Abstract
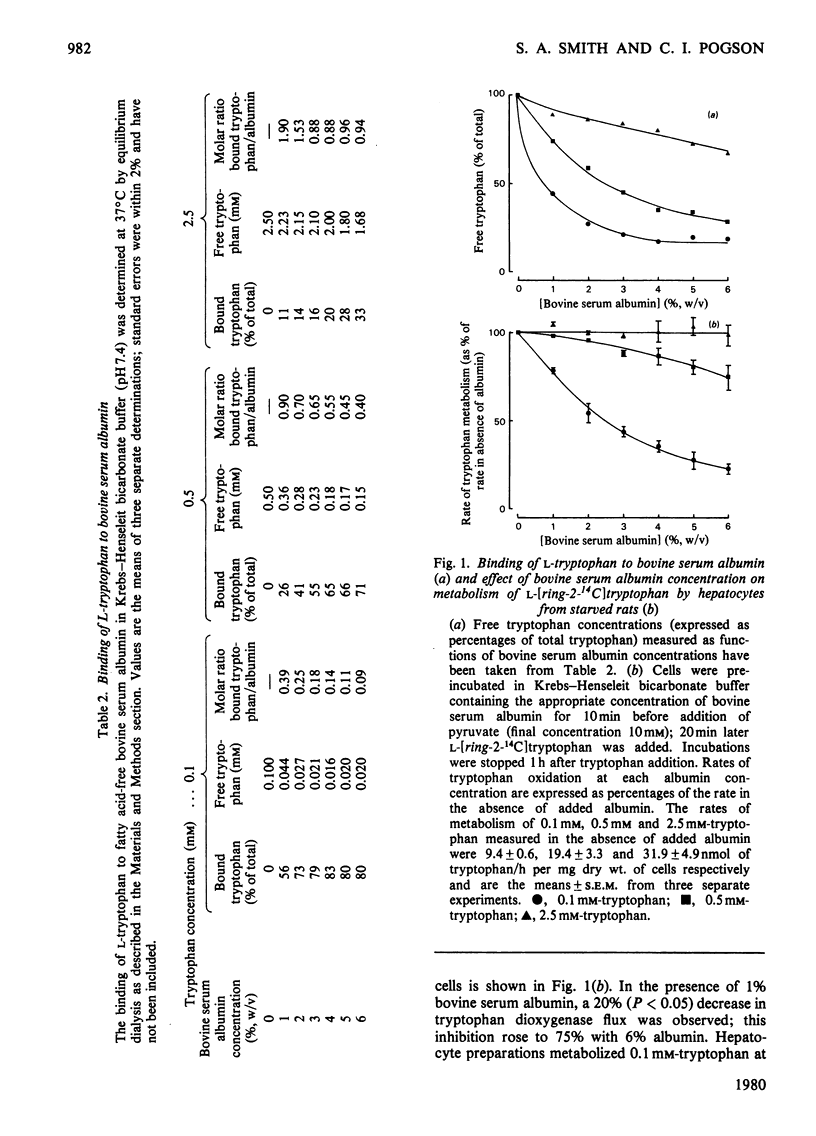
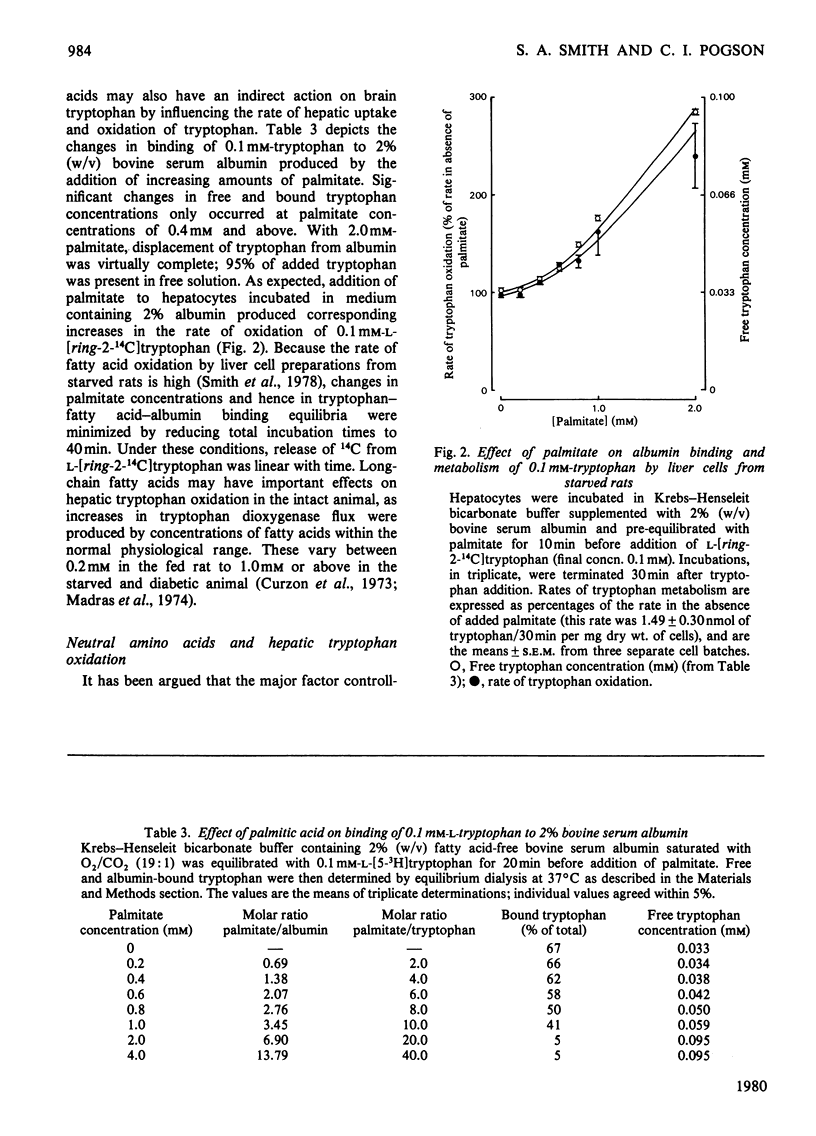
1. Novel methods, using L-[ring-2-14C]tryptophan, are described for the measurement of tryptophan 2,3-dioxygenase activity and tryptophan accumulation in isolated rat liver cells. 2. The effects of bovine serum albumin, non-esterified fatty acids and neutral amino acids on tryptophan oxidation by hepatocytes and on the partition of tryptophan between free and albumin-bound forms were investigated. 3. Oxidation of physiological concentrations (0.1 mM) of tryptophan was inhibited by approx. 50% in the presence of 2% (w/v) bovine serum albumin; no effects were found at tryptophan concentrations of 0.5 mM and above. 4. Increases in free tryptophan concentrations produced by displacement of 0.1 mM-tryptophan from albumin-binding sites by palmitate resulted in increased flux through tryptophan dioxygenase. 5. Addition of a mixture of neutral amino acids, at plasma concentrations, to hepatocyte incubations had no effect on the rate of tryptophan oxidation. 6. It is concluded that alterations in free tryptophan concentrations consequent to changes in albumin binding may be an important factor in regulating tryptophan uptake and catabolism by the liver. The results are briefly discussed with reference to possible consequences on brain tryptophan metabolism.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badawy A. A., Evans M. Regulation of rat liver tryptophan pyrrolase by its cofactor haem: Experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975 Sep;150(3):511–520. doi: 10.1042/bj1500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The role of free serum tryptophan in the biphasic effect of acute ethanol administration on the concentrations of rat brain tryptophan, 5-hydroxytryptamine and 5-hydroxyindol-3-ylacetic acid. Biochem J. 1976 Nov 15;160(2):315–324. doi: 10.1042/bj1600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Punjani N. F., Evans M. Enhancement of rat brain tryptophan metabolism by chronic ethanol administration and possible involvement of decreased liver tryptophan pyrrolase activity. Biochem J. 1979 Mar 15;178(3):575–580. doi: 10.1042/bj1780575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A. The functions and regulation of tryptophan pyrrolase. Life Sci. 1977 Sep 15;21(6):755–768. doi: 10.1016/0024-3205(77)90402-7. [DOI] [PubMed] [Google Scholar]
- Bender D. A., Cockcroft P. M. Increase in brain tryptophan and 5-hydroxytryptamine on administration of phenothiazines to rats. Biochem Soc Trans. 1977;5(1):155–157. doi: 10.1042/bst0050155. [DOI] [PubMed] [Google Scholar]
- Bloxam D. L., Curzon G. A study of proposed determinants of brain tryptophan concentration in rats after portocaval anastomosis or sham operation. J Neurochem. 1978 Nov;31(5):1255–1263. doi: 10.1111/j.1471-4159.1978.tb06250.x. [DOI] [PubMed] [Google Scholar]
