Abstract
Highly conserved signalling pathways such as Notch and Wnt are essential in the regulation of differentiation and proliferation processes during adult tissue homeostasis. Human papillomaviruses (HPVs) have evolved with humans to manipulate these signalling pathways to establish a basal reservoir of infected cells by limiting HPV-infected keratinocyte differentiation whilst ensuring that differentiating cells are in a replication-competent state. Here, we focus on the canonical Notch and Wnt signalling pathways and their crosstalk to ensure cell-fate lineage determination during epithelial homeostasis. We then examine how HPVs use their E6 and E7 proteins to inhibit differentiation and maintain stem-like characteristics using Notch and Wnt in HPV-infected cells. Determining the functions of E6 and E7 in the maintenance of the infected cell reservoir, and the molecular crosstalk between Notch and Wnt is vital for our understanding of HPV persistence, and may represent an important factor in the development of therapeutic agents for HPV-associated disease.
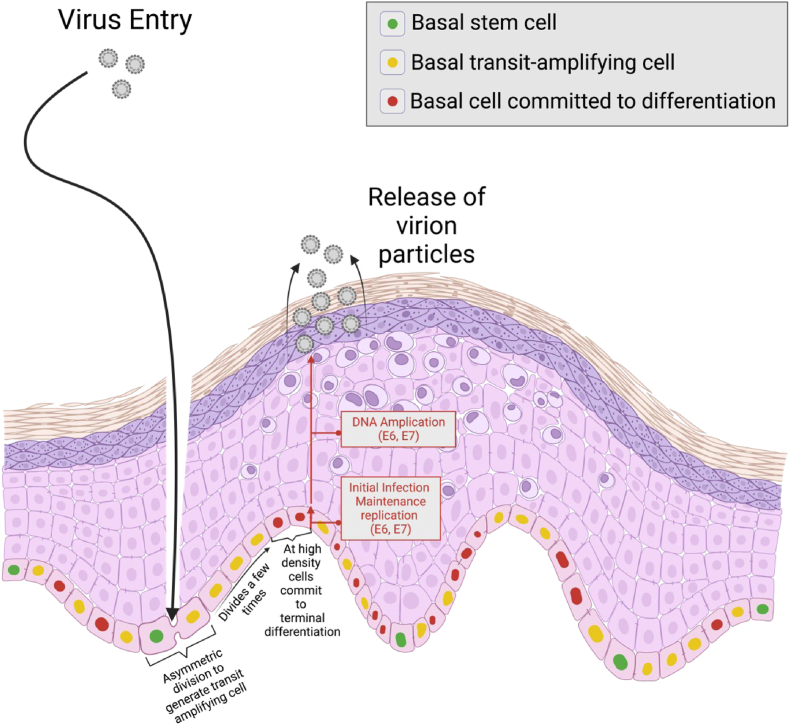
Signalling pathways are vital in the generation of cellular and morphological diversity during animal development and adult tissue homeostasis [1]. Notch and Wnt are two complex pathways that monitor and regulate one another to control cell fate decisions [2]. They often have opposing effects on cell fate determination which control progression along a specific cellular lineage [3]. Human papillomaviruses (HPVs) have co-evolved with a range of different animal species including humans for millions of years [4]. HPVs are highly tissue-restricted as during evolution, they became adapted to specific epithelial niches and their local microenvironments and have hence developed distinct tissue tropisms [[5], [6], [7], [8]]. Lesion formation begins when HPV infects a basal stem cell in the epithelium through microabrasions on the skin surface (Fig. 1) [[9], [10], [11]]. The basal stem cell then proliferates and asymmetrically divides to generate an array of transit amplifying cells and establish a reservoir of virally infected cells, and at a specific cell density allows some infected cells to commit to differentiation (Fig. 1). To ensure a replication-competent environment for genome amplification and packaging of viral particles, HPV uses E6 and E7 proteins for both episomal genome maintenance and the reactivation of cell division among the infected, differentiated cells [[12], [13], [14], [15]].
Fig. 1.
Events during HPV infection in the stratified squamous epithelium. HPV enters the multi-layer squamous epithelium through microabrasions on the skin surface and is thought to infect a basal stem cell. The infected basal stem cell asymmetrically divides to generate transit amplifying cells that undergo a fixed number of divisions prior to differentiation. At a specific cell density, they commit to differentiation and extend into the parabasal layers and eventually HPV virion particles are released from the stratified epithelium. The E6/E7 proteins modulate the timing of differentiation of cells in the epithelial basal layer.
HPV types from different genera use their E6 and E7 proteins to manipulate highly conserved signalling pathways to cause persistent papillomas [[16], [17], [18], [19], [20]]. In high-risk Alpha HPV types, E6 and E7 proteins drive cell proliferation in the basal and parabasal layers. High-risk E6 degrades p53 and PDZ substrates to inhibit differentiation and induce keratinocyte immortalization; high-risk E7 degrades pRb family members including p105 and p107 which control cell cycle entry in the basal layer, and p130 which controls cell cycle entry in the upper epithelial layers [[20], [21], [22], [23]]. Deregulated expressions of E6 and E7 in these types, in particular HPV 16 and 18, cause nearly all cases of cervical cancer and anal cancer as well as being a cause of oropharyngeal cancer [[24], [25], [26], [27], [28]]. During the normal productive cycle of these and other HPV types, E6 and E7's key function is play a role in homeostasis and enforce genome amplification. While low-risk HPVs cannot promote E6-dependent degradation of p53 or PDZ proteins, and low-risk E7 has lower affinities for p105 and 107 than its high-risk counterpart, persistent infection can still progress into problematic pathologies, such as papillomatosis and sometimes even cancers [[29], [30], [31], [32]]. For example, HPV type 11 causes recurrent respiratory papillomatosis in children which has no effective treatment. Its recurrent nature, despite surgical removal, blocks the lower airways and lungs, resulting in significant morbidity and occasional mortality [33,34]. Hence, the primary function of E6 and E7 of all papillomaviruses is to establish a viral reservoir by restricting basal keratinocyte differentiation whilst driving differentiating cells into a replication-competent state [35,36].
