Introduction
Early land plants' ability to adapt to novel environmental pressures associated with an ever‐changing terrestrial habitat was the result of a vast set of evolutionary innovations, including metabolic ones (Wagner, 2011; Bowman et al., 2017). Land plants, as sessile organisms, were driven to evolve integrated and modular metabolic pathways. Several of them were true metabolic network innovations, responsible for synthesizing several novel compounds (Cannell et al., 2020; Dadras et al., 2023b). The new specialized metabolites (SMs) contributed to thrive in these new and frequently hostile environments (Rensing, 2018; Cheng et al., 2019; Han et al., 2019; Buschmann, 2020; Fürst‐Jansen et al., 2020). There is evidence that metabolic plasticity is a key component of a highly complex network in the plant–environment interaction, which also includes morphoanatomical traits. This network largely and ultimately determines the ability of terrestrial plants to escape from the most severe environmental threats, the so‐called ‘flight strategy’ of sessile organisms (Potters et al., 2007; Lauder et al., 2019). While an elaborate metabolic system was already placed in the closest algal ancestors of land plants (Rieseberg et al., 2021; Dadras et al., 2023a), primary and particularly secondary metabolic networks have grown far more sophisticated throughout plant evolution (Keeling et al., 2010; Wang et al., 2015; Maeda, 2019; Bowles et al., 2020; Li et al., 2024). They contributed to land plant distribution toward more challenging habitats (Steemans et al., 2009). For instance, the R2R3MYB family of transcription factors (TFs), which regulates a wide array of biological processes, including the expression of genes involved in the biosynthesis of phenylpropanoids, has been extraordinarily expanded and diversified in the lineage of angiosperms (Feller et al., 2011; Bowman et al., 2017; Albert et al., 2018; Jiang & Rao, 2020; Davies et al., 2021). Enzymes involved in both the ‘decoration’ of basic phenylpropanoid skeletons (e.g. the C6‐C3‐C6 core skeleton of flavonoids) and their transport to different subcellular compartments have also expanded much throughout plant evolution (Kitamura, 2006; Tohge et al., 2018; Alseekh et al., 2020; Davies et al., 2020; Li et al., 2020; Wen et al., 2020). The extraordinary chemical diversity originated from the rise and evolution of multiple SM pathways, coupled with their location in different tissues and cellular compartments, well explains the outstanding plant adaptability to harsh stressful conditions (sensu stricto, that is, distance from pre‐existing homeostasis) associated with the terrestrial habitat (Fürst‐Jansen et al., 2020; Rensing, 2020).
The pivotal role of SMs in the adaptability of land plants depends not only on their extraordinarily high number and diversified skeletons, synthesized by different taxa (Weng et al., 2021), but also on their inherent ability to play multiple functions (Milo & Last, 2012; Ehlers et al., 2020; Mutwil, 2020; Durán‐Medina et al., 2021; Hu et al., 2021; de Vries et al., 2021; Weng et al., 2021). Although SM biosynthesis might have served as a sink for the excess of carbon available to plants during their initial exploration of a highly enriched CO2 atmosphere (Dadras et al., 2023a,b), SMs multifunctionality efficiently compensates for the energetic cost required for their biosynthesis (Kliebenstein, 2013; Erb & Kliebenstein, 2020). The multifunctional nature of SMs and their high responsiveness to abiotic and biotic stressors provide plants with an unlimited defense arsenal, in which each SM may play different roles depending on the severity of the stress events and the degree of plant body complexity. These factors determine the metabolite distribution at the organ, tissue, cellular, and subcellular levels (Schneider et al., 2019; Wang et al., 2019; Shitan & Yazaki, 2020; Weng et al., 2021). In simpler terms, the evolution of multifunctional SM biosynthesis follows the natural tendency to catch as many flies with one clamp as possible (Wink, 1999; Izhaki, 2002).
Here, we focus on the ancient and ubiquitous class of flavonoids (Fig. 1), which are highly responsive to abiotic and biotic environmental stressors and are capable of regulating key steps in plant growth and development (Pollastri & Tattini, 2011; Schneider et al., 2019; Chapman & Muday, 2021; Garagounis et al., 2021; Venegas‐Molina et al., 2021; Daryanavard et al., 2023). However, their multifunctionality makes it difficult to determine the foremost environmental drivers for the emergence and diversification of the flavonoid metabolic network, despite decades of extensive research (Rozema et al., 1997, 2002; Buer et al., 2010; Tripp et al., 2018; Yonekura‐Sakakibara et al., 2019; Davies et al., 2020). We provide a detailed analysis of the complex relationship between the multifunctional nature of flavonoids and the environmental stimuli primarily responsible for the rise of the flavonoid metabolic network, offering conclusive evidence for the structural–functional relationship that is at the root of their functional versatility.
Fig. 1.
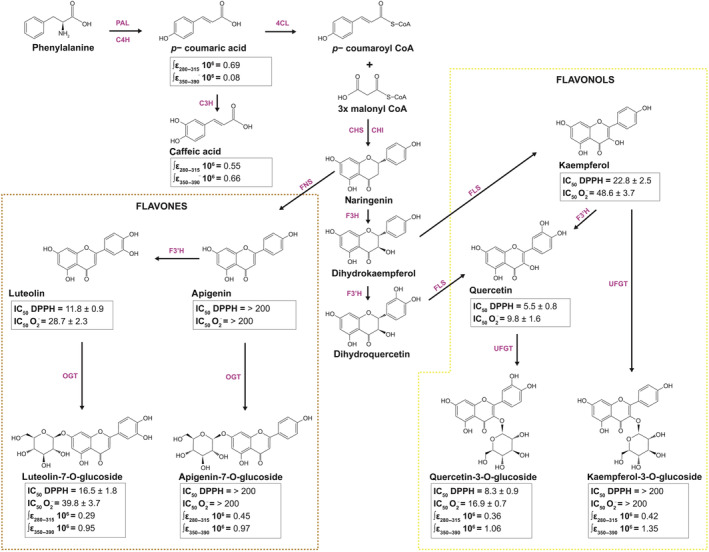
Simplified scheme of the phenylpropanoid pathway leading to the biosynthesis of hydroxycinnamic acid derivatives (HCAs, here reported are p‐coumaric and caffeic acids), mono‐ and dihydroxy B‐ring‐substituted flavones and flavonols (FLAV). The UV‐absorbing capacity of HCAs and FLAV has been measured by integrating individual molar extinction coefficients (ε) over the 280–315 (UV‐B) and 315–390 nm (UV‐A) waveband. The antioxidant capacity of FLAV, both aglycones and glycoside derivatives, has been estimated by calculating the concentration (μM) of individual metabolites capable of reducing by 50% (IC50) that of the synthetic free radical DPPH (2,2‐diphenyl‐1‐picrylhydrazyl) and the superoxide anion (O2 −), following the spectrophotometric protocols of Baratto et al. (2003). Data of IC50 are means ± SD of three replicate measurements. 4CL, 4‐coumaroyl‐CoA ligase; C3H, p‐coumarate 3‐hydroxylase; C4H, cinnamate 4‐hydroxylase; CHI, chalcone isomerase; CHS, chalcone synthase; F3′H, flavonoid 3′‐hydroxylase; F3H, flavanone 3‐hydroxylase; FLS, flavonol synthase; FNS, flavone synthase; OGT; 7‐O‐glucosyl transferase; PAL, phenylalanine ammonia‐lyase; UFGT; UDP glucose‐flavonoid 3‐O‐glucosyl transferase.
Did flavonoid metabolism first emerge in response to biotic pressures?
