Abstract
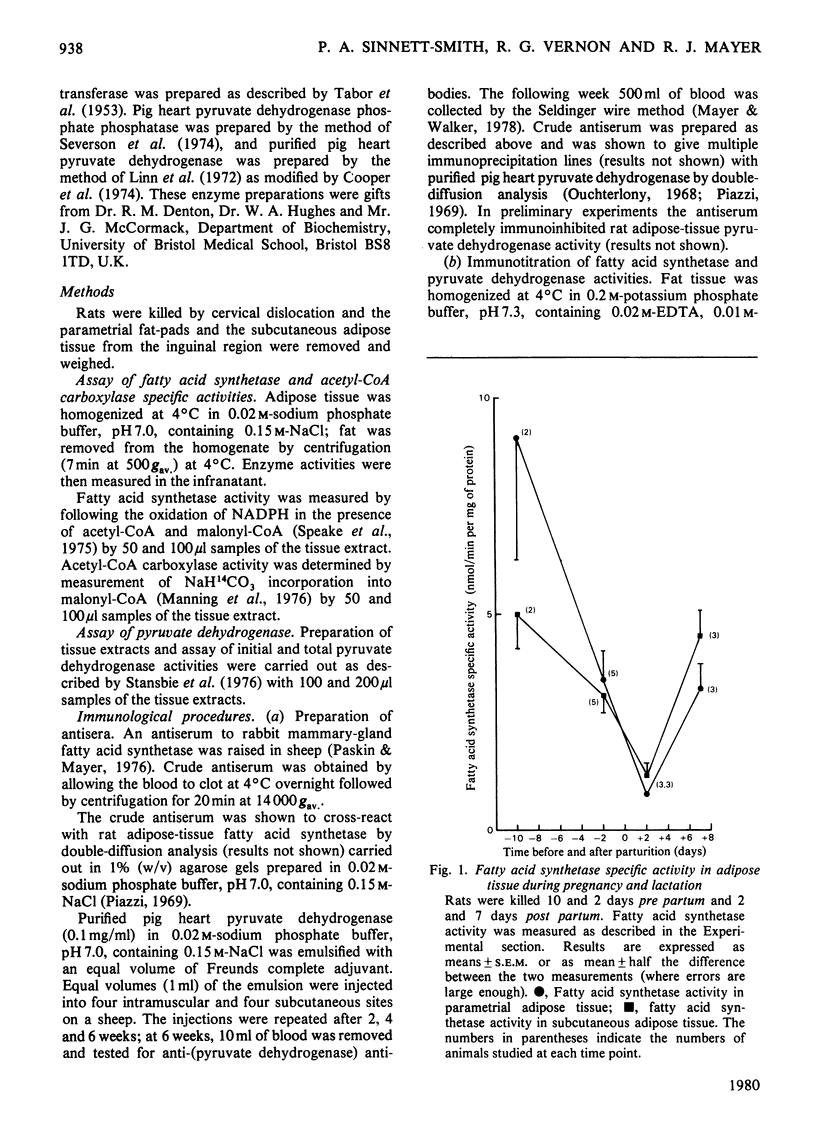
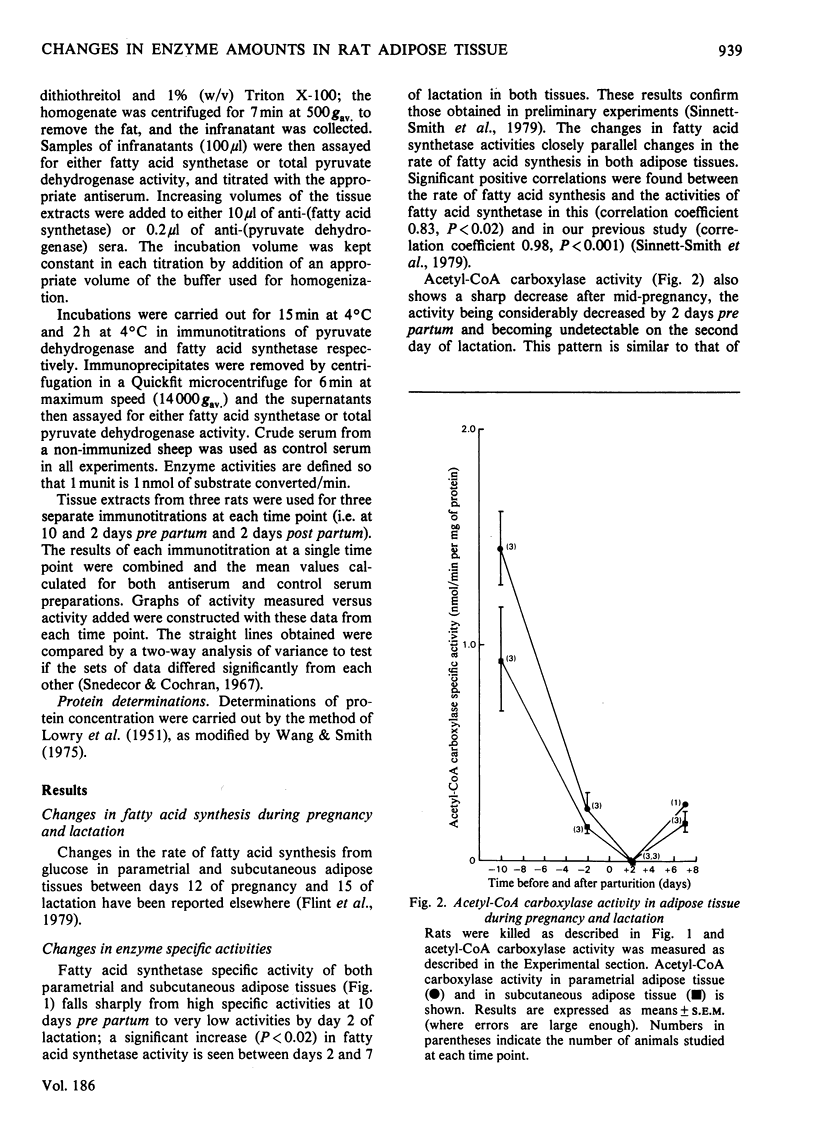
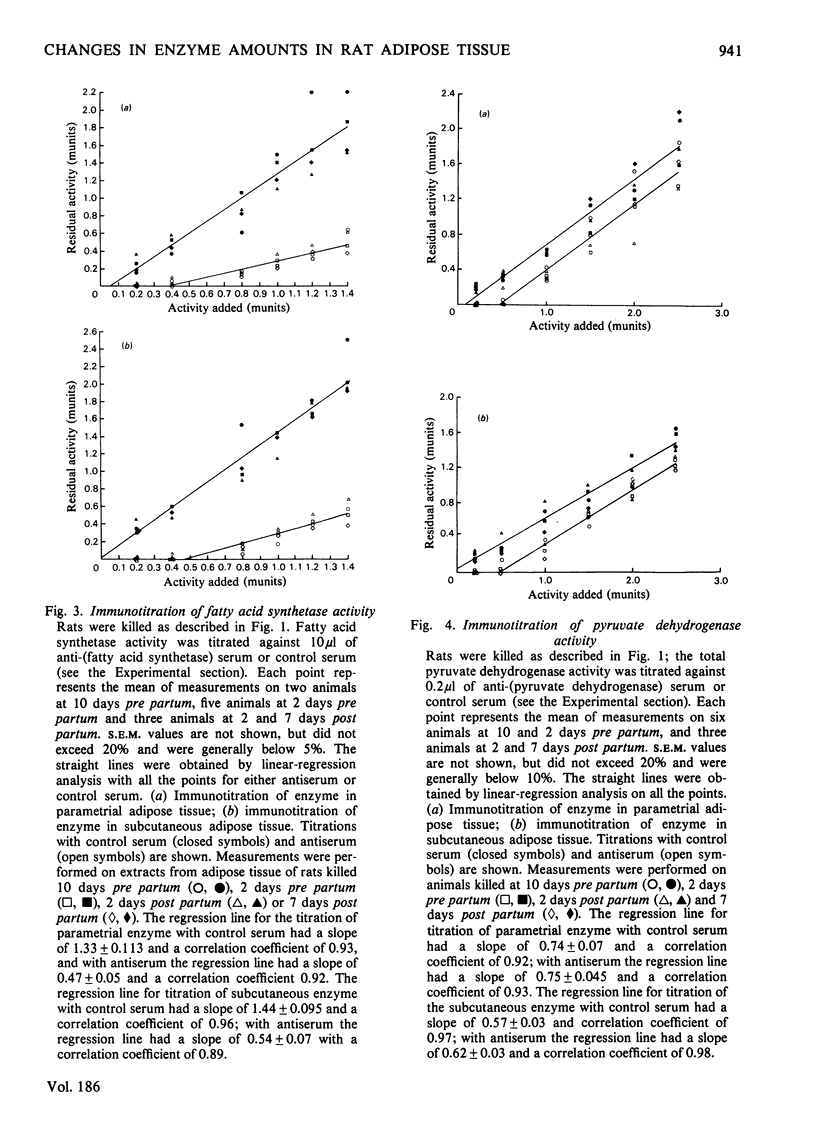
1. The specific activities of fatty acid synthetase, acetyl-CoA carboxylase and pyruvate dehydrogenase were measured in rat adipose-tissue extracts in pregnancy and lactation. Fatty acid synthetase specific activity correlates very closely with the rate of fatty acid synthesis, the enzyme specific activity decreasing after mid-pregnancy in a manner very similar to the rate of fatty acid synthesis. Acetyl-CoA carboxylase specific activity also decreases dramatically after mid-pregnancy. Initial pyruvate dehydrogenase specific activity shows a decrease between 2 days pre partum and 2 days post partum, but total enzyme activity shows no significant change in the same period. 2. Immunotitrations of fatty acid synthetase and pyruvate dehydrogenase activities were carried out; the titrations showed that the change in the fatty acid synthetase activity is due to a change in the enzyme amount; the amount of pyruvate dyhydrogenase does not change. Therefore the decrease in fatty acid biosynthesis in subcutaneous and parametrial adipose tissue in late pregnancy and early lactation is associated with a decrease in the amount of at least one of the enzymes involved in fatty acid biosynthesis. The correlation of these events with known hormonal changes is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEATON G. H., BEARE J., RYU M. H., McHENRY E. W. Protein metabolism in the pregnant rat. J Nutr. 1954 Oct 11;54(2):291–304. doi: 10.1093/jn/54.2.291. