suggests a link between prenatal exposure to individual metals and some mixtures, not all components within a mixture are necessarily equally important.19 In other words, such associations might be only observed in certain subgroups of the population with specific patterns of metal exposures.20,21 Like genetic signatures, metal-clique signatures represent a certain subgroup of the population with uniquely characteristic patterns of metal exposures that essentially identify specific subgroups. As a direct application of the conceptual framework of precision environmental health,22 the metal clique signatures identify subgroups based on patterns of metal exposures. However, few, if any, environmental epidemiology investigations have focused on such subgroup-specific exposure to metals.
Multiple studies have linked individual or mixtures of metal biomarkers with depression symptoms.23,24,25 Yet, few have investigated their interaction through metal cliques, and consequently, the etiological mechanisms are not well understood. Studies in human and animal models have linked dysregulated metal homeostasis to multiple neurological conditions. Altered levels of trace elements, including manganese (Mn), copper (Cu), and zinc (Zn) were associated with worsened neurological health outcomes like bipolar mood disorder, dementia in Alzheimer’s disease, and autism.26,27,28 Recent studies in children with autism spectrum disorder (ASD) suggest that interactions between dysregulated fetal Cu and Zn levels may precede ASD diagnosis.29 Further, altered homeostasis and nutritional deficiencies in trace elements may worsen internalizing symptoms.30,31 A few studies have further hypothesized that metal ion interactions may induce mood-stabilizing effects.32 Therefore, according to the developmental origins of health and disease (DOHaD) hypothesis, such alterations in metal concentrations may occur even during pregnancy and could have a long-term effect on child neurodevelopment.
Exposure to toxic metals is also associated with the composition, diversity, and functional activities of the GM.33,34,35,36 We previously found that higher prenatal lead exposure reduced the diversity and abundance of beneficial taxa within the GM in children during mid-childhood (9–11 years).37,38 Although metals have been linked to both alterations of the GM and increased depression symptoms, it is not well understood whether there are specific sub-populations with worsened symptoms than the rest. Identifying such subgroups is important since (1) not all individuals are equally susceptible to altered levels of metal exposure during pregnancy, and (2) distinguishing such heterogeneous adverse effects is pivotal for public health policy. Since such altered exposure occurred during pregnancy, research is needed to address plausible strategies for intervention to counteract their possible long-term adverse effects that can impact individual lives. Understanding gut microbial exposomics from the window of intervention strategies is a possible way forward. Moreover, given the recent and rapidly developing research on probiotic GM, it is a natural, well-mitigated strategy to study their impact on adverse effects of past metal exposures. Further, with the recent studies of the beneficial effect of A. muciniphila in neurodevelopment, it is quite pivotal to explore its specific role in modifying any association between metal exposure and depression. This study, therefore, investigates the role of A. muciniphila in modifying the association between prenatal metal clique signature and depressive symptoms in late childhood from a precision environmental health perspective.
Results
Characteristics of study population
Data comes from the Programming Research in Obesity, Growth, Environment, and Social Stressors (PROGRESS) cohort, which is an ongoing prospective birth cohort study conducted in Mexico City, Mexico. Table 1 provides the descriptive statistics of the 112 participants included in this study, stratified by the presence/absence of A. muciniphila detected in 9-11-year-old gut samples. The mean (SD) relative abundance of A. muciniphilia in this sample was 0.09 (0.48). A. muciniphilia was present (i.e., defined as an estimated relative abundance more than zero) in 24% of participants. Among those with a non-zero relative abundance of A. muciniphilia, the distribution (as well as the spread) of the relative abundance was 0.0003, 0.0113, 0.0456, 0.2986, and 4.7789, at 0th, 25th, 50th, 75th and 100th percentiles, respectively. Mothers of children with A. muciniphila exhibited a lower BMI of 26.33 kg/m2 in pregnancy. The average t-scored Childhood Depression Inventory (CDI) was 52.97 (range: 40–81). On average, those without gut A. muciniphila had a higher CDI score (i.e., more depressive symptoms). For further analysis, we converted the relative abundance of A. muciniphila as a binary indicator of presence and absence to ward off any influence of outliers. The Spearman correlation between the metal concentrations is presented in Figure S1, where we observed stronger positive correlations between metals within the third trimester, followed by the second trimester. However, the inter-correlation between metal concentrations at the second and third trimesters was relatively lower. There was no significant difference between concentration at the second and third trimesters for most metals, except for Mn, Co, and Zn. Further, the concentrations of all three metals, Mn, Co, and Zn, were higher in the third trimester (Median [Inter Quartile Range] of concentrations are shown in Table S1). Within each trimester, the concentrations of the metals did not significantly differ with respect to the presence (or absence) of A. muciniphila (see Table S2 for stratification by the presence of A. muciniphila).
Table 1.
Descriptive statistics of covariates and depression scores from 9 to 11 years of age PROGRESS participants included in this study (n = 112)
| Overall |
A. muciniphila Absent |
A. muciniphila Present |
p-value | |
|---|---|---|---|---|
| Covariates | ||||
| Child Sex | 0.83 | |||
| Male n(%) | 68 (60.7) | 51 (60.00) | 17 (63.0) | |
| Female n(%) | 44 (39.3) | 34 (40.00) | 10 (37.0) | |
| Maternal SES in pregnancy | 0.71 | |||
| Lower n(%) | 61 (54.5) | 48 (56.5) | 13 (48.2) | |
| Medium n(%) | 41 (36.6) | 29 (34.1) | 12 (44.4) | |
| Higher n(%) | 10 (8.9) | 8 (9.4) | 2 (7.4) | |
| Maternal age at birth (years) mean(Sd) | 28.7 (5.8) | 29.2 (5.9) | 27.1 (5.4) | 0.12 |
| Maternal BMI in pregnancy (kg/m2) mean(Sd) | 27.3 (4.5) | 27.6 (4.6) | 26.3 (4.2) | 0.28 |
| Child age at gut microbial sample collection (years) mean (Sd) | 9.7 (0.9) | 9.6 (0.9) | 9.8 (1.0) | 0.42 |
| Outcome | 0.07 | |||
| t-scored Childhood Depression Inventory mean(Sd) | 53.0 (8.1) | 53.7 (8.1) | 50.7 (7.8) | |
The p-values comparing PROGRESS participants with vs. without gut A. muciniphila were calculated using Fisher’s exact test or the Wilcoxon rank-sum test, depending on the underlying variable.
Individual associations with childhood depressive symptoms
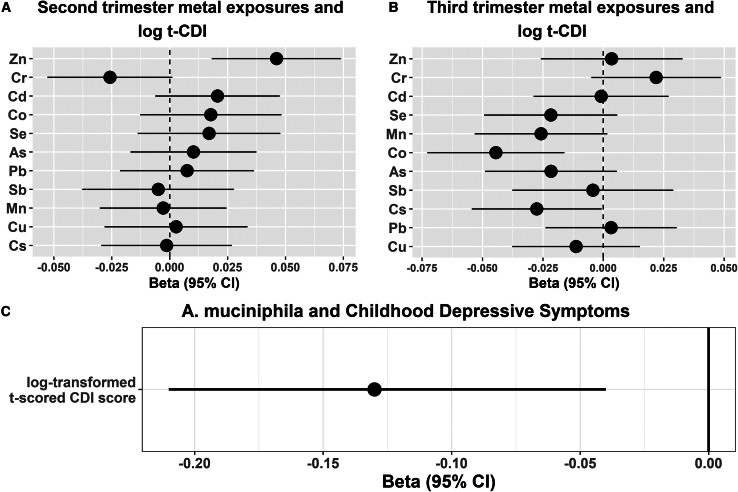
We first presented the preliminary results of individual analyses with metals and the presence of A. muciniphila. The presence of A. muciniphila was associated with significantly decreased t-scored CDI (log-tCDI) (beta = −0.13, 95%CI = [−0.21,−0.04], the randomization-based robust p-value [Prand < 0.0001]) (Figure 1C). The result remained statistically significant and preserved the directionality even on non-log transformed t-scored CDI. Associations with individual prenatal metal exposures and t-scored CDI were previously reported in our previous study.39 However, we decided to present these results because these newly estimated associations were reported on a covariate-balanced dataset (therefore doubly robustness in estimates and systematically differs from the previous ones) and for the continuity of the narrative. A few individual metal exposures during pregnancy were significantly associated with log-tCDI (Figures 1A and 1B). In the 2nd trimester, higher Cr exposure was associated with decreased log(t-CDI) ( [95% CI] = −0.03[-0.05, 0.00],p = 0.05). In contrast, increased Zn exposure was associated with a higher log(t-CDI) score ( [95% CI] = 0.04[0.01, 0.06], p = 0.008). Similarly, in the 3rd trimester, higher Co and As were associated with decreased log(t-CDI) scores ( [95% CI] = -0.04[-0.07, −0.02], p = 0.002 and −0.03[-0.06, −0.01], p = 0.02, respectively), while Cr was associated with increased log(t-CDI) ( [95% CI] = 0.03[0.00, 0.05], p = 0.04). Only the false discovery rate (FDR)-adjusted p-value associated with Co, in the 3rd trimester, was lower than 0.05. These individual associations provide us with a sense of directionality and overall estimates; however, this paper’s primary interest is finding combinations of metal-clique signatures, which we present in the next subsection.