The emergence of flavonoids represented an outstanding major metabolic innovation during the plants' water‐to‐land transition (de Vries et al., 2017; Davies et al., 2020; Dos Santos Nascimento & Tattini, 2022). This rise has been initially hypothesized to have occurred in response to herbivore pressure (Swain, 1977; Cooper Driver, 1980), the long‐known ‘biochemical coevolutionary arms–race theory’ (Ehrlich & Raven, 1964). In brief, the rise and the diversification of flavonoids, in terms of number and structural complexity, paralleled with major changes in plant morphology, would have been a direct consequence of the selective pressure caused by predation and diseases (Levin, 1971; Swain, 1975, 1977). This coevolution hypothesis has been proven for several classes of SMs, but questioned in other instances, such as the case of flavonoids and other phenolics (Jones & Firn, 1991; Close & McArthur, 2002; Davies et al., 2020; Erb & Kliebenstein, 2020). For instance, Rausher (2001) argued that plant enemies are too rare to generate a frequent evolution of defensive features, such as the biosynthesis of many SMs, particularly flavonoids. Close & McArthur (2002) pointed out the relatively minor role of many phenolics, including flavonoids, as anti‐herbivore agents, while providing evidence for their main functions as photo‐protectants. Although tannins have historically been viewed as defense compounds against herbivore insects, relatively new evidence supports their antioxidant role (Salminen & Karonen, 2011; Constabel et al., 2014; Gourlay & Constabel, 2019). Finally, the vast literature concerning the phenylpropanoid biosynthesis in response to herbivores and their role in plant resistance has not provided proof of the predominant role of flavonoids as deterrents for herbivores (Serrano et al., 2012; Garcia‐Molina & Pastor, 2024). For instance, UV‐B radiation, which is known to trigger flavonoid biosynthesis, has been reported to either increase or decrease the resistance to herbivores in a range of species (Izaguiree et al., 2003; Rousseaux et al., 2004; Schneider et al., 2019). The biosynthesis of flavonoids is strongly suppressed by the bacterial flg22, which indeed stimulates other phenylpropanoid biosynthetic branch pathways (Serrano et al., 2012), in agreement with the observation that sinapic and caffeic acid derivatives offer higher herbivory resistance than flavonoids (for a review, see Ballaré, 2014). There is also convincing evidence that most angiosperms prioritize immune responses over stress‐induced flavonoid accumulation under microbial attack, and this might represent an ancient evolutionary regulatory crosstalk mechanism (Lozoya et al., 1991; Lo & Nicholson, 1998; Logemann & Hahlbrock, 2002; Serrano et al., 2012).
It is conceivable that, despite flavonoids' excellent antibacterial properties, resistance to natural enemies driven by greater production of these compounds may merely be a side consequence of chemicals that evolved to perform other ecological purposes (Rausher, 2001; Erb & Kliebenstein, 2020). This hypothesis is reasonable based on both the multifunctional nature of SMs and the vast range of environmental stresses, other than predators, that plants face on land (Rensing, 2018; Donoghue et al., 2021).
The intriguing relationship between flavonoids and oxidative stress
It is worth noting that once plants moved onto land, they were confronted with a novel set of abiotic environmental stresses, such as the scarcity of water and nutrients, high solar irradiance and changing spectral quality of light, and huge fluctuation in air temperature (Fürst‐Jansen et al., 2020; Markham & Greenham, 2021; Xu et al., 2021; Kim et al., 2022). The evolution of a molecular network conferring water stress resistance is indeed the typical feature of all land plants (Rensing, 2020; Schreiber et al., 2022). This supports the view that the simultaneous action of abiotic stressors, predominantly but not exclusively a combination of water scarcity and high sun irradiation, was the fundamental driver for the rise of SM biosynthesis pathways, including for flavonoids (Rensing, 2018; Brunetti et al., 2019; Dixon & Dickinson, 2024). Flavonoid biosynthesis is greatly activated in response to drought stress and high solar irradiation (Tattini et al., 2004, 2015; Nakabayashi et al., 2015; Siipola et al., 2016; Wang et al., 2020), but it is also triggered by nutrient deficiency, salinity and cold (Lillo et al., 2008; Agati et al., 2011; Albert et al., 2018; Bian et al., 2019; Sachdev et al., 2021). This leads to the hypothesis that changes in reactive oxygen species (ROS)/redox homeostasis, as commonly occur in plants exposed to a wide range of environmental stressors (Devireddy et al., 2021; Peláez‐Vico et al., 2022; Dietz & Vogelsang, 2024), may have regulated the flavonoid biosynthesis in land plants (Babu et al., 2003, 2005; Taylor & Grotewold, 2005; Xu et al., 2015). This hypothesis fits well with the notion that the activities of most TFs that regulate the flavonoid biosynthetic genes, including the R2R3MYBs, are under tight ROS/redox control (Heine et al., 2004; He et al., 2018; Imran et al., 2018; Martin et al., 2022; Pratyusha & Sarada, 2022). There is compelling evidence that stress‐induced increase in the excitation pressure on PSII and the subsequent change in the redox status of the photosynthetic electron transport chain (PETC) serve as retrograde signals (chloroplast‐to‐nucleus signaling) to regulate flavonoid biosynthesis (Gerhardt et al., 2008; Akhtar et al., 2010; Richter et al., 2020, 2023).
While ROS/redox regulation of flavonoid production does not necessarily point to a primary function of these molecules as quenchers/scavengers of stress‐induced ROS accumulation (i.e. as antioxidants sensu stricto), flavonoids are components of the integrated antioxidant network, aimed at keeping the ROS level within a sub‐lethal concentration range, under the most severe stressful conditions (to be described later, for details, Agati et al., 2007, 2012, 2020; Nakabayashi et al., 2015; Tattini et al., 2015; Muhlemann et al., 2018; Chapman & Muday, 2021; Martin et al., 2022). We note that the addition of far‐red light (FR), which is known to induce a more oxidized PETC, inhibits the biosynthesis of flavonoids and greatly decreases the ratio of quercetin (Que) to kaempferol (Kae) derivatives, which is the inverse of what happens when Brassica napus is supplemented with UV‐B radiation (Gerhardt et al., 2008). On the contrary, several studies have observed a marked increase in Que to Kae ratio in several angiosperms, such as pea, soybean, and Arabidopsis thaliana, supplemented with red light (R) (Furuya et al., 1962; Falcone Ferreyra et al., 2021; Lim et al., 2023). An increase in Que to Kae derivatives, or in dihydroxy B‐ring (dihydroxy thereafter) to monohydroxy B‐ring‐substituted (monohydroxy) flavonoids, is commonly observed in plant lineages of different complexity (such as bryophytes and angiosperms) in response to a wide range of abiotic stressors, including to high PAR and UV‐B radiation (for review articles see, Pollastri & Tattini, 2011; Neugart & Schreiner, 2018; Agati et al., 2020; Davies et al., 2020; Dos Santos Nascimento & Tattini, 2022; Singh et al., 2023). While Que and Kae aglycones, the last to a considerably lesser extent, have an effective ability to scavenge free radicals and ROS, this is not the case for Kae derivatives, in which the highly reactive 3‐OH (flavonol) group is usually glycosylated (Rice‐Evans et al., 1996; Fig. 1). Glycosylation makes flavonoids soluble in the aqueous cellular milieu, prevents their auto‐oxidation, facilitates their transport from the endoplasmic reticulum (ER) to different cellular compartments, but depresses to some extent their antioxidant capacity (Fig. 1). The ROS‐scavenging activity of flavonoids mostly depends on the presence of the catechol group in the B‐ring, followed by the presence of both C2‐C3 unsaturation and a 4‐oxo function in the C‐ring, just like in Que (Rice‐Evans et al., 1996; Williams et al., 2004). Consistently, Que 3‐O‐glucoside has a lower ROS‐scavenging ability than Que, but considerably higher antioxidant capacity than Kae, whereas Kae 3‐O‐glucoside displays negligible antioxidant capacity (Fig. 1). While we cannot rule out the possibility that glycosylated flavonoids are de‐glycosylated, releasing the most active aglycone forms (e.g. plants contain a plethora of β‐glucosidase that may perform this function, Roepke & Bozzo, 2015; Le Roy et al., 2016; Baba et al., 2017), there is no consistent body of evidence showing the presence of flavonoid aglycones in plant cells prone to oxidative stress, such as in epidermal and sub‐epidermal tissues (Wollenweber et al., 2011; Ketudat Cairns et al., 2015; Baba et al., 2017; Uehara et al., 2018).