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint D. J., Sinnett-Smith P. A., Clegg R. A., Vernon R. G. Role of insulin receptors in the changing metabolism of adipose tissue during pregnancy and lactation in the rat. Biochem J. 1979 Aug 15;182(2):421–427. doi: 10.1042/bj1820421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- Hamosh M., Clary T. R., Chernick S. S., Scow R. O. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta. 1970 Sep 8;210(3):473–482. doi: 10.1016/0005-2760(70)90044-5. [DOI] [PubMed] [Google Scholar]
- Joshi V. C., Sidbury J. B., Jr Hormonal regulation of fatty acid synthetase in chick embryo liver. Arch Biochem Biophys. 1976 Apr;173(2):403–414. doi: 10.1016/0003-9861(76)90278-2. [DOI] [PubMed] [Google Scholar]
- Knopp R. H., Saudek C. D., Arky R. A., O'Sullivan J. B. 2 phases of adipose tissue metabolism in pregnancy: maternal adaptations for fetal growth. Endocrinology. 1973 Apr;92(4):984–988. doi: 10.1210/endo-92-4-984. [DOI] [PubMed] [Google Scholar]
- Lakshmanan M. R., Nepokroeff C. M., Kim M., Porter J. W. Adaptive synthesis of fatty acid synthetase and acetyl-CoA carboxylase by isolated rat liver cells. Arch Biochem Biophys. 1975 Aug;169(2):737–745. doi: 10.1016/0003-9861(75)90219-2. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Manning R., Dils R., Mayer R. J. Purification and some properties of acetyl-coenzyme A carboxylase from rabbit mammary gland. Biochem J. 1976 Feb 1;153(2):463–468. doi: 10.1042/bj1530463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The significance of changes in tissue clearing-factor lipase activity in relation to the lipaemia of