Figure 1.
Associations between individual metal exposures during pregnancy, the presence of A. muciniphila in childhood gut-microbiome, and log-tCDI scores (n = 112) in the PROGRESS children at 9–11 years of age
(A) shows associations (beta estimates and 95% CIs) with metal exposures in the 2nd trimester. Dotted vertical line denotes the null association.
(B) shows associations (beta estimates and 95% CIs) with metal exposures in the 3rd trimester. Dotted vertical line denotes the null association.
(C) shows an association between the presence of A. muciniphila and Childhood Depressive Symptoms. t-CDI: t scored Child Depression Index.
Association between prenatal metal-clique signature and childhood depressive symptoms and effect modification by A. muciniphila
A metal-cliques signature denotes a specific subgroup of children with certain patterns of metal exposure, and therefore, it can be regarded as a metallomics signature only present in certain sub-populations. We obtained 123 unique clique signatures (of order two) from rh-SiRF. Among these, only 3% of cliques have a stability (i.e., frequency of occurrence) of more than 5% (we presented the list of top 10 combinations in Table S3). We chose the top three combinations since those formed a closed-looped network (Figure S2 for a forced-directed graph of this clique). We identified a three-component metal-clique signature composed of (1) high Zn in the 2nd trimester (concentration ≥ 20th percentile of the sample), (2) low Co in the 3rd trimester (concentration ≤ 80th percentile of the sample), and (3) low Cr in the 2nd trimester (concentration ≤ 55th percentile of the sample). This three-component metal-clique signature is essentially a binary indicator that specifies a subgroup of children (comprising almost 40.9% of the sample) with a specific pattern of prenatal metal exposure. Next, this clique signature was put into a regression model to estimate the association with log-tCDI on the covariate-balanced dataset, further controlling for covariates and confounders.
The metal-clique signature high Zn in 2nd trimester-low Co in 3rd trimester-low Cr in 2nd trimester was obtained explicitly from the results of the rh-SiRF algorithm, as this particular combination occurred most frequently among all the trees in the random forest.
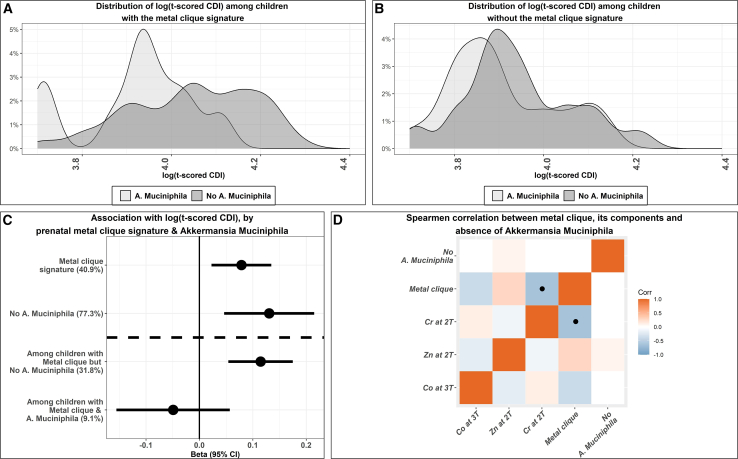
Further, note that this metal-clique signature differs from a mixture association since this unique signature is only identified in a particular subgroup of the children. The distributions of the log-tCDI were shown in Figures 2A and 2B for children with (and without) the metal-clique signature. Within children without the metal-clique signature, the distribution of log-tCDI is similar between children with or without A. muciniphila. In contrast, the distributions of log-tCDI among children with the metal-clique signature differ drastically between those with and without A. muciniphila. The three-component metal-clique signature of high Zn, low Cr in the 2nd trimester, and low Co in the 3rd trimester was significantly associated with an increased depression score ( [95% CI] = 0.08[0.02, 0.13], Prand < 0.0001) (Figure 2C). However, this metal-clique signature was not correlated with the presence of A. muciniphila (Figure 2D), indicating that A. muciniphila is not a mediator between this metal-clique signature and childhood depression scores. However, the presence of A. muciniphila modifies this association. For 31.8% of children with no A. muciniphila, the metal-clique signature was strongly associated with increased depression score ( [95% CI] = 0.11[0.05, 0.18], Prand < 0.0001). In contrast, for children with A. muciniphila, this same metal-clique signature was weakly associated with decreased depression scores in almost 9.1% of children ( [95% CI] = -0.05[-0.16, 0.06], Prand < 0.4). We further estimated the Spearman correlations between the components of this metal-clique signature, the overall indicator of the three-component metal-clique signature, and the absence of A. muciniphila (Figure 2B). Correlations between the metal components were minimal, which implies that correlation between metal concentrations did not have a significant effect in forming the clique, indicating a non-linear interaction. We did not undertake a mediation analysis mainly because (1) the metal-clique signature was not correlated with the presence or absence of A. muciniphila (Figure 2D), (2) the microbiome and the depression outcome were measured at similar time points.
Figure 2.
The distribution and the association with prenatal metal-clique signature and log-tCDI scores and the effect modification by the absence of A. muciniphila in childhood gut microbiome
(A) shows the distribution of log-tCDI scores among children with the metal-clique signature (between those with and without A. muciniphila).
(B) shows the distribution of log-tCDI scores among children without the metal-clique signature (between those with and without A. muciniphila).
(C) shows the beta coefficients and 95% CIs for the association with prenatal metal-clique signature and log-tCDI scores and the effect modification by the absence of A. muciniphila; the proportions of the sample characterized by the clique are shown in brackets on the y axis.
(D) shows the Spearman correlations among the components of the metal-clique signature (individual metal concentrations), the metal-clique signature, and the indicator for the absence of A. muciniphila among the 112 PROGRESS children; the blue colored boxes with black dots imply statistically significant associations (p-value is 0.03). The colored but blank boxes without any symbol denote statistically non-significant correlation coefficients. t-CDI, t scored Child Depression Index.
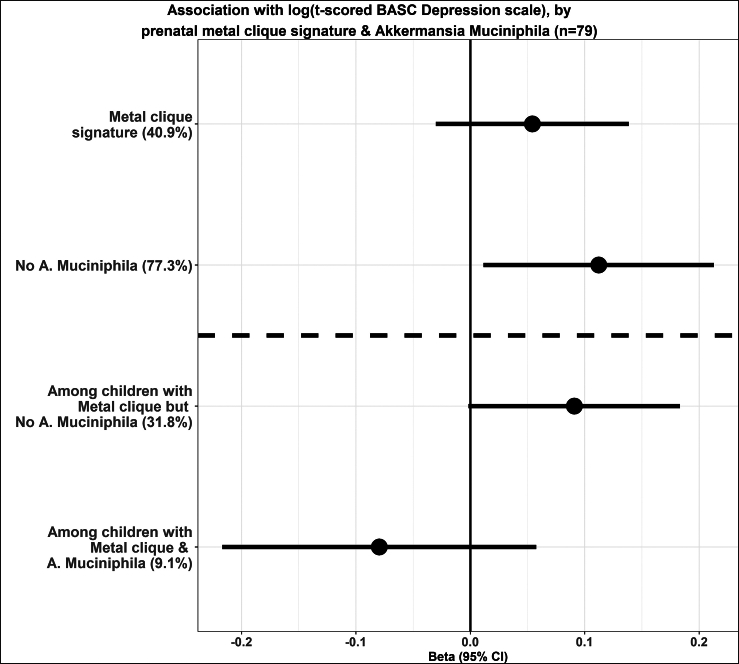
Validation analysis with BASC-2 depression scale
We repeated the analysis with the same metal clique signature and A. muciniphila with the BASC-2 depression score to verify whether the previously observed associations could be replicated. There were 79 children with both scores available; therefore, the analysis was repeated on this sub-sample. The correlation between the two scores was 0.57, indicating a moderate to strong association between both measures. The associations are presented in Figure 3. Overall, we observe a similar pattern of associations and the effect modification by A. muciniphila.
Figure 3.
The beta coefficients and 95% CIs for the association with prenatal metal-clique signature and log-t scored BASC depression and the effect modification by the absence of A. muciniphila; the proportions of the sample characterized by the clique are shown in brackets on the y axis
The sample size for this analysis was 79. BASC: Behavioral Assessment System for Children.