The functional significance of flavonoids as antioxidants in an in planta condition has long been debated (for critical review articles, see Hernández et al., 2009; Agati et al., 2012, 2020), owing to early observations of their almost exclusive location in the vacuoles of epidermal cells (Hrazdina et al., 1982; Caldwell et al., 1983; Hutzler et al., 1998). Instead, flavonoids occur in the vacuoles, the cytoplasm, including the chloroplasts, and the nuclei of parenchymatic cells (Fig. 2; Polster et al., 2006; Agati et al., 2007, 2009, 2012; Böttner et al., 2021) in significantly larger amounts than in the epidermal tissues (Gori et al., 2021; Fig. 3). This makes flavonoids ideal for fine‐tuning the ROS concentration in different subcellular compartments, as widely reported in several species (Ferreres et al., 2011; Muhlemann et al., 2018; Chapman et al., 2019; Agati et al., 2020; Singh et al., 2021; Cerqueira et al., 2023). Agati et al. (2007) provided conclusive evidence that chloroplast‐located dihydroxy flavonoids (Fig. 2) efficiently quenched singlet oxygen generated by a large excess of photosynthetically active radiation. Flavonols distributed in the cytoplasm and the nuclei of stomata guard cells effectively scavenge H2O2 (Watkins et al., 2014, 2017, see the next section for details). Flavonoids' ability to scavenge ROS may be especially advantageous in plants dealing with multiple environmental stresses, such as when solar irradiance causes severe light stress (Fini et al., 2011; Tattini et al., 2015). It is known that plants experience severe photooxidative stress, on a daily and seasonal basis, when light irradiance vastly exceeds that usable for photosynthesis, as occurs during the central hours of the day. Light excess is often accompanied by high temperature and vapor pressure deficit, consequently driving stomata closure. The resulting midday depression of photosynthesis, which results in huge ROS production, is further enhanced due to excess light‐ and heat‐induced reduction in the activity of photosynthetic enzymes (Bagley et al., 2015; Moore et al., 2021). There is evidence that the activity of antioxidant enzymes may fall significantly during the central hours of the day, mostly due to the negative effect of high air temperature (Peltzer & Polle, 2001; Lu et al., 2008; Tattini et al., 2015; Soengas et al., 2018), further enhancing photooxidative stress. The large diurnal variations in flavonoid content recently reported in a range of species, with higher concentrations detected in the midday hours (Barnes et al., 2008, 2016; Gori et al., 2021), equip plants with not only an effective shield against the penetration of higher levels of UV‐B but also with a more efficient ROS‐scavenging system. We have recently provided evidence that the morning‐to‐midday increase in flavonoid content observed at the whole‐leaf level, almost exclusively involves sub‐epidermal tissues and dihydroxy flavonoids (Gori et al., 2021). This is consistent with the common observation that flavonoids with modest ROS‐scavenger capacities respond poorly to light stress and to a variety of other abiotic stimuli (Agati et al., 2012; Fig. 3).
Fig. 2.
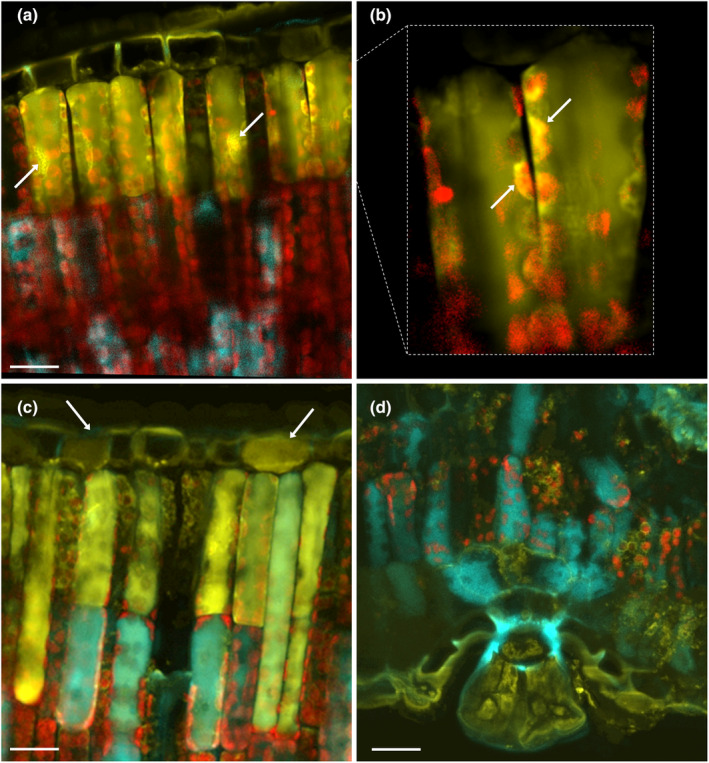
Inter‐ and intra‐cellular distribution of flavonoids (FLAV) and hydroxycinnamic acid derivatives (HCAs) in 6‐month‐old Phillyrea latifolia leaves newly developed in full sunlight. Cross sections were stained with Naturstoff reagent (NR, phosphate‐buffered (pH 6.8) saline (1%, w/v, NaCl) solution of 0.1% (w/v) 2‐amino ethyl diphenyl boric acid) and merged fluorescence images (a–d) result from confocal laser scanning microscopy (CLSM) analysis under the following, sequential, excitation (exc)/emission (em) setups. λexc = 365/λem = 415–485 nm for HCA‐derived blue fluorescence; λexc = 488/λem = 565–535 nm for FLAV‐derived yellow fluorescence; λexc = 638/λem = 690–785 nm for chlorophyll‐derived red fluorescence. FLAV accumulate in the vacuoles and the nuclei of adaxial parenchyma (arrows in a), in the outer envelope membranes of the chloroplasts (arrows in b), and in the vacuoles of adaxial epidermal cells (arrows in c). HCAs occur in abaxial mesophyll cells, which have a palisade‐like morpho‐anatomy (as typically occurs in sun‐adapted leaves), together with yellow fluorescent FLAV (d). The multicellular glandular trichome exclusively accumulates FLAV in the vacuole and likely in the cytoplasm, whereas HCAs are merely distributed in the wall of the trichome stalk cell (d). Bars, 20 μm.
Fig. 3.