Here, we review the modulation of Notch and Wnt signalling pathways by the representatives of the Alpha high-risk and low-risk groups-types 16 and 11 respectively for persistent viral infection which can cause HPV-associated diseases. First, we review our understanding of the Notch and Wnt pathways in animal development, specifically the cell fate decisions required to maintain epithelial homeostasis. Then, we reflect on how HPVs have modulated the Notch and Wnt pathways to ensure viral persistence of the infected cell in the basal layer.
1. Role of Notch in epithelial and HPV-modulated homeostasis
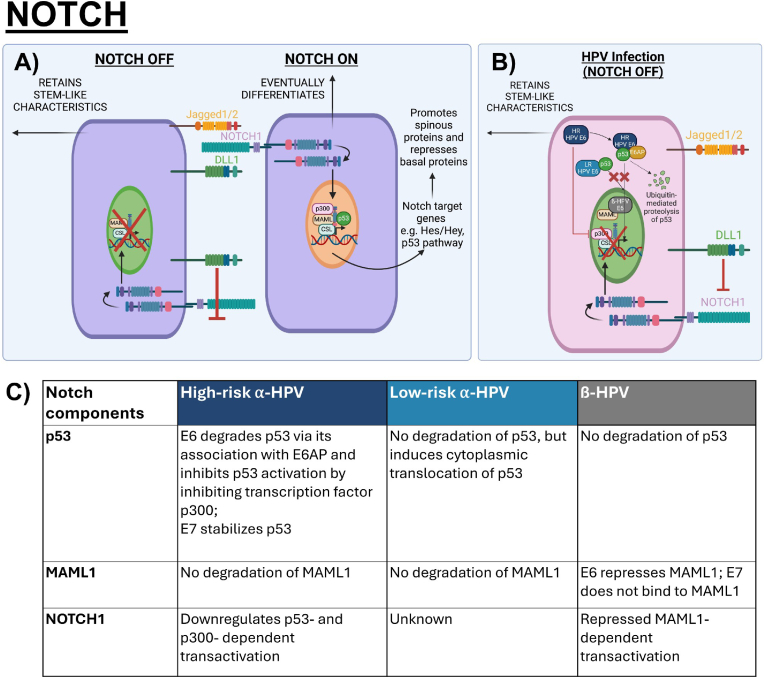
Canonical Notch signalling occurs when a cell-cell interaction between a Notch receptor and its ligands, Delta and Serrate, releases the intracellular domain of Notch (NICD) for nuclear translocation [[37], [38], [39]]. In the nucleus, NICD interacts with transcription factor CBF1/Su(H)/LAG-1 (CSL) to recruit coactivators, such as MAML proteins, to drive the transcription of Notch target gene families- HES and HEY which are associated with differentiation processes in the cervix and uterine endometrium, and the interfollicular epidermis [[40], [41], [42], [43]]. Canonical Notch signalling has been shown to function as the commitment switch from a proliferative basal cell to a terminally differentiated parabasal cell where it represses basal markers such as CK5 and CK14 and promotes spinous differentiation markers such as CK1, CK10 and IVL [[44], [45], [46]]. The Notch ligands detected in the epidermis that can activate Notch signalling are Delta-like 1 (DLL1), Jagged1 and Jagged2 which have variable expression patterns across the epithelial layers and distinct ligand-specific functional effects on cell fate determination. Jagged1 is primarily expressed in the parabasal layers and Jagged2 is expressed in the basal layer [47,48]. Both have been found to induce basal keratinocyte differentiation, but some studies have also functionally linked Jagged1 to increasing stem-like characteristics in cancer cells [47,[49], [50], [51]]. DLL1 expression is highest in basal stem cell clusters of healthy fetal and adult human epidermis, where high-DLL1-expressing basal keratinocytes were unresponsive to Notch activation and retained stem-like characteristics yet induced Notch1 activation and differentiation in neighbouring cells (Fig. 2A) [[52], [53], [54], [55]]. Tumour suppressor p53 can also target Notch signalling by binding to and transactivating the Notch1 promoter, resulting in keratinocyte commitment to differentiation [56,57].
Fig. 2.
Canonical Notch signalling in epithelial homeostasis and HPV-modulated homeostasis. A) Notch activation for the commitment switch to differentiation from the basal-parabasal junction can be activated by Notch ligands Jagged1, Jagged2 or DLL1 to stimulate expression of the Hes/Hey families and its targets. The normal role of p53 also activates Notch signalling by binding to the Notch1 promoter. High-DLL1-expressing basal keratinocyte cis-inhibits Notch signalling to retain stem-like characteristics while inducing Notch1 activation and differentiation in neighbouring cell [43,52,57]. B) To delay differentiation in HPV-infected cells, different genera of HPVs use E6 to inhibit the Notch signalling pathway. High-risk Alpha HPV types degrade p53 using the E6/E6AP complex and further inhibit p53 activity by inhibiting transcription factor p300 to inhibit Notch1 transcription, low-risk types cannot degrade p53 but prevent its nuclear translocation and interaction with Notch target genes, whilst Beta HPVs bind to MAML to repress NICD activation [57,61,63,64]. C) Table summarizes the interactions of Notch components, including p53, MAML1 and NOTCH1, with Alpha- and Beta- HPV E6 and E7.