- Bloxam D. L., Warren W. H. Error in the determination of tryptophan by the method of Denkla and Dewey. A revised procedure. Anal Biochem. 1974 Aug;60(2):621–625. doi: 10.1016/0003-2697(74)90275-9. [DOI] [PubMed] [Google Scholar]
- Bowmer C. J., Lindup W. E. Binding of phenytoin, L-tryptophan and O-methyl red to albumin. Unexpected effect of albumin concentration on the binding of phenytoin and L-tryptophan. Biochem Pharmacol. 1978 Mar 15;27(6):937–942. doi: 10.1016/0006-2952(78)90421-5. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Curzon G., Friedel J., Katamaneni B. D., Greenwood M. H., Lader M. H. Unesterified fatty acids and the binding of tryptophan in human plasma. Clin Sci Mol Med. 1974 Nov;47(5):415–424. doi: 10.1042/cs0470415. [DOI] [PubMed] [Google Scholar]
- Curzon G., Friedel J., Knott P. J. The effect of fatty acids on the binding of tryptophan to plasma protein. Nature. 1973 Mar 16;242(5394):198–200. doi: 10.1038/242198a0. [DOI] [PubMed] [Google Scholar]
- Curzon G., Knott P. J. Effects on plasma and brain tryptophan in the rat of drugs and hormones that influence the concentration of unesterified fatty acid in the plasma. Br J Pharmacol. 1974 Feb;50(2):197–204. doi: 10.1111/j.1476-5381.1974.tb08562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denckla W. D., Dewey H. K. The determination of tryptophan in plasma, liver, and urine. J Lab Clin Med. 1967 Jan;69(1):160–169. [PubMed] [Google Scholar]
- Fehlmann M., Le Cam A., Kitabgi P., Rey J. F., Freychet P. Regulation of amino acid transport in the liver. Emergence of a high affinity transport system in isolated hepatocytes from fasting rats. J Biol Chem. 1979 Jan 25;254(2):401–407. [PubMed] [Google Scholar]
- Fernstrom J. D., Wurtman R. J. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. 1972 Oct 27;178(4059):414–416. doi: 10.1126/science.178.4059.414. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Kappelman A. H., Kaufman S. Partial purification and characterization of tryptophan hydroxylase from rabbit hindbrain. J Biol Chem. 1972 Jul 10;247(13):4165–4173. [PubMed] [Google Scholar]
- GREENGARD O., FEIGELSONP The purification and properties of liver tryptophan pyrrolase. J Biol Chem. 1962 Jun;237:1903–1907. [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., Curzon G. Decrease of 5-hydroxytryptamine in the brain provoked by hydrocortisone and its prevention by allopurinol. Nature. 1968 Dec 14;220(5172):1095–1097. doi: 10.1038/2201095a0. [DOI] [PubMed] [Google Scholar]