pregnancy. Biochem J. 1968 Feb;106(3):677–682. doi: 10.1042/bj1060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskin N., Mayer R. J. A method for the analysis of protein turnover characteristics. Indirect estimation of rates of protein degradation. Biochem J. 1978 Jul 15;174(1):153–161. doi: 10.1042/bj1740153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskin N., Mayer R. J. Molecular weight and subunit size of fatty acid synthetase from rabbit mammary gland. Biochem J. 1976 Oct 1;159(1):181–184. doi: 10.1042/bj1590181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzi S. E. A simple method for preliminary immunodiffusion test of antigen-antibody systems having unknown ratios of reaction. Anal Biochem. 1969 Feb;27(2):281–284. doi: 10.1016/0003-2697(69)90033-5. [DOI] [PubMed] [Google Scholar]
- SPRAY C. M. A study of some aspects of reproduction by means of chemical analysis. Br J Nutr. 1950;4(4):354–360. doi: 10.1079/bjn19500059. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith P. A., Vernon R. G., Mayer R. J. Fatty acid synyhesis and the activities of fatty acid synthesizing enzymes in rat adiposes tissue during pregnancy and lactation [proceedings]. Biochem Soc Trans. 1979 Apr;7(2):388–389. doi: 10.1042/bst0070388. [DOI] [PubMed] [Google Scholar]
- Smith R. W. The effects of pregnancy, lactation and involution on the metabolism of glucose by rat parametrial adipose tissue. J Dairy Res. 1973 Oct;40(3):353–360. doi: 10.1017/s0022029900014722. [DOI] [PubMed] [Google Scholar]