Sensitivity analyses
We carried out multiple sensitivity analyses to verify our results remain robust: (1) for the major associations (except the forest plots for individual metal exposures), we estimated randomization-based p -values (Prand) (that are robust against any assumption of normality) by permuting each of the outcomes 106 times. The significant Prand values were far lower than the model-based p-values (Table S4). (2) Because of the small sample, we chose minimal covariates to adjust in the models; still, we incorporated techniques like “covariate-balancing” to obtain robust results (the covariate-balanced love plot is presented in Figure S3). (3) Moreover, the presence of A. muciniphila remained strongly associated with significantly decreased log-tCDI scores even on the non-covariate balanced dataset ( [95% CI] = -0.10[-0.18,-0.01]). (4) Directions for the metal-clique signature associations (with and without the presence of A. muciniphila) remained similar to the main findings under alterations to each of the thresholds (increase and decrease by ten percentiles) (Figures S4 and S5), implying greater robustness. (5) Results remained robustly similar when we binarized the t-scored CDI (≥75th percentile) and repeated the metal-clique associations (Figure S6). (6) We used a negative control outcome – having a pet (at the time of microbial sample collection), which might have similar sources of potential selection bias but would not be causally related to the identified metal-clique signature. In the association with the metal-clique signature, we found a null association with different directionality (OR[95% CI]:0.7[0.2,2.5]) between the metal-clique signature and having a pet as the outcome. This illustrates a reduced potential of selection and residual confounding biases. (7) Finally, in Figure S7, we show the Spearman correlations among the individual metal concentrations, the metal-clique signature, and the indicator for the absence of A. muciniphila with the non-significant correlations as blank.
Discussion
In this study, we explored the modifying effect of A. muciniphila on the associations between prenatal exposure to a certain metal-clique signature and depressive symptoms in late childhood. Our results suggest that a subgroup of children characterized by the prenatal metal-clique signature of zinc-chromium-cobalt had significantly increased depression scores in late childhood. In the absence of A. muciniphila in childhood GM, children with this metal-clique signature had significantly higher depression symptoms. However, for children with A. muciniphila, this metal-clique signature was weakly associated with lower depression symptoms. This analysis provides the first exploratory evidence that the presence of A. muciniphila likely attenuates the association between specific prenatal exposure to metals and depression in later childhood in certain subgroups of children.
A. muciniphila is a symbiotic bacterium colonizing the intestinal epithelium’s mucosal layer. This mucus layer comprises high-molecular-weight glycoproteins called mucins.40 A. muciniphila breaks down mucins, producing short-chain fatty acids (SCFAs) that facilitate the bacteria’s colonization process and provide energy and neuromodulators for the host.41 These SCFAs contribute to the maturation of the immune and neurological systems.41 As a member of the GM, A. muciniphila communicates with the central nervous system (CNS) via the microbiota-gut-brain axis (MGBA).42,43,44 Reduced A. muciniphila levels have been seen in mice and rats displaying depression-like behavior.45,46 In the FinnBrain Birth Cohort, the prevalence of Akkermansia was inversely associated with maternal postpartum depression symptoms.47 Depression is associated with lower levels of brain-derived neurotrophic factor (BDNF), and the neurotrophic hypothesis connects this decrease in BDNF to the pathophysiology of depression.48,49,50 A. muciniphila counters this by enhancing BDNF expression, fostering synaptic pathways, and reducing depression symptoms.2,13,14 Individuals with depression often have reduced 5-HT (5-hydroxytryptamine or serotonin) levels, aligned with the monoamine deficiency hypothesis, which posits that the fundamental physiological cause of depression involves a reduction in serotonin, norepinephrine, and/or dopamine within the CNS.51,52,53 A. muciniphila positively influences host 5-HT levels in the intestine through factors like its outer membrane protein Amuc_1100, which elevates intestinal 5-HT expression.54,55 When considering our findings with those presented from these animal and human studies, there appears to be a consistent negative correlation between the presence and prevalence of Akkermansia and depressive behavior, with multiple potential biological mechanisms.13 This recurring pattern strongly suggests the potential utility of Akkermansia as a viable strategy to alleviate depression symptoms; however, further longitudinal intervention studies with large samples are needed.13
We found that the presence of a prenatal metal-clique, including high Zn and low Cr in the second trimester and low Co in the third trimester, was associated with a higher depression index in children, and the association was reduced in children who had A. muciniphila in the GM. Previous research has indicated that heavy metal exposure can initiate neuro-inflammation, oxidative stress, hormonal imbalances, and disruptions to neurotransmitters like dopamine and serotonin, all of which can contribute to the etiology of depressive disorders.24,56,57,58 For example, Rokoff et al. show that prenatal exposure to Pb was found to be linked to increased anxiety symptoms during adolescence. In contrast, prenatal exposure to Mn was positively correlated with internalizing symptoms, particularly among girls from mid-childhood through adolescence.18 Gari et al. report that prenatal concentrations of micronutrients, Se and Zn, and neurotoxic metals, Pb and Hg, exert notable influences on the neuropsychological development of children at the age of 7.59 A previous study by our group found that a metal-microbial clique of high Zn in the second trimester, low Co in the third trimester, and high abundance of Bacteroides fragilis and Faecalibacterium prauznitzii in childhood was associated with increased depression scores.20 Co is a component of vitamin B12, which may be associated with depression,60,61 and has been previously associated with A. muciniphila.62 The results of our study in the context of these previous findings suggest that in utero exposure to metals could be, particularly important in contributing to the development of anxiety and depression symptoms.
Exposure to metals can impact GM composition and function, especially during early development. The existing body of knowledge about the interactions between metals and Akkermansia in human physiology is limited, and exposures during the prenatal period appear entirely unexplored with Akkermansia. Our analysis did not indicate strong correlations between the prenatal metal-clique signature and A. muciniphila; however, previous research indicated that toxic metals like Cd, Pb, Cu, and Al reduced A. muciniphila levels in mice and common carp.63,64,65,66,67 Shen et al., found that higher childhood blood Mn may lead to lower mucin degradation and energy generation and is significantly associated with lower Akkermansiaceae.34 Their findings indicate a potential association between metal exposure in childhood and Akkermansia abundance, but they did not find an association with earlier exposures. Although there is limited evidence of interaction between Akkermansia in GM and metal exposure, there is evidence of potential biological mechanisms that may combine to influence human health. Metal exposure may alter the relative abundance of Akkermansia within the GM or reduce its ability to produce SCFAs,68,69 either of which may reduce Akkermansia’s ability to communicate with the CNS and potentially influence depression etiology. A. muciniphila also helps strengthen the epithelial barrier in the gut,70 while metal exposures can negatively impact gut barrier function.71 Our finding of modification by A. muciniphila between metal-cliques and depression may function through improved epithelial barrier strength. Alternative mechanisms may also exist, supporting the need for further investigation in this area.
While this study contributes to the growing body of evidence concerning the adverse link between metal exposure, depression, and human GM, some limitations must be acknowledged. The sample size limited our ability to make more robust conclusions due to a lack of power. Nevertheless, consistent associations across various sensitivity analyses and using causal-inference methods boost the inferences, even though some estimates did not reach statistical significance. The literature points out the importance of physical activity during pregnancy on trace elements, gut microbiota, and childhood depression.72,73 The level of physical activity among study participants has not been evaluated in detail; therefore, we acknowledge this as another limitation of this study. Additionally, measuring prenatal metal exposure through maternal blood during pregnancy is suboptimal as it does not directly gauge fetal metal exposure. For example, the presence of higher Pb levels in placental samples than in the umbilical cord points to the placenta’s role as a potential barrier and to the fact that it probably accumulates Pb throughout pregnancy.74,75,76 Furthermore, placental tissue had the most abundance of metals like Mn, followed by maternal serum, suggesting that Mn crosses the placenta via active transport.74,75,76 In the analysis, we did not control the models by diet-related covariates due to a lack of information collected during the survey. However, in this ongoing PROGRESS cohort, we are now collecting data using a food frequency questionnaire that we will use as part of our future research. We further hypothesize that the modifying effect of Akkermansia will be stronger in a subgroup of children with relatively unhealthy diet patterns, making the currently observed associations will more significant.
Strengths of this study include the novel investigation of Akkermansia as a modifier of prenatal metal exposures and childhood depression. Further, the earliest measurement of postnatal microbiome was available in late childhood; therefore, we could not include earlier stages of microbiome in this current analysis. We performed robust statistical analysis, applying tools from current state-of-the-art machine-learning and causal inference literature and using the presence of A. muciniphila instead of its relative abundance to minimize the plausibility of measurement error. Our analysis of metal-clique signatures reflects the application of a precision environmental framework. It further adds novel insight into combinations of metal exposures that are found in susceptible subgroups of the population, potentially making them more vulnerable to childhood depression. Findings from this study have translational potential, indicating A. muciniphila as an intervention avenue to help prevent depression in children with prenatal metal exposures. Additional future directions of this work include (1) mediation analysis with depression measured later in adolescence and in vitro and animal experiments to help establish the biological plausibility of associations between metal exposures and A. muciniphila and mechanisms of modification; (2) inclusion of additional essential and non-essential metals during pregnancy for a detailed “metallomics” mixture analysis.