- Green A. R., Woods H. F., Joseph M. H. Tryptophan metabolism in the isolated perfused liver of the rat: effects of tryptophan concentration, hydrocortisone and allopurinol on tryptophan pyrrolase activity and kynurenine formation. Br J Pharmacol. 1976 May;57(1):103–114. doi: 10.1111/j.1476-5381.1976.tb07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph M. H., Young S. N., Curzon G. The metabolism of a tryptophan load in rat brain and liver. The influence of hydrocortisone and allopurinol. Biochem Pharmacol. 1976 Dec 1;25(23):2599–2604. doi: 10.1016/0006-2952(76)90515-3. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Bradford N. M., McGivan J. D. Characteristics of the transport of alanine, serine and glutamine across the plasma membrane of isolated rat liver cells. Biochem J. 1978 Dec 15;176(3):827–836. doi: 10.1042/bj1760827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott P. J., Curzon G. Free tryptophan in plasma and brain tryptophan metabolism. Nature. 1972 Oct 20;239(5373):452–453. doi: 10.1038/239452a0. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Piras M. M. Tryptophan pyrrolase of liver. 3. Conjugation in vivo during cofactor induction by tryptophan analogues. J Biol Chem. 1967 Jun 25;242(12):2959–2965. [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Tyler B. The regulation of folate and methionine metabolism. Biochem J. 1976 Aug 15;158(2):341–353. doi: 10.1042/bj1580341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letter A. A., Zombor G., Henderson J. F. Tryptophan as a source of one-carbon units for purine biosynthesis de novo. Can J Biochem. 1973 Apr;51(4):486–488. doi: 10.1139/o73-058. [DOI] [PubMed] [Google Scholar]
- Lipsett D., Madras B. K., Wurtman R. J., Munro H. N. Serum tryptophan level after carbohydrate ingestion: selective decline in non-albumin-bound tryptophan coincident with reduction in serum free fatty acids. Life Sci II. 1973 Jan 22;12(2):57–64. doi: 10.1016/0024-3205(73)90027-1. [DOI] [PubMed] [Google Scholar]
- MCMENAMY R. H., LUND C. C., VAN MARCKE J., ONCLEY J. L. The binding of L-tryptophan in human plasma at 37 degrees C. Arch Biochem Biophys. 1961 Apr;93:135–139. doi: 10.1016/0003-9861(61)90325-3. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H., KNOX W. E. The conversion of tryptophan to kynurenine in liver. II. The enzymatic hydrolysis of formylkynurenine. J Biol Chem. 1950 Nov;187(1):431–438. [PubMed] [Google Scholar]
- Madras B. K., Cohen E. L., Messing R., Munro H. N., Wurtman R. J. Relevance of free tryptophan in serum to tissue tryptophan concentrations. Metabolism. 1974 Dec;23(12):1107–1116. doi: 10.1016/0026-0495(74)90027-4. [DOI] [PubMed] [Google Scholar]
- McArthur J. N., Dawkins P. D. The effect of sodium salicylate on the binding of L-tryptophan to serum proteins. J Pharm Pharmacol. 1969 Nov;21(11):744–750. doi: 10.1111/j.2042-7158.1969.tb08163.x. [DOI] [PubMed] [Google Scholar]
- McMENAMY R. H., ONCLEY J. L. The specific binding of L-tryptophan to serum albumin. J Biol Chem. 1958 Dec;233(6):1436–1447. [PubMed] [Google Scholar]