Across different genera of HPVs, the Notch pathway is a common target of the E6 protein, emphasizing its important deregulation to ensure viral persistence and a HPV-modulated homeostasis [58,59]. Moreover, during the progression of cervical cancer, down-modulation of Notch1 is required for sustained transcription of the E6/E7 genes and subsequent malignant transformation [60]. For the high-risk Alpha HPV types, E6-dependent degradation of p53 with E6AP downregulates p53-dependent transactivation of the Notch1 promoter, resulting in deregulated differentiation. Moreover, high-risk E6 inhibits transcriptional activity of co-activator p300, which inhibits p53 and Notch1 activation [61,62]. For Beta HPV types, repressed transactivation of the Notch signalling occurs through inhibition of MAML1-a coactivator of Notch signalling, resulting in delayed differentiation (Fig. 2B and C) [57,58,63]. Although low-risk Alpha types cannot degrade p53, their E6 proteins have been found to induce cytoplasmic translocation of p53, hence interfering with its nuclear function [29,64]. The role of the E7 protein on the Notch pathway in disrupting differentiation is less clear, but it is reported that HPV16 E7 stabilizes p53, suggesting the possibility that high-risk E7 may rescue Notch1 expression [[65], [66], [67], [68]]. β-genus E7 does not bind to MAML1 and regulate Notch1 expression, but other critical associations between E7 and Notch targets that drive keratinocyte differentiation have yet to be fully explored.
2. Role of Wnt in epithelial and HPV-modulated homeostasis
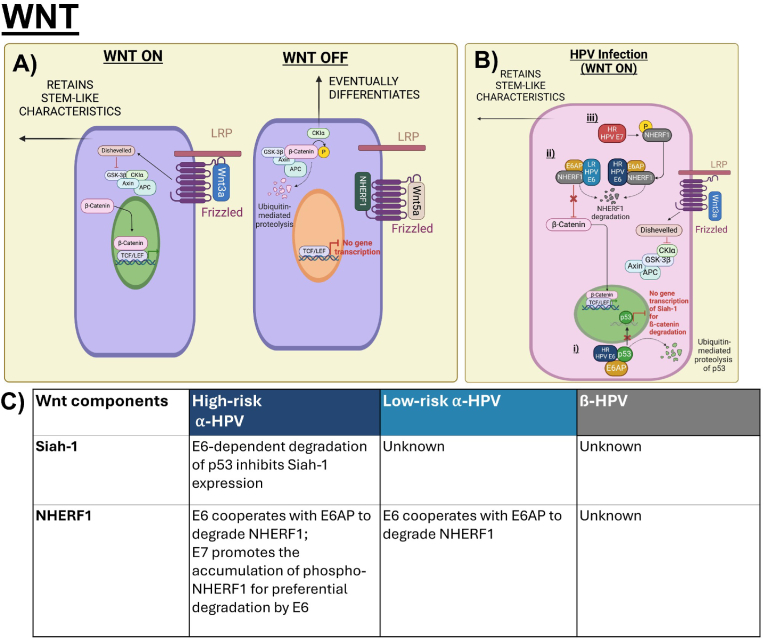
The canonical Wnt/β-Catenin signalling pathway is activated by the secreted Wnt1 class ligands, including Wnt2, Wnt3, Wnt3a and Wnt8a, which are released by signalling cells, that bind to Frizzled receptors (FZD1-10) and LRP5/6 proteins which recruits Dishevelled to inhibit the Axin destruction complex, hence allowing the accumulation of cytoplasmic β-catenin and subsequent nuclear translocation [69,70]. In the nucleus, β-catenin binds with transcription factors and recruits co-activators to drive the expression of target genes, such as Axin2 and c-Myc, to facilitate cell proliferation, survival, differentiation and migration [[71], [72], [73]]. The pathway has been reported to regulate stem cell clusters by safeguarding epigenetic stability and controlling cell proliferation whereas during oncogenesis, β-Catenin expression is deregulated in cancer stem cells [[74], [75], [76]]. The canonical Wnt pathway is highly conserved and can only be activated via the binding of the secreted canonical Wnt1 class ligands, such as Wnt3a (Fig. 3A) [77,78]. Like Notch, Wnt ligands also exhibit different functional effects on proliferation and differentiation. Wnt3a has been found to rescue self-renewal and inhibit differentiation in epidermal, embryonic and haematopoietic stem cells [[79], [80], [81]]. As negative regulators of canonical Wnt signalling, noncanonical ligand Wnt5a and tumour suppressor NHERF1 compete with Wnt3a for the binding to Frizzled. This promotes the degradation of β-catenin and inhibits ß-catenin-dependent Wnt signalling, subsequently inducing differentiation in stem cells (Fig. 3A) [[82], [83], [84], [85]].
Fig. 3.
Canonical Wnt/β-catenin signalling in epithelial homeostasis and HPV-modulated homeostasis. A) Signalling cells secrete canonical Wnt ligands, such as Wnt3a, to activate the Wnt/β-catenin signalling which promotes self-renewal and inhibits differentiation in stem cells. Non-canonical Wnt5a ligand and tumour suppressor NHERF1 bind to Frizzled, thereby inhibiting Wnt3a-dependent LRP phosphorylation and inhibiting accumulation of β-catenin. Inhibition of canonical Wnt signalling has been reported to induce epithelial cell differentiation [79,82,85]. B) HPVs use E6 and E7 to increase canonical Wnt/β-catenin signalling. i) High-risk E6-dependent degradation of p53 prevents the transcriptional activation of ubiquitin ligase Siah-1 which degrades β-catenin; ii) High and low-risk E6 cooperate with E6AP to degrade NHERF1 which subsequently activates the Wnt/β-catenin signalling pathway; iii) High-risk E7 increases the accumulation of phosphorylated NHERF1 which is preferentially targeted by E6 for degradation [89,90,94]. C) Table summarizes the interactions of Wnt components, including Siah-1 and NHERF1, with Alpha- and Beta- HPV E6 and E7.