In conclusion, this study suggests that subgroups of children with specific patterns of metal exposures during pregnancy are more likely to have higher depression symptoms, and the presence of A. muciniphila may be associated with attenuating that detrimental effect. Further observational, experimental, and translational investigation is needed to fully understand the occurrence, mechanisms, and potential interventions along this pathway.
Limitations of the study
Although our study incorporates multiple layers of exposomics and provides a unique perspective on prenatal metal exposure, childhood GM, and depressive symptoms, there are a few limitations to note. The cross-sectional nature of GM data and depression symptom measurements prevents us from drawing any inferences regarding causality. Additionally, the study’s sample size is relatively small, even though we observed significant associations. But, it should be noted that a smaller sample size can lead to higher type 2 errors and, therefore, lower power to detect small effect sizes. Furthermore, the findings need to be replicated in a separate cohort with possibly different geographical locations (with varying patterns of metal exposures). Lastly, the lack of data on maternal and child diet and physical activity levels needs to be considered while interpreting the results.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Vishal Midya (vishal.midya@mssm.edu).
Material availability
This study did not generate new unique reagents or materials.
Data and code availability
-
•
Metagenomic data are publicly available at https://www.ncbi.nlm.nih.gov/sra/PRJNA975184. All other data on the PROGRESS cohort are available upon request at robert.wright@mssm.edu.
-
•
A schematic of the algorithm used and R code with illustrations on a simulated dataset is provided on GitHub (https://github.com/vishalmidya/MiCA-Microbial-Co-occurrence-Analysis/blob/main/MiCA-vignette.md).
Acknowledgments
The authors would like to acknowledge the entire PROGRESS study team, as well as the participants. We would also like to thank Jeremiah Faith and the Microbiome Translational Center at the Icahn School of Medicine at Mount Sinai. Additionally, this work was supported in part through the computational and data resources and staff expertise provided by Scientific Computing and Data at the Icahn School of Medicine at Mount Sinai and supported by the Clinical and Translational Science Awards (CTSA) grant UL1TR004419 from the National Center for Advancing Translational Sciences. Research reported in this publication was also supported by the Office of Research Infrastructure of the National Institutes of Health under award numbers S10OD026880 and S10OD030463. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the National Institute of Environmental Health Sciences (R00ES032884, P30ES023515, R01ES013744, R35ES030435, U2CES026555).
Author contributions
V.M.: writing—review and editing, writing—original draft, visualization, validation, software, methodology, investigation, formal analysis, conceptualization, funding acquisition. K.N.: writing—review and editing, writing—original draft, investigation. J.M.L.: writing—review and editing, investigation. L.A.T.-O.: writing—review and editing, resources, project administration, investigation, data curation. G.G.M.: writing—review and editing, resources, investigation. M.K.H.: writing—review and editing, investigation. C.G.: writing—review and editing, supervision, methodology, investigation, funding acquisition. M.M.T.-R.: writing—review and editing, supervision, resources, investigation, data curation. R.O.W.: writing—review and editing, supervision, resources, investigation, funding acquisition, data curation. M.A.: writing—review and editing, supervision, investigation, funding acquisition. S.E.: writing—review and editing, writing—original draft, validation, resources, methodology, investigation, funding acquisition, formal analysis, conceptualization.
Declaration of interests
M.A. is an employee and equity holder of Linus Biotechnology Inc., a start-up company of Mount Sinai Health System. The company develops tools for the detection of ASD and related conditions. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human gut microbial Raw 16S rRNA data |
Eggers et al.37 | SRA: PRJNA975184 |
| Blood metal measurement (included manganese (Mn), cesium (Cs), antimony (Sb), arsenic (As), selenium (Se), cadmium (Cd), zinc (Zn), chromium (Cr), copper (Cu), cobalt (Co), and lead (Pb)) | Rodosthenous et al.77 | National Institute of Environmental Health Sciences: The Programming Research in Obesity, GRowth, Environment and Social Stress (PROGRESS) Cohort (nih.gov) |
| Software and algorithms | ||
| Code for rh-SiRF | This paper | https://github.com/vishalmidya/MiCA-Microbial-Co-occurrence-Analysis/blob/main/MiCA-vignette.md |
| R version 4.1.1 | Open Source | https://www.r-project.org/ |
| Other | ||
| Programming Research in Obesity, Growth, Environment, and Social Stressors (PROGRESS) cohort | PROGRESS - Institute for Climate Change, Environmental Health, and Exposomics (mountsinaiexposomics.org) | National Institute of Environmental Health Sciences: The Programming Research in Obesity, GRowth, Environment and Social Stress (PROGRESS) Cohort (nih.gov) |
Experimental model and study participant details
This study included data on 112 children (68 males and 44 females) aged 9-11 years from an ongoing prospective birth cohort study conducted in Mexico City, Mexico. Summarized deidentified details on demographics are provided in Table 1. The research protocols for both the main PROGRESS study and its microbiome sub-study underwent thorough review and were granted approval by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai in New York and the National Institute of Public Health in Cuernavaca, Mexico.
Method details
Study population
PROGRESS is an ongoing prospective birth cohort study conducted in Mexico City, Mexico. During early pregnancy, this study enrolled 948 women affiliated with the Mexican Social Security Institute. PROGRESS cohort is a well-characterized ongoing longitudinal birth cohort that has been continuously funded since 2007.78 It closely tracked the development of the infants during their early years, with assessments every six months initially and later biannually. Comprehensive longitudinal follow-up included surveys, physical examinations, and psychological/behavioral evaluations. Biological samples from mothers and children, including blood, were collected and archived at each visit. Additionally, a convenience subsample of participants (n = 123) contributed stool samples from children aged 9–11 years.37 Of the 123 participants with stool samples, a subset of 112 participants had completed outcome data. The current samples were chosen using a convenience sampling strategy due to time restrictions and the cost of gut microbial sample analysis. As a posthoc power estimation, given the sample size and the observed effect sizes, this analysis is well-powered to detect moderate to large standardized effect sizes at 80% power.
Blood metal measurements
Details on the blood metal measurements are available in our previous works and were discussed in detail.79,80 Briefly, during the 2nd and 3rd-trimester visits (18.3 and 31.6 gestational weeks, respectively), maternal blood samples were collected using standard venipuncture.81 Blood specimens were drawn using tubes free from trace metals and stored at temperatures between 2°C and 6°C until analysis. At the “trace metals laboratory of the Icahn School of Medicine at Mount Sinai” and using the “Agilent 8800 ICP Triple Quad (ICP-QQQ) in MS/MS mode”, multiple metal concentrations were measured, which included manganese (Mn), cesium (Cs), antimony (Sb), arsenic (As), selenium (Se), cadmium (Cd), zinc (Zn), chromium (Cr), copper (Cu), cobalt (Co), and lead (Pb). The limits of detection for each metal were: 0.391 μg/L for As, 0.113 μg/L for Cd, 0.117 μg/L for Co, 0.901 μg/L for Cr, 0.122 μg/L for Cs, 1.862 μg/L for Cu, 0.442 μg/L for Mn, 0.377 μg/L for Pb, 0.159 μg/L for Sb, 0.665 μg/L for Se, and 5.053 μg/L for Zn. Measurements were taken in five replicates and reported as an average. The laboratory elemental analysis quality assurance and quality control (QA/QC) procedures included the analyses of calibration standards in the range of 0.001 to 50 ng/ml, initial verification standards, and continuous calibration verification standards. The QC samples consisted of (1) mixed element standards purchased from a different vendor as the calibration standards at two different concentration levels, (2) procedural blanks, (3) 5% of samples prepared and analyzed in duplicate, and (4) in-house pooled blood (IHB) samples at three levels to monitor the accuracy. Lastly, the certified reference materials were from Seronorm Blood (L2, L3) - SERO AS, Billingstad, Norway, and NIST SRM 955c (L1, L2, L3, L4) Toxic Metal in Caprine Blood, Gaithersburg, US. In order to evaluate the inter-day variability of the elemental analysis, two samples were prepared in large quantities, and five aliquots of each were analyzed in every analysis batch: (1) one QC solution containing the same matrix, spiked to a final concentration of 5 ng/ml, and (2) one in-house no-spiked pooled blood sample that contains the elements studied below the 20 percentile of the US reported levels for each analyte. These two described the inter-day variability at a low but detectable exposure level and an ultra-trace low level for each element. Further, these two concentrations help to illustrate the overall performance from one batch to another. Since there is no certified value for the IHB material, only the variabilities were reported. As the levels approach the method limit of detection, the variability is expected to increase; a % relative standard deviation (RSD) below 25% is generally accepted as sufficient. In our case, all of the five ng/ml QC samples fulfilled this criterion, while some ultra-trace elements in the IHB material were below the method limit of detection and, therefore, showed high %RSD values. This method's Lab recovery rates for QC were 90% to 110%, and inter-day and intra-day precision is below 6% for samples with concentrations above the limit of quantification.82 The method applied undergoes periodical proficiency testing accreditation by the Quebec Multielement External Quality Assessment Scheme and the New York State Biomonitoring Program for Trace Elements.