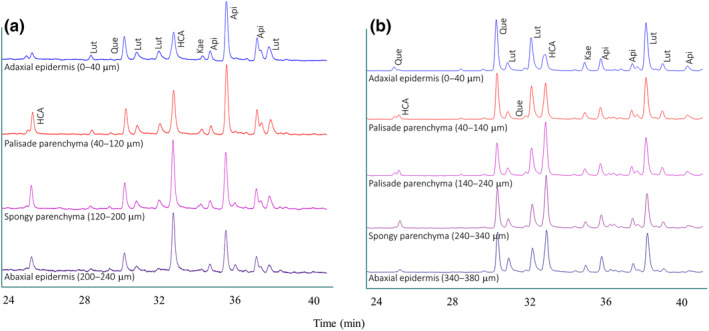
Representative high performance liquid chromatography (HPLC)‐DAD chromatograms of different tissue layers of 3‐month‐old Phyllirea latifolia leaves newly developed in partial shading (25% full sunlight, a) or in full sunlight (b), showing large light‐induced changes in phenylpropanoid composition. While hydroxycinnamic acid (HCA) and apigenin (Api) derivatives mostly contribute to the phenylpropanoid pool in shaded leaves, quercetin (Que) and luteolin (Lut) derivatives largely represent the phenylpropanoids synthesized by full‐sun exposed leaves. Of note, HCAs accumulate poorly in the mostly light‐exposed adaxial epidermis in leaves exposed to the greatest UV‐B irradiance (b), despite their greatest ability to absorb solar UV‐B wavelengths. Longitudinal sections were cut with a cryo‐microtome following the protocols of Ålenius et al. (1995) and Tattini et al. (2015) from leaves sampled at midday. The quali‐ and quantitative analysis of phenylpropanoids were performed using the protocol of Tattini et al. (2015) and chromatograms (recorded at 350 nm) were normalized based on the fresh weight of different tissue layers.
While flavonoids have been reported to effectively counter oxidative stress of different origins in a wide range of angiosperms (Agati et al., 2020), there is no conclusive evidence for this role in the bryophyte lineages. Stafford (1991) speculated that a fledgling flavonoid metabolism was unlikely to provide flavonoid concentrations suitable for efficient ROS scavenging. However, it is worth noting that flavonoid concentrations in the low μM range are sufficient to effectively counter the oxidative stress, and the extant bryophyte lineage accumulate appreciable concentrations of flavonoids (high nmol to low μmol g−1 DW, Albert et al., 2018; Liu et al., 2022). A recent study has shown that the DELLA TF promotes the exclusive biosynthesis of luteolin 7‐O‐glucuronide in Marchantia polymorpha and enhances its tolerance to oxidative stress induced by methyl viologen (which mostly generates superoxide anion and hydroxyl radical, Hernández‐García et al., 2021). The increase in luteolin 7‐O to apigenin 7‐O‐glucuronide in UV‐B‐treated M. polymorpha also poses an antioxidant role of flavonoids in UV photoprotection (Markham et al., 1998, see the next section). This supports the hypothesis of the effective antioxidant role of flavonoids during the evolution of land plant lineages challenged by a wide range of environmental injuries.
Flavonoids in UV photoprotection: a primary antioxidant function?
There is vast, relatively old, literature supporting the idea that an increase in UV, particularly UV‐B irradiance, was the primary driver for the rise of flavonoid metabolism when plants moved from freshwater to colonize land, which is consistent with the notion that UV‐B radiation greatly enhances flavonoid biosynthesis (Wellmann, 1976; Robberecht & Caldwell, 1978; Caldwell, 1979). It has been inferred that the accumulation of flavonoids in land plants is to primarily equip these plants with an efficient shield against the penetration of the shortest wavelengths of solar radiation. Nonetheless, a very recent UV‐omics investigation indicates that UV radiation likely plays a secondary role compared with water availability during plant terrestrialization (for a review, see Martínez‐Abaigar & Núñez‐Olivera, 2022). In other words, while the biosynthesis of protective sunscreens is an ancestral molecular adaptation of land plants (Rensing, 2018), this does not necessarily favor a primary UV‐B absorbing function of flavonoids in the photoprotection systems of different land plant lineages, including the bryophytes (Agati & Tattini, 2010). Even though early lineages of land plants did experience an increase in UV‐B irradiance when moving from freshwater, it is worth noting that all flavonoids have a relative minimum absorbance at the UV‐B portion (280–315 nm), while maximally absorbing at the UV‐A region of the solar spectrum (usually in the range 330–365 nm; Fig. 1; Agati et al., 2009, 2013). This leads to the hypothesis that flavonoids are unlikely to fulfill a primary UV‐B screening function in land plants of varying complexity (Cockell & Knowland, 1999). It is a prerequisite for a metabolite to serve a primary screening function in the overlap between its absorbance spectrum and the light spectrum responsible for its biosynthesis. The biosynthesis of acyl flavonoids, which absorb effectively over the entire range of solar UV wavelengths (Fischbach et al., 1999) is a derived trait of land plants, and it is limited to a few species (Tohge et al., 2016; Alseekh et al., 2020; Wen et al., 2020). For instance, we have reported unusual mono‐ and di‐coumaroyl derivatives of Kae 3‐O‐glucoside, with outstanding capacity to absorb effectively over the entire solar UV spectrum, in the cell walls of stellate trichomes in leaves of Cistus salvifolius (a shrub inhabiting most unfavorable areas of Mediterranean basin, Saracini et al., 2005; Tattini et al., 2007).
Furthermore, we observe that hydroxycinnamic acid derivatives (HCA), whose concentrations are comparable to those of flavonoids under low UV‐B radiation, are almost unresponsive to increasing UV‐B fluence (Burchard et al., 2000; Tattini et al., 2000, 2004; Fig. 3). This finding, which conforms to the general observation of UV‐B‐induced increase in flavonoids to HCAs ratio (Agati & Tattini, 2010; Fig. 3), offers conclusive support to the idea of a relatively minor role of flavonoids as UV‐B absorbers in UV‐B photoprotection. HCAs display the greatest absorption capacity over the UV‐B portion of the solar spectrum among the phenylpropanoid pool synthesized by most taxa (Agati et al., 2013; Neugart et al., 2014; Fig. 1). We note that HCAs distributed on the cuticle matrix, in both the walls and the vacuole of epidermal cells, may effectively limit the entry of UV‐B photons in the leaf, when present in constitutively (i.e. in tissue exposed to low fluence of UV‐B irradiance) high concentrations (Schnitzler et al., 1996; Clarke & Robinson, 2008; González Moreno et al., 2022). The presence of cuticular HCAs may represent an ancestral mechanism for efficient energy dissipation (Renault et al., 2017), based on the observation that the level of cuticular HCA (mainly p‐coumaric and ferulic acids) of most bryophytes largely exceed that of the majority of angiosperms (González Moreno et al., 2022). The functional significance of HCAs, especially those associated with the cuticle and the epidermal cell walls, in UV‐B photoprotection, has been often underestimated (Mazza et al., 2000; Kolb et al., 2001; Fabón et al., 2010; Monforte et al., 2018). However, the matter is of interest, especially when examining the photoprotection mechanisms of land plants at a low degree of body complexity (Renault et al., 2017).