To reduce commitment of differentiation and maintain self-renewal processes of epidermal stem cells, high- and low-risk HPV E6 proteins have been found to upregulate the β-catenin/TCF signalling response which regulate genes involved in cell polarity, proliferation, migration, and differentiation like c-myc, Cyclin D1, and Axin-2 [72,[86], [87], [88]]. High-risk E6 cooperates with E6AP to induce p53 degradation, which inhibits the expression of ubiquitin ligase Siah-1 that degrades β-catenin (Fig. 3B). Loss of p53 and subsequent loss of Siah-1 expression prevents the degradation of β-catenin and allows for its accumulation (Fig. 3B) [89]. Low-risk E6 proteins do not degrade p53, but both high and low-risk E6 cooperate with E6AP to degrade NHERF1- a highly conserved PDZ protein to activate Wnt signalling [86,90,91]. NHERF1 is tumour-suppressive and regulates cellular processes of differentiation and inhibits cervical cancer cell proliferation, thus HPV degrades NHERF1 to delay differentiation and augment proliferation [92,93]. Although the specific roles of E7 in Wnt signalling have been less well-characterized, it has been suggested that high-risk E7 promotes the accumulation of phosphorylated NHERF1 for preferential degradation by E6 (Fig. 3B and C) [94]. Some studies also suggest E7 may contribute to β-catenin stabilization in the cytoplasm where E7 may bind to PP2A-a phosphatase that induces β-catenin degradation [95,96].
3. Role of Notch & Wnt in epithelial and HPV-modulated homeostasis
Crosstalk between the Notch and Wnt signalling pathways where they directly and indirectly regulate each other is vital in many developmental processes. This is particularly important in the process of cell fate determination and cell lineage [2,3,97]. For example, Notch promotes the differentiation of epithelial cells by inhibiting the Wnt signal that promotes their self-renewal [98].
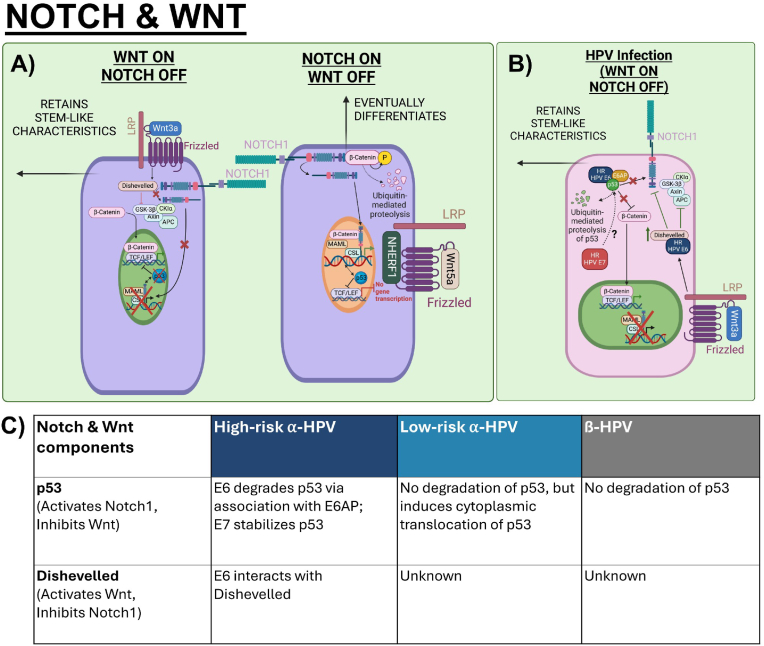
Since both signalling pathways regulate proliferation and differentiation, strict controls of either Notch-ON/Wnt-OFF or Notch-OFF/Wnt-ON are required to avoid conflicts between the two signalling pathways. Notch downregulates Wnt signalling by promoting the degradation of β-catenin to promote differentiation of epidermal stem cells and inhibit their self-renewal processes whilst Wnt inhibits Notch to control cell-fate specification during epidermal development [[98], [99], [100]]. It is suggested that most convergence of the pathways rely on common transcriptional targets, but direct interactions between the pathways have also been reported (Fig. 4A) [[101], [102], [103]]. To achieve Notch-ON/Wnt-OFF, Notch1 can antagonize activated β-catenin activity by isolating β-catenin at the membrane, and active NICD can form a complex with β-catenin via CSL in the nucleus, hence deactivating β-catenin [104]. To achieve Wnt-ON/Notch-OFF, Dishevelled directly interacts with the carboxyl terminal of Notch1 and inhibits CSL transcription factors to antagonize Notch signalling [99,100]. Besides direct interactions between Notch and Wnt, an overlapping player between the pathways could be tumour suppressor p53. Notch1 and p53 are co-regulators of each other in epithelial cells whereas p53 downregulates β-catenin activation by inducing expression of ubiquitin ligase Siah-1 that degrades β-catenin [56,57,105,106]. However, the exact mechanisms between these molecular interactions using p53 remain to be clearly elucidated.
Fig. 4.
Crosstalk of Notch and Wnt in epithelial homeostasis and HPV-modulated homeostasis. A) Notch and Wnt regulate one another to achieve Wnt-ON/Notch-OFF or Notch-ON/Wnt-OFF states. For Wnt-ON/Notch-OFF, Dishevelled interacts with Notch1 and inhibition of NICD-dependent transcription of Notch target genes leads to loss of p53. Loss of p53 subsequently enables accumulation and activation of β-catenin. For Notch-ON/Wnt-OFF, the Notch1 receptor sequesters β-catenin at the membrane. Moreover, NICD can form a complex with β-catenin and CSL in the nucleus to deactivate β-catenin. p53-dependent activation of Notch and inhibition of β-catenin induces the cell to commit to differentiation [99,104,105]. B) HPV's modulation on the crosstalk between Notch and Wnt depends on mutual effectors in the pathways. High-risk E6 degrades p53 which has an activating function on Notch1 and inhibitory function on β-catenin. High-risk E6 also binds to and stabilizes Dishevelled which inhibits Notch1 and activates Wnt by inhibiting the Axin destruction complex. The role of E7 and its cooperative effects with E6 on the crosstalk remain to be well-characterized [108,109]. C) Table summarizes the interactions of Notch & Wnt components, including p53 and Dishevelled, with Alpha- and Beta- HPV E6 and E7.