Note that although the PROGRESS cohort currently has more than 20 available metal measures from the prenatal period, many were measured in separate laboratories and using different instruments. We chose to include only this set of 11 metals to ensure uniformity of instruments and similar lab procedures and to minimize any plausible batch effects.
Akkermansia muciniphila measurement
Details of the gut microbiome sampling and processing procedures have been previously published.37 Briefly, participants were recruited during the 9-11-year PROGRESS study visit. Stool samples were collected by participants at home, processed using the Fast method at the clinic in Mexico City, and promptly stored at -70°C. Frozen samples were then shipped to the Microbiome Translational Center at Mount Sinai for microbiome sequencing analysis. The subsequent steps involved the processing and sequencing of the samples in two distinct batches: the first batch containing 50 samples and the 2nd containing 73 samples. The NEBNext DNA Library Prep kit was used for shotgun metagenomic sequencing, and DNA sequencing was purified using the Illumina HiSeq platform. The sequencing reads were processed using KneadData,83 including trimming using Trimmomatic,84 and eliminating any human-associated reads by alignment to a human reference genome using bowtie2.85 The remaining reads underwent analysis with MetaPhlAn2 and StrainPhlAn to ascertain detailed microbial taxonomy down to the species and strain levels.79,80 Results of the sequencing process have been previously published.37 Only children with no antibiotic use within the past month were included in this sub-study. We used the presence/absence of A. muciniphila for this analysis from the sequencing data; this was done to minimize the effect of any measurement error in raw relative abundance data.
Depressive symptom measurement
As a part of the neurobehavioral follow-up in PROGRESS, childhood depressive symptoms were assessed using the Childhood Depression Inventory (CDI) 2 Self-Report Short Version at ages 9-11 years.86 Part of the CDI form was administered to mothers about their children, and part was administered directly to the children. The CDI questionnaire is appropriate for participants aged 7-17 years and was validated in Spanish.87 Initially, the CDI was validated and standardized for U.S. children; however, the full-length validation of CDI-2 in Spanish ensures that it could also be used in the Mexican children of the PROGRESS cohort. On a scale normalized from 0 to 100, items are scored with higher scores implying worse depression symptoms. We also used the Depression subscale from the Behavioral Assessment System for Children (BASC-2) for validation analysis.88,89 In a home and community setting, BASC is useful in assessing children’s adaptive and behavioral problems.89 The Spanish version of BASC-2 was administered to the children (self-reported) of ages 9-11 years. Multiple scales and composite scores are used within BASC-2, such as the BASC-2 Developmental Social Disorders scale, the externalizing problems, internalizing problems, and the behavioral symptoms index. Since our aim was to use BASC-2 as a validation tool, we used the depression scale within the internalizing composite scores. The raw scores from the scales were converted to age- age-standardized t-scores. Further, similar to CDI scores, a higher score on BASC-2 depression t-score indicates worse depression symptoms. Further details on using BASC-2 in the PROGRESS cohort have been described previously.90
Covariate data
We followed a similar set of minimal covariates in line with our previous work.38,91,92 Potential confounding variables used in this analysis encompassed a range of factors identified in previous literature and prioritized using DAGs. These included (1) the child’s reported sex at birth, (2) age at the time of stool sample collection, (3) the socioeconomic status (SES) of the mother during pregnancy, (4) the mother’s age at childbirth, (5) the mother’s 2nd-trimester body mass index (BMI) while pregnant, and (6) the batch of microbiome analysis (two batches). Please find more details about SES categories in our previous works. All of these covariates were adjusted for in the statistical models. This sub-study utilized convenience sampling due to proximity to the study location, availability of mothers and their children at a given time, or their willingness to participate in the study. Although there is potential selection bias and residual confounding bias, we utilized advanced causal inference techniques to address such issues to the best of our ability. Our analyses utilized the covariate-balancing techniques and negative control outcomes to address any systematic and potential selection and residual confounding biases. A covariate-balancing using propensity score is used in observational studies to eliminate the risk of confounding by balancing the covariates between exposed and non-exposed groups, such that hypothetically, the exposure was randomly assigned.93 A negative control outcome should not be causally related to the exposure of interest, and any observed effect may indicate a potential for unmeasured confounding or bias.94
Quantification and statistical analysis
The Pearson correlation coefficient was used to estimate the correlation between prenatal metal exposures. The t-scored CDI was log-transformed (log-tCDI) (later in the sensitivity analyses, similar results were replicated with non-log-transformed t-scored CDI). Some covariates (maternal age and age at the time of stool collection) included less than 5% missing values; therefore, under the assumption of missing at random, we imputed the missing values using predictive mean matching as implemented in the “MICE” R package.95 A false discovery rate was used to adjust for multiple comparison errors. The demographic details, including the sample size and distribution of the outcome, are reported in Table 1.
The analysis was conducted in three stages. First, to set up the narrative, we used linear regression models to estimate the association between each individual metal and log-tCDI. The results were presented through a forest plot with beta estimates and corresponding 95%CI. Second, we estimated the association between the presence of A. muciniphila and log-tCDI using linear regression. Third, using an interpretable machine-learning algorithm and regression-based framework, we identified a metal-clique signature that characterizes a certain subgroup of children and then estimated their associations with log-tCDI. Any two-tailed p-value less than 0.05 will be considered statistically significant. Results from these analyses are presented in Figure 1.
Although the details can be found in previous works, we present and discuss this method comprehensively for interpretability and further replicability.38,91,92 A metal-cliques signature denotes a specific subgroup of children with certain patterns of metal exposure. Therefore, it can be regarded as an indicator of metal combinations. We used the “repeated holdout signed-iterated Random Forest”(rh-SiRF),38,91,92 with the metal exposures during both the trimesters as predictors and log(t-CDI) as the primary outcome. The rh-SiRF algorithm utilizes “Iterative Random Forests” and the “Random Intersection Trees” to search for combinations of metals that are both predictive and associated with log(t-CDI) scores.96,97,98 A unique benefit of this model is that this algorithm finds the most frequently occurring combinations on the decision path instead of searching for all possible combinations (231 two-order, 1540 three-order, for example). This algorithm uses a metric named “stability”, which intuitively estimates how frequent a particular combination of predictors is among all the trees. On a training/test data partitioning of 60%/40%, the algorithm was repeated 500 times with 250 bootstraps implemented in each iteration. The list of most stable metal combinations (top 5%), along with their frequency of occurrence, is presented in Table S3. We chose the top three from the list of most stable metal combinations. We presented a closed-loop network of this combination through the Fruchterman-Reingold Layout99 implemented through the igraph package in R.100,101 A schematic of this closed-loop network is presented in Figure S2. Finally, this combination is transformed into a binarized indicator using a “quantile-based threshold-finding” algorithm.
For improved inference, we used a matched-sampling strategy typically applied in causal inference analysis to obtain similar covariate distribution between children with or without A. muciniphila.102 The assumption is that, given the covariates, this balancing approach can potentially create “exchangeable” groups of children with or without A. muciniphila such that they are hypothetically randomly assigned, and most importantly, the covariates did not confound the group assignment.39 We used a “subclass matching” procedure as implemented in the R package “MatchIt”.103 This approach uses propensity scores based on all the covariates to classify participants into subclasses, which were then weighted to balance the influence of the covariates for participants with vs. without A. muciniphila. We used “love plots” of the differences in standardized means in covariates to examine the extent of the balancing.39 This love plot is presented in Figure S3. All the regression analyses were based on this covariate-balanced matched sample. Results from this regression-based rh-SiRF method can be found in Figure 2. We further adjusted all the models with the previously described covariates. Lastly, we used the BASC-2 depression score to ensure that the results obtained with CDI scores remain similar (see Figure 3).
Published: November 6, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111335.