- Ng C. Y., Hagino Y., Swan P. B., Henderson L. M. Metabolism of tryptophan in isolated perfused rat liver. J Nutr. 1969 Dec;99(4):465–473. doi: 10.1093/jn/99.4.465. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Invest. 1974 Feb;33(1):5–10. doi: 10.3109/00365517409114190. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Kinetics of competitive inhibition of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1977 Jan;28(1):103–108. doi: 10.1111/j.1471-4159.1977.tb07714.x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H. Transport of metabolic substrates through the blood-brain barrier. J Neurochem. 1977 Jan;28(1):5–12. doi: 10.1111/j.1471-4159.1977.tb07702.x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. The role of blood-brain barrier transport of tryptophan and other neutral amino acids in the regulation of substrate-limited pathways of brain amino acid metabolism. J Neural Transm Suppl. 1979;(15):43–54. doi: 10.1007/978-3-7091-2243-3_4. [DOI] [PubMed] [Google Scholar]
- Rodden F. A., Berg C. P. Enzymatic conversion of L- and D-tryptophan to kynurenine by rat liver. J Nutr. 1974 Feb;104(2):227–238. doi: 10.1093/jn/104.2.227. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E. Valine uptake and incorporation into protein in isolated rat hepatocytes. Nature of the precursor pool for protein synthesis. Eur J Biochem. 1978 Apr;85(1):15–25. doi: 10.1111/j.1432-1033.1978.tb12208.x. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Differential effects of tryptophan on glucose synthesis in rats and guinea pigs. Biochem J. 1978 Dec 15;176(3):817–825. doi: 10.1042/bj1760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart K. K., Doherty R. F. Resolution of DL-tryptophan by affinity chromatography on bovine-serum albumin-agarose columns. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2850–2852. doi: 10.1073/pnas.70.10.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. N., Oravec M., Sourkes T. L. The effect of theophylline on tryptophan pyrrolase in the hypophysectomized rat and some observations on the validity of tryptophan pyrrolase assays. J Biol Chem. 1974 Jun 25;249(12):3932–3936. [PubMed] [Google Scholar]
- Young S. N., Sourkes T. L. Tryptophan catabolism by tryptophan pyrrolase in rat liver. The effect of tryptophan loads and changes in tryptophan pyrrolase activity. J Biol Chem. 1975 Jul 10;250(13):5009–5014. [PubMed] [Google Scholar]
- Young S. N., St-Arnaud-McKenzie D., Sourkes T. L. Importance of tryptophan pyrrolase and aromatic amino acid decarboxylase in the catabolism of tryptophan. Biochem Pharmacol. 1978 Mar 1;27(5):763–767. doi: 10.1016/0006-2952(78)90517-8. [DOI] [PubMed] [Google Scholar]
- Yuwiler A., Oldendorf W. H., Geller E., Braun L. Effect of albumin binding and amino acid competition on tryptophan uptake into brain. J Neurochem. 1977 May;28(5):1015–1023. doi: 10.1111/j.1471-4159.1977.tb10664.x. [DOI] [PubMed] [Google Scholar]