Recent evidence of highly conserved mechanisms for sensing and signaling UV‐B radiation in the liverwort M. polymorpha, the moss Physcomitrella patens, and the flowering plant A. thaliana is of interest and conforms to the notion that the UVR8‐signaling pathway has already originated during the movement of plants from the deeper sea to shallow water (Han et al., 2019). All the species use the UVR8 photoreceptor and the b‐ZIP TF HY5, a master regulator of light signaling and photomorphogenesis, to acclimate to changes in UV‐B wavelengths (Albert et al., 2018; Soriano et al., 2018; Podolec et al., 2021). Moreover, UV‐B radiation similarly changes the flavonoid pool in both bryophytes and angiosperms, since only the biosynthesis of dihydroxy flavones and flavonols is stimulated by UV‐B radiation (Markham et al., 1998; Agati & Tattini, 2010; Wolf et al., 2010; Agati et al., 2012; Fig. 3). It has been therefore inferred that flavonoids are more involved in countering the photooxidative stress generated by UV‐B radiation, through their ROS‐scavenging capacity, than in avoiding photooxidative stress by acting as sunscreens (Ryan et al., 2001; Agati et al., 2012; Emiliani et al., 2013; Dadras et al., 2023b). As a corollary, this offers additional support to early views that high UV‐B irradiance is sensed as an oxidative stress (Landry et al., 1995; Jenkins, 2009), just as occurs when plants experience a wide array of abiotic and biotic stressors. Consistently, the very same effective antioxidant flavonoids accumulate to a similar extent in response to high visible or UV‐B radiation in a range of species (Agati et al., 2009, 2011; Siipola et al., 2016; Albert et al., 2018; Taulavuori et al., 2018; Zhang et al., 2018; Falcone Ferreyra et al., 2021). The antioxidant role of flavonoids due to high light intensity may well explain why surface organs such as glandular trichomes, which are autonomous in phenylpropanoid biosynthesis, preferentially accumulate dihydroxy flavonoids at the expense of HCAs in sun‐adapted Phillyrea latifolia leaves (Tattini et al., 2000; Agati et al., 2002; Fig. 2). It is additionally consistent with the primary ROS‐scavenging functions recently attributed to Que 3‐O‐rutinoside in glandular trichomes of tomato (Sugimoto et al., 2022).
We suggest that following the diversification and efficiency of flavonoid metabolism, which led to the sequential production of flavones, flavonols, and anthocyanins (Li et al., 2020) coupled with a versatile transport system, plants had a vast arsenal of metabolites available, capable of limiting the generation (avoidance through light‐screening) and allowing the scavenging of ROS once they are formed. This enabled plants to reverse efficiently photooxidative stress of increasing severity, allowing their successful adaptation in more challenging habitats (Pollastri & Tattini, 2011; Dos Santos Nascimento & Tattini, 2022).
Flavonoids as signaling molecules: a robust ‘antioxidant‐dependent’ function
The notion that flavonoids act as signaling metabolites has been widely reported in animal cells, and this ability is primarily responsible for the health benefits usually attributed to flavonoids (Williams et al., 2004). The capacity of flavonoids to modulate the activity of a range of proteins that may act as downstream components in diverse signaling pathways (mostly of oxidative nature) has been explored to a lesser extent in plants, especially in aboveground organs (Taylor & Grotewold, 2005; Peer & Murphy, 2006; Brunetti et al., 2018, 2019; Daryanavard et al., 2023). Nonetheless, Helen Stafford proposed, three decades ago, that flavonoids had key functions as internal physiological regulators and chemical messengers, rather than acting as UV‐screening pigments during the colonization of land by plants (Stafford, 1991). She speculated indeed that: (1) a still‐evolving flavonoid metabolism combined with an undeveloped transport system is unlikely to furnish the vacuolar compartment with flavonoid concentrations sufficient to allow optimal UV‐B screening in early land plants; and (2) a primary UV‐screening role does not fit with the extraordinary degree of glycosylation of the flavonoid backbone observed in most plant species. On the contrary, low amounts of flavonoids, which Stafford hypothesized as having been likely synthesized by the first land plants, should have been sufficient to modulate auxin signaling, by acting on both its transport and degradation. Flavonoids had been identified as endogenous regulators of phytochrome‐induced asymmetrical auxin (IAA) distribution, through their ability to modulate the activity of IAA oxidase, in early, seminal experiments conducted at Galston's Lab at Yale University and by Stafford at Reed College in Portland (Furuya et al., 1962; Furuya & Thomas, 1964; Bottomley et al., 1965; Stafford, 1965). Notably, both low red light and white light supplementation promoted asymmetrical IAA distribution, without affecting Kae glycosides biosynthesis, while strongly inducing Que derivatives biosynthesis in Pisum sativum (Bottomley et al., 1965). In the same species, Kae derivatives were observed to act as cofactors of IAA oxidase, while Que derivatives successfully hindered the enzyme activity (Furuya et al., 1962; Galston, 1969). At the time of Stafford's hypothesis, there was additional evidence of antioxidant flavonoids being also most effective in modulating IAA efflux, based on their ability to inhibit the binding of the synthetic auxin transport inhibitor N‐1‐naphthylphthalamic acid (NPA) to a plasma membrane protein (Jacobs & Rubery, 1988). As flavonoids modulate IAA movement and local auxin concentrations at extremely low concentration ranges (from nM to low μM), Stafford speculated this was the ancestral role of flavonoids during plant terrestrialization (Stafford, 1991). Furthermore, she argued that flavonoids might serve these functions in the cytoplasm, near the site of their biosynthesis, that is, the cytoplasmic face of the ER. This argumentation received support later when ancestral IAA auxin efflux PIN proteins, such as the short‐chain PIN5 and PIN8, were discovered to be localized at the ER (Mravec et al., 2009; Viaene et al., 2014; Ung et al., 2022). Incidentally, ER is also the site of IAA biosynthesis (Kriechbaumer et al., 2017; Brunetti et al., 2018).
There is evidence of plasma membrane‐associated PIN trafficking and polarization mechanisms in M. polymorpha and P. patens (Skokan et al., 2019; Tang et al., 2024), and auxin has been reported to influence cell growth and differentiation in both bryophytes (Flores‐Sandoval et al., 2024). These findings support Stafford's opinion of an ancestral role of flavonoids as modulators of intra‐ and intercellular IAA movement. We have also hypothesized that flavonoids served a major function as chemical messengers during plant terrestrialization (Brunetti et al., 2018), but this matter is far from being fully elucidated, as we discuss below.
The role of flavonoids as chemical messengers has been widely reported for the growth of belowground organs in angiosperms (Hassan & Mathesius, 2012; Ng et al., 2020; Ghitti et al., 2022), such as in the arbuscular mycorrhizal (AM) association. The effects of flavonoids on AM result from their ability to modulate both local IAA gradients and the level of downstream components of the auxin signaling pathway, as occurs during nodulation (Zhang et al., 2009; Abdel‐Lateif et al., 2013). The finding that flavonoid aglycones, which are usually exuded by roots, are more effective in promoting AM compared with corresponding glycosylated forms (Zhang et al., 2009; Tian et al., 2021; Kumar et al., 2024), adds further support to the idea that the multifunctionality of flavonoids relates with their antioxidant character. AM association was an event of crucial significance for the adaptability of rootless bryophytes in water‐ and nutrient‐depleted terrestrial habitats (for recent reviews, see Dos Santos Nascimento & Tattini, 2022; Gille et al., 2024; Martin & van der Heijden, 2024). Although the putative role of flavonoids in AM association in bryophytes is an attractive suggestion, the strong relationship between flavonoids and auxin observed in angiosperms needs conclusive support in bryophytes. Nonetheless, flavonoids have been recently reported to block auxin transport and inhibit auxin response, thus contributing to 2D‐3D transition in P. patens (Moody et al., 2021). There is also evidence that SHORT‐LEAF, a member of the Tandem direct repeat‐containing (TDR) proteins regulates gametophore development in P. patens by mediating the auxin distribution pattern through its strong influence on flavonoid biosynthesis (Palit et al., 2024). These findings are remarkable and open the possibility of a putative role of flavonoids as modulators of auxin response and signaling in bryophytes.