Understanding how HPV modulates the Notch and Wnt pathways independently is only the first step in learning how HPV orchestrates the signalling pathways to maintain a HPV-modulated homeostasis. First, we consider how HPVs use E6 to alter key effectors involved in both pathways (Fig. 4B and C). For example, p53 has a dual function in activating Notch1 and inhibiting Wnt [23,57,107]. Hence, high-risk E6 degradation of p53 results in inhibition of Notch and upregulation of Wnt [86,108]. Moreover, high-risk E6 also targets NHERF1 for degradation, further enhancing Wnt [90]. Another example is Dishevelled which enables β-catenin accumulation and inhibits the Notch1 receptor. HPV16 E6 has been found to interact with Dishevelled, therefore augmenting Wnt and perhaps inhibiting Notch [109,110]. Second, we examine how E7 alters key effectors during the crosstalk between Notch and Wnt. The functions of E7 in Notch and Wnt are less established, but high-risk E7 may stabilize p53 hence increasing p53 transactivation of the Notch1 promoter and Siah-1 expression that degrades B-catenin.
The roles of highly conserved Notch and Wnt signalling pathways in epithelial homeostasis are well defined; they are essential for cell fate determination and regulating differentiation and proliferation processes. HPVs use their E6 and E7 proteins to disrupt Notch and Wnt signalling independently as well as interfering with their crosstalk to ensure the maintenance of a viral reservoir in the basal layer. However, it is important to consider that significant variations of E6 and E7 expression levels exist between cells due to a fine balance between splicing factors and cis-regulatory RNA elements on HPV pre-mRNA [111,112]. The varying levels of E6 and E7 expression in individual cells may affect its level of regulation on Notch and Wnt signalling, which may give rise to the heterogeneity required for effective signalling. Further understanding of how the levels of E6 and E7 affect the Notch and Wnt pathways which impact differentiation and proliferation processes of HPV-infected cells is critical. Determining the functions of E6 and E7 alone and in cooperation with each other during this molecular crosstalk between Notch and Wnt is vital for our understanding of HPV persistence. Key players in this crosstalk that are modulated by E6 and E7 have yet to be well-characterized; p53 and Dishevelled hold promise whilst new players may yet to be revealed, representing an important consideration in the development of targeting agents for HPV-associated disease.
CRediT authorship contribution statement
June See Chong: Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. John Doorbar: Writing – review & editing, Supervision, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The support and encouragement of Dr. Nagayasu Egawa (University of Cambridge, UK) is greatly acknowledged. I would also like to thank Dr. Heather Griffin (University of Cambridge, UK) for helpful discussion.
Data availability
No data was used for the research described in the article.
References
- 1.Pires-daSilva A., Sommer R.J. The evolution of signalling pathways in animal development. Nat. Rev. Genet. 2003;4(1):39–49. doi: 10.1038/nrg977. [DOI] [PubMed] [Google Scholar]
- 2.Munoz Descalzo S., Martinez Arias A. The structure of Wntch signalling and the resolution of transition states in development. Semin. Cell Dev. Biol. 2012;23(4):443–449. doi: 10.1016/j.semcdb.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Hayward P., Kalmar T., Arias A.M. Wnt/Notch signalling and information processing during development. Development. 2008;135(3):411–424. doi: 10.1242/dev.000505. [DOI] [PubMed] [Google Scholar]
- 4.Bravo I.G., de Sanjose S., Gottschling M. The clinical importance of understanding the evolution of papillomaviruses. Trends Microbiol. 2010;18(10):432–438. doi: 10.1016/j.tim.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Doorbar J., et al. Principles of epithelial homeostasis control during persistent human papillomavirus infection and its deregulation at the cervical transformation zone. Curr Opin Virol. 2021;51:96–105. doi: 10.1016/j.coviro.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Gariglio P., et al. The role of retinoid deficiency and estrogens as cofactors in cervical cancer. Arch. Med. Res. 2009;40(6):449–465. doi: 10.1016/j.arcmed.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Kranjec C., Doorbar J. Human papillomavirus infection and induction of neoplasia: a matter of fitness. Curr Opin Virol. 2016;20:129–136. doi: 10.1016/j.coviro.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Tomaic V. Functional roles of E6 and E7 oncoproteins in HPV-induced malignancies at diverse anatomical sites. Cancers. 2016;8(10) doi: 10.3390/cancers8100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doorbar J. The papillomavirus life cycle. J. Clin. Virol. 2005;32(Suppl 1):S7–S15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Egawa K. Do human papillomaviruses target epidermal stem cells? Dermatology. 2003;207(3):251–254. doi: 10.1159/000073085. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt A., et al. The primary target cells of the high-risk cottontail rabbit papillomavirus colocalize with hair follicle stem cells. J. Virol. 1996;70(3):1912–1922. doi: 10.1128/jvi.70.3.1912-1922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doorbar J., et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 13.Munger K., et al. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004;78(21):11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park R.B., Androphy E.J. Genetic analysis of high-risk e6 in episomal maintenance of human papillomavirus genomes in primary human keratinocytes. J. Virol. 2002;76(22):11359–11364. doi: 10.1128/JVI.76.22.11359-11364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Songock W.K., Kim S.M., Bodily J.M. The human papillomavirus E7 oncoprotein as a regulator of transcription. Virus Res. 2017;231:56–75. doi: 10.1016/j.virusres.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonsson A., et al. General acquisition of human papillomavirus infections of skin occurs in early infancy. J. Clin. Microbiol. 2003;41(6):2509–2514. doi: 10.1128/JCM.41.6.2509-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonsson A., et al. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J. Virol. 2000;74(24):11636–11641. doi: 10.1128/jvi.74.24.11636-11641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh S.T., Longworth M.S., Laimins L.A. Roles of the E6 and E7 proteins in the life cycle of low-risk human papillomavirus type 11. J. Virol. 2004;78(5):2620–2626. doi: 10.1128/JVI.78.5.2620-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Middleton K., et al. Organization of human papillomavirus productive cycle during neoplastic progression provides a basis for selection of diagnostic markers. J. Virol. 2003;77(19):10186–10201. doi: 10.1128/JVI.77.19.10186-10201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duensing S., Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 2002;62(23):7075–7082. [PubMed] [Google Scholar]