Supplemental information
References
- 1.Shorey S., Ng E.D., Wong C.H.J. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br. J. Clin. Psychol. 2022;61:287–305. doi: 10.1111/bjc.12333. [DOI] [PubMed] [Google Scholar]
- 2.Zhu F., Tu H., Chen T. The Microbiota-Gut-Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients. 2022;14:2081. doi: 10.3390/nu14102081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang T., Li Q., Cheng L., Buch H., Zhang F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019;12:1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collado M.C., Derrien M., Isolauri E., de Vos W.M., Salminen S. Intestinal Integrity and Akkermansia muciniphila, a Mucin-Degrading Member of the Intestinal Microbiota Present in Infants, Adults, and the Elderly. Appl. Environ. Microbiol. 2007;73:7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collado M.C., Laitinen K., Salminen S., Isolauri E. Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatr. Res. 2012;72:77–85. doi: 10.1038/pr.2012.42. [DOI] [PubMed] [Google Scholar]
- 6.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.-M., et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas L.V., Ockhuizen T., Suzuki K. Exploring the influence of the gut microbiota and probiotics on health: a symposium report. Br. J. Nutr. 2014;112:S1–S18. doi: 10.1017/S0007114514001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drell T., Larionova A., Voor T., Simm J., Julge K., Heilman K., Tillmann V., Štšepetova J., Sepp E. Differences in Gut Microbiota Between Atopic and Healthy Children. Curr. Microbiol. 2015;71:177–183. doi: 10.1007/s00284-015-0815-9. [DOI] [PubMed] [Google Scholar]
- 10.Derrien M., Collado M.C., Ben-Amor K., Salminen S., de Vos W.M. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008;74:1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosscher D., Lu Z., Janssens G., Van Caillie-Bertrand M., Robberecht H., De Rycke H., De Wilde R., Deelstra H. In vitro availability of zinc from infant foods with increasing phytic acid contents. Br. J. Nutr. 2001;86:241–247. doi: 10.1079/bjn2001384. [DOI] [PubMed] [Google Scholar]
- 12.Si J., Kang H., You H.J., Ko G. Revisiting the role of Akkermansia muciniphila as a therapeutic bacterium. Gut Microb. 2022;14 doi: 10.1080/19490976.2022.2078619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu R., Zhang Y., Chen S., Zeng Y., Fu X., Chen T., Luo S., Zhang X. The role of the probiotic Akkermansia muciniphila in brain functions: insights underpinning therapeutic potential. Crit. Rev. Microbiol. 2023;49:151–176. doi: 10.1080/1040841X.2022.2044286. [DOI] [PubMed] [Google Scholar]
- 14.Ding Y., Bu F., Chen T., Shi G., Yuan X., Feng Z., Duan Z., Wang R., Zhang S., Wang Q., et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021;105:8411–8426. doi: 10.1007/s00253-021-11622-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen T., Wang R., Duan Z., Yuan X., Ding Y., Feng Z., Bu F., Liu L., Wang Q., Zhou J., et al. Akkermansia muciniphila Protects Against Psychological Disorder-Induced Gut Microbiota-Mediated Colonic Mucosal Barrier Damage and Aggravation of Colitis. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.723856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendelsohn A.L., Dreyer B.P., Fierman A.H., Rosen C.M., Legano L.A., Kruger H.A., Lim S.W., Courtlandt C.D. Low-level lead exposure and behavior in early childhood. Pediatrics. 1998;101:E10. doi: 10.1542/peds.101.3.e10. [DOI] [PubMed] [Google Scholar]
- 17.Alghadir A.H., Gabr S.A., Al-Eisa E. Effects of Physical Activity on Trace Elements and Depression Related Biomarkers in Children and Adolescents. Biol. Trace Elem. Res. 2016;172:299–306. doi: 10.1007/s12011-015-0601-3. [DOI] [PubMed] [Google Scholar]
- 18.Rokoff L.B., Shoaff J.R., Coull B.A., Enlow M.B., Bellinger D.C., Korrick S.A. Prenatal exposure to a mixture of organochlorines and metals and internalizing symptoms in childhood and adolescence. Environ. Res. 2022;208 doi: 10.1016/j.envres.2022.112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrico C., Gennings C., Wheeler D.C., Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2015;20:100–120. doi: 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Midya V., Nagdeo K., Lane J.M., Torres-Olascoaga L.A., Torres-Calapiz M., Gennings C., Horton M.K., Téllez-Rojo M.M., Wright R.O., Arora M., Eggers S. Prenatal metal exposures and childhood gut microbial signatures are associated with depression score in late childhood. Sci. Total Environ. 2024;916 doi: 10.1016/j.scitotenv.2024.170361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Midya V., Agrawal M., Lane J.M., Gennings C., Tarassishin L., Torres-Olascoaga L.A., Eggers J., Gregory J.K., Picker M., Peter I., et al. Association between Exposure to Metals during Pregnancy, Childhood Gut Microbiome, and Risk of Intestinal Inflammation in Late Childhood. Environ. Health. 2024;2:739–749. doi: 10.1021/envhealth.4c00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baccarelli A., Dolinoy D.C., Walker C.L. A precision environmental health approach to prevention of human disease. Nat. Commun. 2023;14:2449. doi: 10.1038/s41467-023-37626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fard F.E., Mirghafourvand M., Mohammad-Alizadeh Charandabi S., Farshbaf-Khalili A., Javadzadeh Y., Asgharian H. Effects of zinc and magnesium supplements on postpartum depression and anxiety: A randomized controlled clinical trial. Women Health. 2017;57:1115–1128. doi: 10.1080/03630242.2016.1235074. [DOI] [PubMed] [Google Scholar]
- 24.Fu X., Li H., Song L., Cen M., Wu J. Association of urinary heavy metals co-exposure and adult depression: Modification of physical activity. Neurotoxicology. 2023;95:117–126. doi: 10.1016/j.neuro.2023.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Racette B.A., Nelson G., Dlamini W.W., Hershey T., Prathibha P., Turner J.R., Checkoway H., Sheppard L., Searles Nielsen S. Depression and anxiety in a manganese-exposed community. Neurotoxicology. 2021;85:222–233. doi: 10.1016/j.neuro.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtin P., Neufeld J., Curtin A., Austin C., Isaksson J., Remnelius K.L., Norrman H.N., Arora M., Bölte S. Associations between Elemental Metabolic Dynamics and Default Mode Network Functional Connectivity Are Altered in Autism. J. Clin. Med. 2023;12:1022. doi: 10.3390/jcm12031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González-Domínguez R., García-Barrera T., Gómez-Ariza J.L. Homeostasis of metals in the progression of Alzheimer’s disease. Biometals. 2014;27:539–549. doi: 10.1007/s10534-014-9728-5. [DOI] [PubMed] [Google Scholar]
- 28.Mustak M.S., Rao T.S.S., Shanmugavelu P., Sundar N.M.S., Menon R.B., Rao R.V., Rao K.S.J. Assessment of serum macro and trace element homeostasis and the complexity of inter-element relations in bipolar mood disorders. Clin. Chim. Acta. 2008;394:47–53. doi: 10.1016/j.cca.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Arora M., Reichenberg A., Willfors C., Austin C., Gennings C., Berggren S., Lichtenstein P., Anckarsäter H., Tammimies K., Bölte S. Fetal and postnatal metal dysregulation in autism. Nat. Commun. 2017;8 doi: 10.1038/ncomms15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baj J., Bargieł J., Cabaj J., Skierkowski B., Hunek G., Portincasa P., Flieger J., Smoleń A. Trace Elements Levels in Major Depressive Disorder—Evaluation of Potential Threats and Possible Therapeutic Approaches. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms242015071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielińska M., Łuszczki E., Dereń K. Dietary Nutrient Deficiencies and Risk of Depression (Review Article 2018–2023) Nutrients. 2023;15:2433. doi: 10.3390/nu15112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geisler A., Mørk A. In: Lithium and Cell Physiology. Bach R.O., Gallicchio V.S., editors. Springer; 1990. The Interaction of Lithium with Magnesium-Dependent Enzymes; pp. 125–136. [DOI] [Google Scholar]