The physicochemical features, especially the presence of the catechol group in the B‐ring, confer flavonoids (and other polyphenols) the potential to scavenge ROS and interact with a range of macromolecules as well (Pollastri & Tattini, 2011). For instance, flavonoids may inhibit the activities of a wide array of proteins, including protein kinases by strongly competing with their ATP‐binding sites (structural similarity), as well as acting at the ATP noncompetitive binding site through the formation of both hydrogen bonds and van der Waals interactions (Barron et al., 2002; Bode & Dong, 2013). There is compelling evidence that the 3′‐OH group as seen in dihydroxy flavones and flavonols is pivotal for hydrogen bonds with protein kinase backbone amide groups (for a review, see Hou & Kumamoto, 2010). This conforms to the observation that Que and luteolin are more active than Kae and apigenin, respectively, in inhibiting the activities of a range of tyrosine kinases (Chin et al., 2013; Alizadeh & Ebrahimzadeh, 2022). There is consensus that these features are significantly more important than the conventional hydrogen‐donating capacity (antioxidant role sensu stricto) to explain the effects of flavonoids in the modulation of human cell growth and metabolism (Hou & Kumamoto, 2010; Gu et al., 2019). Flavonoids can regulate and modulate the activities of a wide range of proteins in plant cells, including but not limited to protein kinases. For instance, flavonoids inhibit the activity of PIDs, which are serine/threonine kinases that phosphorylate the PIN, IAA efflux carriers (Henrichs et al., 2012; Adamowski & Friml, 2015), thus determining their asymmetrical distribution at the plasma membrane, and hence the intercellular IAA fluxes, the well‐known polar IAA transport (PAT). However, flavonoids may also modulate the activities of several ATP‐binding cassette B subfamily (ABCB)‐type IAA transporters (multidrug resistance (MDR) P‐glycoproteins, Blakeslee et al., 2005) through bifunctional interactions at both the vicinal ATP‐binding site and the steroid‐interacting region within the protein cytosolic domain (Conseil et al., 1998; Ferreira et al., 2015). In turn, flavonoids could synergistically inhibit both PIN‐ and ABCB‐based major IAA streams (Mellor et al., 2022), through direct association with PINs (Teale et al., 2020; Kurepa et al., 2023). Indeed, the synthetic inhibitor of IAA transport NPA was shown to lead to conformational perturbation in PIN and hence to decreases in PIN activity (Abas et al., 2021). It is not surprising that the antioxidant dihydroxy flavonoids, particularly the flavonol Que, display the greatest inhibitory effect on the activities of PIN and MDR P‐glycoproteins proteins (Mohana et al., 2016), and hence in determining IAA gradients in different tissues and cells (Peer & Murphy, 2006, 2007; Michniewicz et al., 2007; Bailly et al., 2008; Adamowski & Friml, 2015). This may well explain the term ‘developmental regulators’, coined for flavonols by Taylor & Grotewold (2005), a robust function of these molecules in both plants and animals.
We observe that flavonoids may influence IAA gradients in shoots and roots not only by modifying hormone transport at the organ, tissue, cellular, and subcellular levels, but also by influencing IAA catabolism. Early research established that some flavonoids block IAA oxidase (Furuya et al., 1962; Bottomley et al., 1966), a peroxidase for which flavonoids display strong affinity, as is also the case for vacuolar peroxidases that use flavonoids as preferential substrates to detoxify hydrogen peroxide (H2O2, Yamasaki et al., 1997). This has strong similarities with the mechanisms through which flavonoids inhibit IAA oxidase activity, that is, by serving as preferential substrates compared to IAA for IAA oxidase, and by scavenging H2O2 generated during early steps of auxin oxidation (Galston et al., 1950; Mathesius, 2001). It is not surprising, therefore, that Que and its derivatives are much more potent inhibitors of IAA oxidase than the corresponding Kae‐derived compounds, these last behaving indeed as cofactors of IAA oxidase at certain concentrations (Furuya et al., 1962; Bottomley et al., 1966). The largely different action of Que and Kae derivatives on IAA oxidase activity may be in part explained by the capacity of Que, but not of Kae derivatives, to chelate Mn (II), a well‐known cofactor of IAA oxidase (Morgan et al., 1966). The ability of dihydroxy flavonoids to chelate transition metal ions (De Souza & De Giovani, 2004) has also been used to explain their ability to prevent irreversible oxidative damage in plant nuclei. Dihydroxy flavonoids may efficiently chelate Fe(II)‐ions involved in the Fenton reaction (Fe(II) + H2O2 → Fe(III) + OH*), thus limiting the formation of hydroxyl radical (OH*) (Agati et al., 2012). Recent findings suggest that the major route through which IAA is oxidized in early and modern land plants is by the action of DIOXYGENASE for AUXIN OXIDATION1 protein (DAO1, Zhang et al., 2016), a member of the 2‐oxoglutarate and Fe(II)‐dependent (2OG Fe(II)) oxygenase superfamily. Interestingly, an Arabidopsis mutant overaccumulating the antioxidant flavonol Que displayed the lowest level of ox‐IAA (Peer et al., 2013), likely due to the effective inhibition of DAO activity and scavenging of ROS (Zhang & Peer, 2017). The strong inhibitory effect of antioxidant flavonoids on the activity of proteins regulating IAA‐oxidation is suggested as being of greater significance than their modulation of inter‐ and intra‐cellular auxin movement in determining auxin gradients at cellular and subcellular levels and, hence, in regulating plant growth (Zhang & Peer, 2017).
Overall, this evidence implies that flavonoids play a critical role in modulating the auxin‐signaling network beyond influencing the distribution of IAA at both inter‐ and intra‐cellular levels. Furthermore, relatively recent findings support the notion that flavonoids act as components of a regulatory circuit of the auxin‐signaling pathway. Grunewald et al. (2012) have shown that IAA enhances the synthesis of Que derivatives, by acting on the WRKY23 TF and, in turn, Que may fine‐tune IAA distribution, in a PIN‐independent manner. The auxin–flavonol relationship is strong (Blilou et al., 2005; Lewis et al., 2011) and very recent findings provide conclusive evidence that the IAA repressor IAA17.1, a repressor of early IAA response genes, together with the heat shock protein HSFA5a, promote flavonol biosynthesis and decrease ROS accumulation in salt‐treated roots of Populus tomentosa (Song et al., 2024).
There is also recent evidence of a robust relationship between flavonols and the abscisic acid (ABA)‐signaling pathway (Gao et al., 2021; Segarra‐Medina et al., 2023), which may have contributed greatly to the adaptation of plants to the harsh terrestrial habitat (Brunetti et al., 2019). The high integration of ABA‐ and light signaling, which occurs at the level of primary signaling components, such as the bZIP TFs ABA Insensitive 5 (ABI5) and HY5 (Chen et al., 2008), may well explain the ABA‐induced activation of flavonol biosynthesis, especially of quercetin, in a vast range of species (Berli et al., 2010; Alonso et al., 2016; Song et al., 2022; Castro‐Cegrí et al., 2023). It is noted that the crosstalk between ABA and light signaling is an ancient and robust trait of terrestrial plants as the structure and function of HY5 and ABI5 are conserved among early and current‐day land plants (Komatsu et al., 2013; Gangappa & Botto, 2016). Flavonols, in turn, regulate the ABA signaling, acting at the level of downstream network components, such as H2O2 and MAPKs (Brunetti et al., 2019). Studies conducted at Gloria Muday's Lab have conclusively shown that flavonols, accumulated (and likely synthesized) in the cytoplasm and nucleus of stomata guard cells, antagonize the closure of stomata by greatly decreasing the levels of H2O2, a well‐known downstream messenger of the ABA signaling network (Watkins et al., 2014, 2017). However, it cannot be excluded that flavonols additionally inhibit the activity of MAPKs that operate downstream of H2O2 to induce stomata closure (Jammes et al., 2009; De Zelicourt et al., 2016; Brunetti et al., 2019).