- 21.Carestiato F.N., et al. Analysis of molecular biology techniques for the diagnosis of human papillomavirus infection and cervical cancer prevention. Rev. Soc. Bras. Med. Trop. 2006;39(5):428–432. doi: 10.1590/s0037-86822006000500002. [DOI] [PubMed] [Google Scholar]
- 22.Isaacson Wechsler E., et al. Reconstruction of human papillomavirus type 16-mediated early-stage neoplasia implicates E6/E7 deregulation and the loss of contact inhibition in neoplastic progression. J. Virol. 2012;86(11):6358–6364. doi: 10.1128/JVI.07069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheffner M., et al. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 24.Bosch F.X., et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J. Natl. Cancer Inst. 1995;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 25.Walboomers J.M., et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Herrero R., et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J. Natl. Cancer Inst. 2003;95(23):1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 27.Chaturvedi A.K., et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2023;41(17):3081–3088. doi: 10.1200/JCO.22.02625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C., Franceschi S., Clifford G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 2018;18(2):198–206. doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietsch E.C., Murphy M.E. Low risk HPV-E6 traps p53 in the cytoplasm and induces p53-dependent apoptosis. Cancer Biol. Ther. 2008;7(12):1916–1918. doi: 10.4161/cbt.7.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egawa N., Doorbar J. The low-risk papillomaviruses. Virus Res. 2017;231:119–127. doi: 10.1016/j.virusres.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Klingelhutz A.J., Roman A. Cellular transformation by human papillomaviruses: lessons learned by comparing high- and low-risk viruses. Virology. 2012;424(2):77–98. doi: 10.1016/j.virol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felsani A., Mileo A.M., Paggi M.G. Retinoblastoma family proteins as key targets of the small DNA virus oncoproteins. Oncogene. 2006;25(38):5277–5285. doi: 10.1038/sj.onc.1209621. [DOI] [PubMed] [Google Scholar]
- 33.Ivancic R., et al. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol. 2018;3(1):22–34. doi: 10.1002/lio2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goon P., et al. Recurrent respiratory papillomatosis: an overview of current thinking and treatment. Eur. Arch. Oto-Rhino-Laryngol. 2008;265(2):147–151. doi: 10.1007/s00405-007-0546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan N., et al. Immunoinformatics approach for design novel multiepitope prophylactic and therapeutic vaccine based on capsid proteins L1 and L2 and oncoproteins E6 and E7 of human papillomavirus 16 and human papillomavirus 18 against cervical cancer. Osong Public Health Res Perspect. 2024;15(4):307–328. doi: 10.24171/j.phrp.2024.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rampias T., et al. Activation of Wnt signaling pathway by human papillomavirus E6 and E7 oncogenes in HPV16-positive oropharyngeal squamous carcinoma cells. Mol. Cancer Res. 2010;8(3):433–443. doi: 10.1158/1541-7786.MCR-09-0345. [DOI] [PubMed] [Google Scholar]
- 37.Bray S.J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 38.Ehebauer M., Hayward P., Arias A.M. Notch, a universal arbiter of cell fate decisions. Science. 2006;314(5804):1414–1415. doi: 10.1126/science.1134042. [DOI] [PubMed] [Google Scholar]
- 39.Ehebauer M., Hayward P., Martinez-Arias A. Notch signaling pathway. Sci. STKE. 2006;2006(364):cm7. doi: 10.1126/stke.3642006cm7. [DOI] [PubMed] [Google Scholar]
- 40.Kopan R. Notch: a membrane-bound transcription factor. J. Cell Sci. 2002;115(Pt 6):1095–1097. doi: 10.1242/jcs.115.6.1095. [DOI] [PubMed] [Google Scholar]
- 41.Orzechowska M., Anusewicz D., Bednarek A.K. Functional gene expression differentiation of the notch signaling pathway in female reproductive tract tissues-A comprehensive review with analysis. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.592616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowell C., Radtke F. Cutaneous Notch signaling in health and disease. Cold Spring Harb Perspect Med. 2013;3(12):a017772. doi: 10.1101/cshperspect.a017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watt F.M., Estrach S., Ambler C.A. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr. Opin. Cell Biol. 2008;20(2):171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanpain C., et al. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20(21):3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thelu J., Rossio P., Favier B. Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 2002;2:7. doi: 10.1186/1471-5945-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rangarajan A., et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20(13):3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nickoloff B.J., et al. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9(8):842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- 48.Powell B.C., et al. The Notch signalling pathway in hair growth. Mech. Dev. 1998;78(1–2):189–192. doi: 10.1016/s0925-4773(98)00177-4. [DOI] [PubMed] [Google Scholar]