- 33.Laue H.E., Moroishi Y., Jackson B.P., Palys T.J., Madan J.C., Karagas M.R. Nutrient-toxic element mixtures and the early postnatal gut microbiome in a United States longitudinal birth cohort. Environ. Int. 2020;138 doi: 10.1016/j.envint.2020.105613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Y., Laue H.E., Shrubsole M.J., Wu H., Bloomquist T.R., Larouche A., Zhao K., Gao F., Boivin A., Prada D., et al. Associations of Childhood and Perinatal Blood Metals with Children’s Gut Microbiomes in a Canadian Gestation Cohort. Environ. Health Perspect. 2022;130 doi: 10.1289/EHP9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sitarik A.R., Arora M., Austin C., Bielak L.F., Eggers S., Johnson C.C., Lynch S.V., Kyun Park S., Hank Wu K.-H., Yong G.J.M., Cassidy-Bushrow A.E. Fetal and early postnatal lead exposure measured in teeth associates with infant gut microbiota. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eggers S., Safdar N., Sethi A.K., Suen G., Peppard P.E., Kates A.E., Skarlupka J.H., Kanarek M., Malecki K.M.C. Urinary lead concentration and composition of the adult gut microbiota in a cross-sectional population-based sample. Environ. Int. 2019;133 doi: 10.1016/j.envint.2019.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eggers S., Midya V., Bixby M., Gennings C., Torres-Olascoaga L.A., Walker R.W., Wright R.O., Arora M., Téllez-Rojo M.M. Prenatal lead exposure is negatively associated with the gut microbiome in childhood. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1193919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Midya V., Lane J.M., Gennings C., Torres-Olascoaga L.A., Gregory J.K., Wright R.O., Arora M., Téllez-Rojo M.M., Eggers S. Prenatal Lead Exposure Is Associated with Reduced Abundance of Beneficial Gut Microbial Cliques in Late Childhood: An Investigation Using Microbial Co-Occurrence Analysis (MiCA) Environ. Sci. Technol. 2023;57:16800–16810. doi: 10.1021/acs.est.3c04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z., Kim H.J., Lonjon G., Zhu Y., written on behalf of AME Big-Data Clinical Trial Collaborative Group Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019;7:16. doi: 10.21037/atm.2018.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen A. Mucus—a protective secretion of complexity. Trends Biochem. Sci. 1983;8:169–173. [Google Scholar]
- 41.Liu M.-J., Yang J.-Y., Yan Z.-H., Hu S., Li J.-Q., Xu Z.-X., Jian Y.-P. Recent findings in Akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin. Nutr. 2022;41:2333–2344. doi: 10.1016/j.clnu.2022.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Lei W., Cheng Y., Gao J., Liu X., Shao L., Kong Q., Zheng N., Ling Z., Hu W. Akkermansia muciniphila in neuropsychiatric disorders: friend or foe? Front. Cell. Infect. Microbiol. 2023;13 doi: 10.3389/fcimb.2023.1224155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke G., Stilling R.M., Kennedy P.J., Stanton C., Cryan J.F., Dinan T.G. Minireview: Gut microbiota: the neglected endocrine organ. Mol. Endocrinol. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 45.McGaughey K.D., Yilmaz-Swenson T., Elsayed N.M., Cruz D.A., Rodriguiz R.M., Kritzer M.D., Peterchev A.V., Roach J., Wetsel W.C., Williamson D.E. Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety- and depressive-like behavior following social defeat in mice. Sci. Rep. 2019;9:3281. doi: 10.1038/s41598-019-40140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park Y.S., Kim S.H., Park J.W., Kho Y., Seok P.R., Shin J.-H., Choi Y.J., Jun J.-H., Jung H.C., Kim E.K. Melatonin in the colon modulates intestinal microbiota in response to stress and sleep deprivation. Int. Res. 2020;18:325–336. doi: 10.5217/ir.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aatsinki A.-K., Keskitalo A., Laitinen V., Munukka E., Uusitupa H.-M., Lahti L., Kortesluoma S., Mustonen P., Rodrigues A.J., Coimbra B., et al. Maternal prenatal psychological distress and hair cortisol levels associate with infant fecal microbiota composition at 2.5 months of age. Psychoneuroendocrinology. 2020;119 doi: 10.1016/j.psyneuen.2020.104754. [DOI] [PubMed] [Google Scholar]
- 48.Takebayashi N., Maeshima H., Baba H., Nakano Y., Satomura E., Kita Y., Namekawa Y., Nomoto H., Suzuki T., Arai H. Duration of last depressive episode may influence serum BDNF levels in remitted patients with major depression. Depress. Anxiety. 2012;29:775–779. doi: 10.1002/da.21933. [DOI] [PubMed] [Google Scholar]
- 49.Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., Wang W., Tang W., Tan Z., Shi J., et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Yohn C.N., Gergues M.M., Samuels B.A. The role of 5-HT receptors in depression. Mol. Brain. 2017;10:28. doi: 10.1186/s13041-017-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tundo A., de Filippis R., Proietti L. Pharmacologic approaches to treatment resistant depression: Evidences and personal experience. World J. Psychiatr. 2015;5:330–341. doi: 10.5498/wjp.v5.i3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon N., Goelman G. Understanding alterations in serotonin connectivity in a rat model of depression within the monoamine-deficiency and the hippocampal-neurogenesis frameworks. Behav. Brain Res. 2016;296:141–148. doi: 10.1016/j.bbr.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Delgado P.L. Depression: the case for a monoamine deficiency. J. Clin. Psychiatry. 2000;61:7–11. [PubMed] [Google Scholar]
- 54.Cheng R., Xu W., Wang J., Tang Z., Zhang M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila alleviates the depression-like behavior of depressed mice induced by chronic stress. Biochem. Biophys. Res. Commun. 2021;566:170–176. doi: 10.1016/j.bbrc.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Xu W., Wang R., Cheng R., Tang Z., Zhang M. The outer membrane protein Amuc\_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021;12:3597–3610. doi: 10.1039/d1fo00115a. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y., Dai Y., Li M., Guo L., Cao C., Huang Y., Ma R., Qiu S., Su X., Zhong K., et al. Exposure to cadmium induces neuroinflammation and impairs ciliogenesis in hESC-derived 3D cerebral organoids. Sci. Total Environ. 2021;797 doi: 10.1016/j.scitotenv.2021.149043. [DOI] [PubMed] [Google Scholar]
- 57.Lestaevel P., Dhieux B., Delissen O., Benderitter M., Aigueperse J. Uranium modifies or not behavior and antioxidant status in the hippocampus of rats exposed since birth. J. Toxicol. Sci. 2015;40:99–107. doi: 10.2131/jts.40.99. [DOI] [PubMed] [Google Scholar]
- 58.Tao C., Li Z., Fan Y., Li X., Qian H., Yu H., Xu Q., Lu C. Independent and combined associations of urinary heavy metals exposure and serum sex hormones among adults in NHANES 2013-2016. Environ. Pollut. 2021;281 doi: 10.1016/j.envpol.2021.117097. [DOI] [PubMed] [Google Scholar]
- 59.Garí M., Grzesiak M., Krekora M., Kaczmarek P., Jankowska A., Król A., Kaleta D., Jerzyńska J., Janasik B., Kuraś R., et al. Prenatal exposure to neurotoxic metals and micronutrients and neurodevelopmental outcomes in early school age children from Poland. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112049. [DOI] [PubMed] [Google Scholar]
- 60.Peppard L., Oh K.M., Gallo S., Milligan R. Risk of depression in pregnant women with low-normal serum Vitamin B12. Res. Nurs. Health. 2019;42:264–272. doi: 10.1002/nur.21951. [DOI] [PubMed] [Google Scholar]
- 61.Esnafoglu E., Ozturan D.D. The relationship of severity of depression with homocysteine, folate, vitamin B12, and vitamin D levels in children and adolescents. Child Adolesc. Ment. Health. 2020;25:249–255. doi: 10.1111/camh.12387. [DOI] [PubMed] [Google Scholar]
- 62.Al-Musharaf S., Aljuraiban G.S., Al-Ajllan L., Al-Khaldi N., Aljazairy E.A., Hussain S.D., Alnaami A.M., Sabico S., Al-Daghri N. Vitamin B12 Status and Gut Microbiota among Saudi Females with Obesity. Foods. 2022;11:4007. doi: 10.3390/foods11244007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giambò F., Italia S., Teodoro M., Briguglio G., Furnari N., Catanoso R., Costa C., Fenga C. Influence of toxic metal exposure on the gut microbiota. World Acad. Sci. J. 2021;3:1. [Google Scholar]
- 64.Feng S., Liu Y., Huang Y., Zhao J., Zhang H., Zhai Q., Chen W. Influence of oral administration of Akkermansia muciniphila on the tissue distribution and gut microbiota composition of acute and chronic cadmium exposure mice. FEMS Microbiol. Lett. 2019;366:fnz160. doi: 10.1093/femsle/fnz160. [DOI] [PubMed] [Google Scholar]
- 65.Chang X., Li H., Feng J., Chen Y., Nie G., Zhang J. Effects of cadmium exposure on the composition and diversity of the intestinal microbial community of common carp (Cyprinus carpio L.) Ecotoxicol. Environ. Saf. 2019;171:92–98. doi: 10.1016/j.ecoenv.2018.12.066. [DOI] [PubMed] [Google Scholar]