Conclusions: not all flavonoids are equally multifunctional
The functional significance of the diversity and complexity of specialized metabolism has been focused mostly on plant–herbivore interactions and based upon the notions that: (1) most SMs synthesized within specific pathways have low biological activity; and (2) the deployment of a mixture of SMs provides functional synergisms and evolutionary stability (Firn & Jones, 2000; Steppuhun & Baldwin, 2008; Heiling et al., 2022; Blanchard & Holeski, 2024).
Consequently, the extraordinary chemical diversity within the flavonoid class, caused by the vast range of glycosylation and substitution patterns of the C6‐C3‐C6 skeleton, complicates a deterministic estimation of their multifunctionality. As previously stated, flavonoids differ significantly in antioxidant capacity, especially when considering the forms found in plant cells. Monohydroxy flavonoid derivatives, for example glycosides of apigenin and Kae, are poor antioxidants (Fig. 1), and their putative effects in an in planta condition have been erroneously inferred from studies conducted in vitro or ex‐vivo using flavonoid aglycones in too many instances (Williamson, 2002). While studies involving flavonoid aglycones may reveal the functions of distinct flavonoid classes in belowground processes (e.g. lateral root emergence; symbiotic nodulation and/or mycorrhizal association Zhang et al., 2009; Chapman & Muday, 2021), this is not the case for aboveground organs, which often accumulate flavonoid glycosides in their tissues. Once again, we emphasize that Que 3‐O‐glucoside has a lower antioxidant capacity than Que, but has a higher ROS‐scavenging ability than Kae. The antioxidant capacity of Kae 3‐O‐glucoside is indeed negligible in a concentration range consistent with its solubility in the aqueous cellular milieu (Fig. 1).
Accordingly, monohydroxy flavones and flavonols have significantly lesser multifunctional potential than their dihydroxy counterparts. It may not be a mere coincidence that in plants exposed to a variety of environmental stresses, including the increase in UV‐B or visible light irradiance, the biosynthesis of antioxidant flavonoids is activated, while the monohydroxy flavonoid pool remains unchanged (for extensive reviews see Agati & Tattini, 2010; Agati et al., 2012, 2020; Fig. 3). Data here reported support flavonoids' key activities in both preventing irreversible stress‐induced oxidative damage and modulating different oxidative stress‐induced signaling pathways. Flavonoids tune both ROS levels and the activity of downstream components of oxidative signaling pathways, such as a wide range of protein kinases, in plants and animals. The antioxidant function of flavonoids is, therefore, robust and strongly tied to the plant's ability to evolve (i.e. evolvability, sensu Lesne, 2008; Wagner, 2011) in an ever‐changing terrestrial habitat.
In fact, antioxidant flavonoids play a role in stress‐induced morphogenic responses (SIMR), a typical feature of plants exposed to a wide range of stresses (Jansen, 2002; Potters et al., 2007), which are, indeed, strongly dependent on ROS (and IAA) signals (Gayomba & Muday, 2020; Martin et al., 2022). Flavonoids regulate the auxin‐signaling pathway by severely reducing the activity of proteins that regulate IAA‐oxidation while determining IAA gradients by acting on proteins that escort IAA at intra‐ and intercellular levels. Consistently, flavonoids have been recognized as modulating plant development (reviewed recently in Daryanavard et al., 2023), particularly root growth and architecture (Mathesius, 2018; Gayomba & Muday, 2020). Studies examining the involvement of flavonoids in the development of aboveground organs, such as shoot architecture, have yielded conflicting results (Beveridge et al., 2007; Buer & Djordjevic, 2009; Buer et al., 2013; Fraser et al., 2017). This is because most research has been conducted under growth conditions different enough from those often experienced by plants concomitantly facing multiple stressors in their natural solar irradiation when SIMR truly makes sense (Robson et al., 2015). For example, high levels of sunlight and UV‐B stimulate or inhibit IAA biosynthesis and signaling, respectively (Hersch et al., 2012; Hayes et al., 2014; Huq, 2018), whereas both light regimes stimulate the biosynthesis of antioxidant flavonoids (Agati et al., 2020). In Arabidopsis, a high light‐induced increase in IAA biosynthesis also triggers the biosynthesis of flavonols, particularly of Que (Lewis et al., 2011; Grunewald et al., 2012). In turn, Que may attenuate local auxin signaling, thus inhibiting apical dominance, as typically occurs in UV‐B‐treated plants under natural conditions (Hayes et al., 2014; Robson et al., 2015). The mutual regulation of auxin biosynthesis/signaling and flavonoids usually observed in angiosperms is still lacking to be properly described in bryophytes, but very recent studies open new perspectives on this intriguing matter (Moody et al., 2021; Palit et al., 2024).
The functional significance of the regulatory roles of flavonols on the ABA signaling network has not yet received enough attention, despite the fact they have the potential to significantly regulate the gas exchange performance of plants facing multiple environmental pressures associated with rapid climate change, such as a combination of transient heat waves and rainfall scarcity in high light‐stressed habitats. However, the matter is of primary significance for the ecology of plants with highly diverse complexity.
Overall, we have shown that while flavonoids with varying physicochemical properties have similar abilities to absorb UV radiation and repel herbivores, they differ greatly in their ability to scavenge ROS and hence to modulate both hormone and oxidative signaling pathways. We have provided conclusive evidence that these antioxidant‐related properties, coupled with the distribution in different tissues and cellular compartments, confer only to antioxidant flavonoids the ability to efficiently serve several functions in plants undergoing changes in cellular homeostasis because of a variety of external stimuli. The observation that the biosynthesis of antioxidant flavonoids is a common response of different land plants lineages when confronted with a range of environmental pressures is remarkable, implying that this might represent an ancient feature of land plants.
Competing interests
None declared.
Author contributions
LBSN and MT conceived the structure and wrote the MS. CB and AG performed HPLC analysis of phenylpropanoids and estimated the scavenger ability of individual flavonoids for DPPH radical and superoxide anion. GA and ELP determined the UV‐absorbing capacities of individual phenylpropanoids and performed CLSM analyses. All the authors revised and edited the MS.
Acknowledgements
We are indebted to Prof. Marco Landi and Prof. Lucia Guidi (University of Pisa) for helpful comments on the MS. Open access publishing facilitated by Consiglio Nazionale delle Ricerche, as part of the Wiley ‐ CRUI‐CARE agreement.