- 49.Thambyrajah R., et al. Cis inhibition of NOTCH1 through JAGGED1 sustains embryonic hematopoietic stem cell fate. Nat. Commun. 2024;15(1):1604. doi: 10.1038/s41467-024-45716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodilla V., et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. U. S. A. 2009;106(15):6315–6320. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X., et al. Jagged1 expression regulated by Notch3 and Wnt/beta-catenin signaling pathways in ovarian cancer. Oncotarget. 2010;1(3):210–218. doi: 10.18632/oncotarget.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lowell S., et al. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 2000;10(9):491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- 53.Chakrabarti R., et al. Notch ligand Dll1 mediates cross-talk between mammary stem cells and the macrophageal niche. Science. 2018;360(6396) doi: 10.1126/science.aan4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y., et al. Oscillations of Delta-like1 regulate the balance between differentiation and maintenance of muscle stem cells. Nat. Commun. 2021;12(1):1318. doi: 10.1038/s41467-021-21631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Negri V.A., et al. Single-cell RNA sequencing of human epidermis identifies Lunatic fringe as a novel regulator of the stem cell compartment. Stem Cell Rep. 2023;18(11):2047–2055. doi: 10.1016/j.stemcr.2023.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lefort K., et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev. 2007;21(5):562–577. doi: 10.1101/gad.1484707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yugawa T., et al. Regulation of Notch1 gene expression by p53 in epithelial cells. Mol. Cell Biol. 2007;27(10):3732–3742. doi: 10.1128/MCB.02119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brimer N., et al. Cutaneous papillomavirus E6 oncoproteins associate with MAML1 to repress transactivation and NOTCH signaling. Oncogene. 2012;31(43):4639–4646. doi: 10.1038/onc.2011.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weijzen S., et al. HPV16 E6 and E7 oncoproteins regulate Notch-1 expression and cooperate to induce transformation. J. Cell. Physiol. 2003;194(3):356–362. doi: 10.1002/jcp.10217. [DOI] [PubMed] [Google Scholar]
- 60.Talora C., et al. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 2002;16(17):2252–2263. doi: 10.1101/gad.988902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel D., et al. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999;18(18):5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oswald F., et al. p300 acts as a transcriptional coactivator for mammalian Notch-1. Mol. Cell Biol. 2001;21(22):7761–7774. doi: 10.1128/MCB.21.22.7761-7774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan M.J., et al. Cutaneous beta-human papillomavirus E6 proteins bind Mastermind-like coactivators and repress Notch signaling. Proc. Natl. Acad. Sci. U. S. A. 2012;109(23):E1473–E1480. doi: 10.1073/pnas.1205991109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun L., et al. Two different HPV-11E6 fusion proteins trap p53 in the cytoplasm and induce apoptosis. Cancer Biol. Ther. 2008;7(12):1909–1915. doi: 10.4161/cbt.7.12.6941. [DOI] [PubMed] [Google Scholar]
- 65.Seavey S.E., et al. The E7 oncoprotein of human papillomavirus type 16 stabilizes p53 through a mechanism independent of p19(ARF) J. Virol. 1999;73(9):7590–7598. doi: 10.1128/jvi.73.9.7590-7598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., et al. Abrogation of p53-induced G1 arrest by the HPV 16 E7 protein does not inhibit p53-induced apoptosis. Oncogene. 1996;12(12):2731–2735. [PubMed] [Google Scholar]
- 67.Demers G.W., Halbert C.L., Galloway D.A. Elevated wild-type p53 protein levels in human epithelial cell lines immortalized by the human papillomavirus type 16 E7 gene. Virology. 1994;198(1):169–174. doi: 10.1006/viro.1994.1019. [DOI] [PubMed] [Google Scholar]
- 68.Jones D.L., Thompson D.A., Munger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997;239(1):97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- 69.Tolwinski N.S., Wieschaus E. A nuclear function for armadillo/beta-catenin. PLoS Biol. 2004;2(4):E95. doi: 10.1371/journal.pbio.0020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banziger C., et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125(3):509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 71.Molenaar M., et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86(3):391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 72.Jho E.H., et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell Biol. 2002;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He T.C., et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 74.Theka I., et al. Wnt/beta-catenin signaling pathway safeguards epigenetic stability and homeostasis of mouse embryonic stem cells. Sci. Rep. 2019;9(1):948. doi: 10.1038/s41598-018-37442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rao T.P., Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ. Res. 2010;106(12):1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 76.Vatansever H.S., et al. The role of stem/progenitor cells and Wnt/beta-catenin signaling pathway in the patients with prostate cancer. Minerva Urol. Nefrol. 2014;66(4):249–255. [PubMed] [Google Scholar]
- 77.Chae W.J., Bothwell A.L.M. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 2018;39(10):830–847. doi: 10.1016/j.it.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin K., et al. Canonical and noncanonical Wnt signaling: multilayered mediators, signaling mechanisms and major signaling crosstalk. Genes Dis. 2024;11(1):103–134. doi: 10.1016/j.gendis.2023.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jia L., et al. Effects of Wnt3a on proliferation and differentiation of human epidermal stem cells. Biochem. Biophys. Res. Commun. 2008;368(3):483–488. doi: 10.1016/j.bbrc.2008.01.097. [DOI] [PubMed] [Google Scholar]
- 80.Xu Z., et al. Wnt/beta-catenin signaling promotes self-renewal and inhibits the primed state transition in naive human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2016;113(42):E6382–E6390. doi: 10.1073/pnas.1613849113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luis T.C., et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113(3):546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- 82.Sato A., et al. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010;29(1):41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schizas N.P., et al. Inhibition versus activation of canonical Wnt-signaling, to promote chondrogenic differentiation of Mesenchymal Stem Cells. A review. Orthop. Rev. 2021;13(2) doi: 10.52965/001c.27098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y., Huang X., Yuan Y. MicroRNA-410 promotes chondrogenic differentiation of human bone marrow mesenchymal stem cells through down-regulating Wnt3a. Am J Transl Res. 2017;9(1):136–145. [PMC free article] [PubMed] [Google Scholar]