- 66.Meng X.-L., Li S., Qin C.-B., Zhu Z.-X., Hu W.-P., Yang L.-P., Lu R.-H., Li W.-J., Nie G.-X. Intestinal microbiota and lipid metabolism responses in the common carp (Cyprinus carpio L.) following copper exposure. Ecotoxicol. Environ. Saf. 2018;160:257–264. doi: 10.1016/j.ecoenv.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 67.Zhai Q., Li T., Yu L., Xiao Y., Feng S., Wu J., Zhao J., Zhang H., Chen W. Effects of subchronic oral toxic metal exposure on the intestinal microbiota of mice. Sci. Bull. 2017;62:831–840. doi: 10.1016/j.scib.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 68.Claus S.P., Guillou H., Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2 doi: 10.1038/npjbiofilms.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campana A.M., Laue H.E., Shen Y., Shrubsole M.J., Baccarelli A.A. Assessing the role of the gut microbiome at the interface between environmental chemical exposures and human health: Current knowledge and challenges. Environ. Pollut. 2022;315 doi: 10.1016/j.envpol.2022.120380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reunanen J., Kainulainen V., Huuskonen L., Ottman N., Belzer C., Huhtinen H., de Vos W.M., Satokari R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015;81:3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen X.-L., Xu Y.-M., Lau A.T.Y. Toxic metals in the regulation of epithelial-mesenchymal plasticity: demons or angels? Cancer Cell Int. 2022;22:237. doi: 10.1186/s12935-022-02638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Susukida R., Usuda K., Hamazaki K., Tsuchida A., Matsumura K., Nishi D., Inadera H., Japan Environment and Children’s Study JECS Group Association of prenatal psychological distress and postpartum depression with varying physical activity intensity: Japan Environment and Children’s Study (JECS) Sci. Rep. 2020;10:6390. doi: 10.1038/s41598-020-63268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petrovic D., Perovic M., Lazovic B., Pantic I. Association between walking, dysphoric mood and anxiety in late pregnancy: A cross-sectional study. Psychiatr. Res. 2016;246:360–363. doi: 10.1016/j.psychres.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Stojsavljević A., Rovčanin M., Rovčanin B., Miković Ž., Jeremić A., Perović M., Manojlović D. Human biomonitoring of essential, nonessential, rare earth, and noble elements in placental tissues. Chemosphere. 2021;285 doi: 10.1016/j.chemosphere.2021.131518. [DOI] [PubMed] [Google Scholar]
- 75.Nandakumaran M., Al-Sannan B., Al-Sarraf H., Al-Shammari M. Maternal-fetal transport kinetics of manganese in perfused human placental lobule in vitro. J. Matern. Fetal Neonatal Med. 2016;29:274–278. doi: 10.3109/14767058.2014.998193. [DOI] [PubMed] [Google Scholar]
- 76.Jagodić J., Pavlović S., Borković-Mitić S., Perović M., Miković Ž., Đurđić S., Manojlović D., Stojsavljević A. Examination of trace metals and their potential transplacental transfer in pregnancy. Int. J. Mol. Sci. 2022;23:8078. doi: 10.3390/ijms23158078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodosthenous R.S., Burris H.H., Svensson K., Amarasiriwardena C.J., Cantoral A., Schnaas L., Mercado-García A., Coull B.A., Wright R.O., Téllez-Rojo M.M., Baccarelli A.A. Prenatal lead exposure and fetal growth: Smaller infants have heightened susceptibility. Environ.Int. 2017;99:228–233. doi: 10.1016/j.envint.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.PROGRESS Institute for Climate Change Environmental Health, and Exposomics. https://mountsinaiexposomics.org/progress/
- 79.Truong D.T., Franzosa E.A., Tickle T.L., Scholz M., Weingart G., Pasolli E., Tett A., Huttenhower C., Segata N. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods. 2015;12:902–903. doi: 10.1038/nmeth.3589. [DOI] [PubMed] [Google Scholar]
- 80.Truong D.T., Tett A., Pasolli E., Huttenhower C., Segata N. Microbial strain-level population structure and genetic diversity from metagenomes. Genome Res. 2017;27:626–638. doi: 10.1101/gr.216242.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heiss J.A., Téllez-Rojo M.M., Estrada-Gutiérrez G., Schnaas L., Amarasiriwardena C., Baccarelli A.A., Wright R.O., Just A.C. Prenatal lead exposure and cord blood DNA methylation in PROGRESS: an epigenome-wide association study. Environ. Epigenet. 2020;6 doi: 10.1093/eep/dvaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zota A.R., Ettinger A.S., Bouchard M., Amarasiriwardena C.J., Schwartz J., Hu H., Wright R.O. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20:367–373. doi: 10.1097/EDE.0b013e31819b93c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.KneadData – The Huttenhower Lab. https://huttenhower.sph.harvard.edu/kneaddata/
- 84.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kovacs M. The Encyclopedia of Clinical Psychology. John Wiley & Sons, Ltd; 2015. Children’s Depression Inventory (CDI and CDI 2) pp. 1–5. [DOI] [Google Scholar]
- 87.Cumba-Avilés E., López-Robledo Y.M., Caro-González W., Rosario-Nieves I. Pilot Validation Study for the Spanish-language CDI-2 among Adolescents from Puerto Rico. Rev. Puertorriquena Psicol. 2020;31:110–126. [PMC free article] [PubMed] [Google Scholar]
- 88.Kamphaus R.W. The Encyclopedia of Clinical Psychology. John Wiley & Sons, Ltd; 2015. Behavior Assessment System for Children, Second Edition (BASC-2) pp. 1–6. [DOI] [Google Scholar]
- 89.Reynolds C.R. The Corsini Encyclopedia of Psychology. American Cancer Society; 2010. Behavior Assessment System for Children; pp. 1–2. [DOI] [Google Scholar]
- 90.McGuinn L.A., Bellinger D.C., Colicino E., Coull B.A., Just A.C., Kloog I., Osorio-Valencia E., Schnaas L., Wright R.J., Téllez-Rojo M.M., et al. Prenatal PM2. 5 exposure and behavioral development in children from Mexico City. Neurotoxicology. 2020;81:109–115. doi: 10.1016/j.neuro.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Midya V., Alcala C.S., Rechtman E., Gregory J.K., Kannan K., Hertz-Picciotto I., Teitelbaum S.L., Gennings C., Rosa M.J., Valvi D. Machine Learning Assisted Discovery of Interactions between Pesticides, Phthalates, Phenols, and Trace Elements in Child Neurodevelopment. Environ. Sci. Technol. 2023;57:18139–18150. doi: 10.1021/acs.est.3c00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Midya V., Gennings C. Detecting Shape-based Interactions among Environmental Chemicals using an Ensemble of Exposure-Mixture Regression and Interpretable Machine Learning Tools. Stat. Biosci. 2023;16:395–415. [Google Scholar]
- 93.Imai K., Ratkovic M. Covariate Balancing Propensity Score. J. Roy. Stat. Soc. B Stat. Methodol. 2014;76:243–263. doi: 10.1111/rssb.12027. [DOI] [Google Scholar]
- 94.Arnold B.F., Ercumen A. Negative Control Outcomes: A Tool to Detect Bias in Randomized Trials. JAMA. 2016;316:2597–2598. doi: 10.1001/jama.2016.17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buuren S.v., Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J. Stat. Software. 2011;45:1–67. [Google Scholar]
- 96.Basu S., Kumbier K., Brown J.B., Yu B. Iterative random forests to discover predictive and stable high-order interactions. Proc. Natl. Acad. Sci. USA. 2018;115:1943–1948. doi: 10.1073/pnas.1711236115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumbier K., Basu S., Brown J.B., Celniker S., Yu B. Refining interaction search through signed iterative random forests. arXiv. 2018 doi: 10.1101/467498. Preprint at. [DOI] [Google Scholar]
- 98.Shah R.D., Meinshausen N. Random intersection trees. J. Mach. Learn. Res. 2014;15:629–654. [Google Scholar]
- 99.Kobourov S.G. Spring Embedders and Force Directed Graph Drawing Algorithms. arXiv. 2012 doi: 10.48550/arXiv.1201.3011. Preprint at. [DOI] [Google Scholar]
- 100.Csardi G., Nepusz T. The igraph software package for complex network research. J. Complex Syst. 2006;1695:1–9. [Google Scholar]
- 101.Csárdi G., Nepusz T., Müller K., Horvát S., Traag V., Zanini F., Noom D. igraph for R: R interface of the igraph library for graph theory and network analysis. 2023. [DOI]
- 102.Greifer N. 2023. Covariate balance tables and plots: A guide to the cobalt package. [Google Scholar]
- 103.Ho D.E., Imai K., King G., Stuart E.A. MatchIt: nonparametric preprocessing for parametric causal inference. J. Stat. Software. 2011;42:1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Metagenomic data are publicly available at https://www.ncbi.nlm.nih.gov/sra/PRJNA975184. All other data on the PROGRESS cohort are available upon request at robert.wright@mssm.edu.
-
•
A schematic of the algorithm used and R code with illustrations on a simulated dataset is provided on GitHub (https://github.com/vishalmidya/MiCA-Microbial-Co-occurrence-Analysis/blob/main/MiCA-vignette.md).