- Smith S., Easter D. J., Dils R. Fatty acid biosynthesis. 3. Intracellular site of enzymes in lactating-rabbit mammary gland. Biochim Biophys Acta. 1966 Dec 7;125(3):445–455. [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differention. Studies on the effects of hormones on the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1975 May;148(2):309–320. doi: 10.1042/bj1480309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbie D., Denton R. M., Bridges B. J., Pask H. T., Randle P. J. Regulation of pyruvate dehydrogenase and pyruvate dehydrogenase phosphate phosphatase activity in rat epididymal fat-pads. Effects of starvation, alloxan-diabetes and high-fat diet. Biochem J. 1976 Jan 15;154(1):225–236. doi: 10.1042/bj1540225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter-Dub M. T., Leclercq R., Sutter B. C., Jacquot R. Plasma glucose, progesterone and immunoreactive insulin levels in the lactating rat. Horm Metab Res. 1974 Jul;6(4):297–300. doi: 10.1055/s-0028-1093852. [DOI] [PubMed] [Google Scholar]
- TABOR H., MEHLER A. H., STADTMAN E. R. The enzymatic acetylation of amines. J Biol Chem. 1953 Sep;204(1):127–138. [PubMed] [Google Scholar]
- Volpe J. J., Lyles T. O., Roncari D. A., Vagelos P. R. Fatty acid synthetase of developing brain and liver. Content, synthesis, and degradation during development. J Biol Chem. 1973 Apr 10;248(7):2502–2513. [PubMed] [Google Scholar]
- Volpe J. J., Marasa J. C. Hormonal regulation of fatty acid synthetase, acetyl-CoA carboxylase and fatty acid synthesis in mammalian adipose tissue and liver. Biochim Biophys Acta. 1975 Mar 24;380(3):454–472. doi: 10.1016/0005-2760(75)90113-7. [DOI] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]