- 85.Wheeler D.S., et al. Direct interaction between NHERF1 and Frizzled regulates beta-catenin signaling. Oncogene. 2011;30(1):32–42. doi: 10.1038/onc.2010.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lichtig H., et al. HPV16 E6 augments Wnt signaling in an E6AP-dependent manner. Virology. 2010;396(1):47–58. doi: 10.1016/j.virol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 87.Aghbash P.S., et al. The effect of Wnt/beta-catenin signaling on PD-1/PDL-1 axis in HPV-related cervical cancer. Oncol. Res. 2022;30(3):99–116. doi: 10.32604/or.2022.026776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vlad-Fiegen A., et al. The Wnt pathway destabilizes adherens junctions and promotes cell migration via beta-catenin and its target gene cyclin D1. FEBS Open Bio. 2012;2:26–31. doi: 10.1016/j.fob.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsuzawa S.I., Reed J.C. Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell. 2001;7(5):915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 90.Drews C.M., Case S., Vande Pol S.B. E6 proteins from high-risk HPV, low-risk HPV, and animal papillomaviruses activate the Wnt/beta-catenin pathway through E6AP-dependent degradation of NHERF1. PLoS Pathog. 2019;15(4) doi: 10.1371/journal.ppat.1007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yin W., et al. HPV E6 inhibits E6AP to regulate epithelial homeostasis by modulating keratinocyte differentiation commitment and YAP1 activation. PLoS Pathog. 2023;19(6) doi: 10.1371/journal.ppat.1011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu L., et al. Na+/H+ exchanger regulatory factor 1 (NHERF1) directly regulates osteogenesis. J. Biol. Chem. 2012;287(52):43312–43321. doi: 10.1074/jbc.M112.422766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Q., et al. NHERF1 inhibits beta-catenin-mediated proliferation of cervical cancer cells through suppression of alpha-actinin-4 expression. Cell Death Dis. 2018;9(6):668. doi: 10.1038/s41419-018-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Accardi R., et al. E6 and E7 from human papillomavirus type 16 cooperate to target the PDZ protein Na/H exchange regulatory factor 1. J. Virol. 2011;85(16):8208–8216. doi: 10.1128/JVI.00114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pim D., et al. Activation of the protein kinase B pathway by the HPV-16 E7 oncoprotein occurs through a mechanism involving interaction with PP2A. Oncogene. 2005;24(53):7830–7838. doi: 10.1038/sj.onc.1208935. [DOI] [PubMed] [Google Scholar]
- 96.Li X., et al. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001;20(15):4122–4131. doi: 10.1093/emboj/20.15.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Collu G.M., Hidalgo-Sastre A., Brennan K. Wnt-Notch signalling crosstalk in development and disease. Cell. Mol. Life Sci. 2014;71(18):3553–3567. doi: 10.1007/s00018-014-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu A.J., Watt F.M. beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126(10):2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]
- 99.Collu G.M., et al. Dishevelled limits Notch signalling through inhibition of CSL. Development. 2012;139(23):4405–4415. doi: 10.1242/dev.081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Axelrod J.D., et al. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science. 1996;271(5257):1826–1832. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- 101.Kim H.A., et al. Notch1 counteracts WNT/beta-catenin signaling through chromatin modification in colorectal cancer. J. Clin. Invest. 2012;122(9):3248–3259. doi: 10.1172/JCI61216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwon C., et al. Notch post-translationally regulates beta-catenin protein in stem and progenitor cells. Nat. Cell Biol. 2011;13(10):1244–1251. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kwon C., et al. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat. Cell Biol. 2009;11(8):951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Acar A., et al. Inhibition of Wnt signalling by Notch via two distinct mechanisms. Sci. Rep. 2021;11(1):9096. doi: 10.1038/s41598-021-88618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levina E., Oren M., Ben-Ze'ev A. Downregulation of beta-catenin by p53 involves changes in the rate of beta-catenin phosphorylation and Axin dynamics. Oncogene. 2004;23(25):4444–4453. doi: 10.1038/sj.onc.1207587. [DOI] [PubMed] [Google Scholar]
- 106.Nair P., Somasundaram K., Krishna S. Activated Notch1 inhibits p53-induced apoptosis and sustains transformation by human papillomavirus type 16 E6 and E7 oncogenes through a PI3K-PKB/Akt-dependent pathway. J. Virol. 2003;77(12):7106–7112. doi: 10.1128/JVI.77.12.7106-7112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim N.H., et al. p53 and microRNA-34 are suppressors of canonical Wnt signaling. Sci. Signal. 2011;4(197):ra71. doi: 10.1126/scisignal.2001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dotto G.P. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat. Rev. Cancer. 2009;9(8):587–595. doi: 10.1038/nrc2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bonilla-Delgado J., et al. The E6 oncoprotein from HPV16 enhances the canonical Wnt/beta-catenin pathway in skin epidermis in vivo. Mol. Cancer Res. 2012;10(2):250–258. doi: 10.1158/1541-7786.MCR-11-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scarth J.A., et al. The human papillomavirus oncoproteins: a review of the host pathways targeted on the road to transformation. J. Gen. Virol. 2021;102(3) doi: 10.1099/jgv.0.001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosty C., et al. Identification of a proliferation gene cluster associated with HPV E6/E7 expression level and viral DNA load in invasive cervical carcinoma. Oncogene. 2005;24(47):7094–7104. doi: 10.1038/sj.onc.1208854. [DOI] [PubMed] [Google Scholar]
- 112.Tang S., et al. The E7 oncoprotein is translated from spliced E6∗I transcripts in high-risk human papillomavirus type 16- or type 18-positive cervical cancer cell lines via translation reinitiation. J. Virol. 2006;80(9):4249–4263. doi: 10.1128/JVI.80.9.4249-4263.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.