References
- Abas L, Kolb M, Stadlmann J, Janacek D, Lukic K, Schwechheimer C, Savanov LA, Mach L, Friml J, Hammes UZ. 2021. Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proceedings of the National Academy of Sciences, USA 118: e2020857118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel‐Lateif K, Vaissavre V, Gherbi H, Verries C, Meudec E, Perrine‐Walker F, Cheynier V, Svistoonoff S, Frnache C, Bogusz D et al. 2013. Silencing of the chalcone synthase gene in Casuarina glauca highlights the important role of flavonoids during nodulation. New Phytologist 199: 1012–1021. [DOI] [PubMed] [Google Scholar]
- Adamowski M, Friml J. 2015. PIN‐dependent auxin transport: action, regulation, and evolution. Plant Cell 27: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agati G, Azzarello E, Pollastri S, Tattini M. 2012. Flavonoids as antioxidants in plants: location and functional significance. Plant Science 196: 67–76. [DOI] [PubMed] [Google Scholar]
- Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M. 2011. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. Journal of Plant Physiology 168: 204–212. [DOI] [PubMed] [Google Scholar]
- Agati G, Brunetti C, Di Ferdinando M, Ferrini F, Pollastri S, Tattini M. 2013. Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiology and Biochemistry 72: 35–45. [DOI] [PubMed] [Google Scholar]
- Agati G, Brunetti C, Fini A, Gori A, Guidi L, Landi M, Sebastiani F, Tattini M. 2020. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 9: 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agati G, Galardi C, Gravano E, Romani A, Tattini M. 2002. Flavonoid distribution in tissues of Phillyrea latifolia L. leaves as estimated by microspectrofluorometry and multispectral fluorescence microimaging. Photochemistry and Photobiology 76: 350–360. [DOI] [PubMed] [Google Scholar]
- Agati G, Matteini P, Goti A, Tattini M. 2007. Chloroplast‐located flavonoids can scavenge singlet oxygen. New Phytologist 174: 77–89. [DOI] [PubMed] [Google Scholar]
- Agati G, Stefano G, Biricolti S, Tattini M. 2009. Mesophyll distribution of ‘antioxidant’ flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Annals of Botany 104: 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agati G, Tattini M. 2010. Multiple functional roles of flavonoids in photoprotection. New Phytologist 186: 786–793. [DOI] [PubMed] [Google Scholar]
- Akhtar TA, Lees HA, Lampi MA, Enstone D, Brain RA, Greenberg BM. 2010. Photosynthetic redox imbalance influences flavonoid biosynthesis in Lemna gibba . Plant, Cell & Environment 33: 1205–1219. [DOI] [PubMed] [Google Scholar]
- Albert NW, Thrimawithana AH, McGhie TK, Clayton WA, Deroles SC, Schwinn KE, Bowman JL, Jordan BR, Davies KM. 2018. Genetic analysis of the liverwort Marchantia polymorpha reveals that R2R3MYB activation of flavonoid production in response to abiotic stress is an ancient character in land plants. New Phytologist 218: 554–566. [DOI] [PubMed] [Google Scholar]
- Ålenius CM, Vogelmann TC, Bornman JF. 1995. A three‐dimensional representation of the relationship between penetration of u.v.‐B radiation and u.v.‐screening pigments in leaves of Brassica napus . New Phytologist 131: 287–302. [Google Scholar]
- Alizadeh SR, Ebrahimzadeh MA. 2022. Quercetin derivatives: drug design, development, and biological activities, a review. European Journal of Medicinal Chemistry 229: 114068. [DOI] [PubMed] [Google Scholar]
- Alonso R, Berli FJ, Fontana A, Piccoli P, Bottini R. 2016. Malbec grape (Vitis vinifera L.) responses to the environment: berry phenolics as influenced by solar UV‐B, water deficit and sprayed abscisic acid. Plant Physiology and Biochemistry 109: 84–90. [DOI] [PubMed] [Google Scholar]
- Alseekh S, Perez de Souza L, Benina M, Fernie AR. 2020. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 174: 112347. [DOI] [PubMed] [Google Scholar]
- Baba SA, Wishwakarma RA, Ashra N. 2017. Functional characterization of CsBGlu12, a β‐glucosidase from Crocus sativus, provides insights into its role in abiotic stress through accumulation of antioxidant flavonols. Journal of Biological Chemistry 292: 4700–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu TS, Akhtar T, Lampi MA, Tripuranthakam S, Dixon DG, Greenberg BM. 2003. Similar stress responses are elicited by copper and ultraviolet radiation in the aquatic plant Lemna gibba: implication of reactive oxygen species as common signals. Plant & Cell Physiology 44: 1320–1329. [DOI] [PubMed] [Google Scholar]
- Babu TS, Tripuranhakam S, Greenberg BM. 2005. Biochemical responses of the aquatic higher plant Lemna gibba to a mixture of copper and 1,2‐dihydroxyanthraquinone: synergistic toxicity via reactive oxygen species. Environmental Toxicology & Chemistry 24: 3030–3036. [DOI] [PubMed] [Google Scholar]
- Bagley J, Rosenthal DM, Ruiz‐Vera UM, Siebers MH, Kumar P, Ort DR, Bernacchi CJ. 2015. The influence of photosynthetic acclimation to rising CO2 and warmer temperatures on leaf and canopy photosynthesis models. Global Biogeochemical Cycles 29: 194–206. [Google Scholar]
- Bailly A, Sovero V, Vincenzetti V, Santelia D, Bartnik D, Koenig BW, Mancuso S, Martinoia E, Geisler M. 2008. Modulation of P‐glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. Journal of Biological Chemistry 283: 21817–21826. [DOI] [PubMed] [Google Scholar]
- Ballaré CL. 2014. Light regulation of plant defense. Annual Review of Plant Biology 65: 335–363. [DOI] [PubMed] [Google Scholar]
- Baratto MC, Tattini M, Galardi C, Pinelli P, Romani A, Visiol F, Basosi R, Pogni R. 2003. Antioxidant activity of galloyl quinic derivatives isolated from P. lentiscus leaves. Free Radical Research 37: 405–412. [DOI] [PubMed] [Google Scholar]
- Barnes PW, Tobler MA, Keefover‐Ring K, Flint SD, Barkley AE, Ryels RJ, Lindroth RL. 2016. Rapid modulation of ultraviolet shielding in plants is influenced by solar ultraviolet radiation and linked to alterations in flavonoids. Plant, Cell & Environment 39: 222–230. [DOI] [PubMed] [Google Scholar]
- Barnes W, Flint SD, Slusser JR, Gao W, Ryel RJ. 2008. Diurnal changes in epidermal UV transmittance of plants in naturally high UV environments. Physiologia Plantarum 133: 363–372. [DOI] [PubMed] [Google Scholar]
- Barron D, Di Pietro A, Dumontet C, McIntosh D. 2002. Isoprenoid flavonoids are new leads in the modulation of chemoresistance. Phytochemistry Reviews 1: 325–332. [Google Scholar]
- Berli FJ, Moreno D, Piccoli P, Hespanhol‐Viana L, Silva MF, Bressan‐Smith R, Cavagnaro JB, Bottini R. 2010. Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet‐B radiation by enhancing ultraviolet‐absorbing compounds, antioxidant enzymes and membrane sterols. Plant, Cell & Environment 33: 1–10. [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Mathesius U, Rose RJ, Gresshoff PM. 2007. Common regulatory themes in meristem development and whole‐plant homeostasis. Current Opinion in Plant Biology 10: 44–51. [DOI] [PubMed] [Google Scholar]
- Bian H‐X, Li W, Niu C‐F, Wei W, Hu Y, Han J‐Q, Lu X, Tao J‐J, Jin M, Qin H et al. 2019. A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytologist 225: 268–283. [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Peer WA, Murphy AS. 2005. Auxin transport. Current Opinion in Plant Biology 8: 494–500. [DOI] [PubMed] [Google Scholar]
- Blanchard M, Holeski LM. 2024. Consequences and costs of chemical complexity: the evolutionary ecology of direct phytochemical defense against herbivores. International Journal of Plant Sciences 185: 3–14. [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44. [DOI] [PubMed] [Google Scholar]
- Bode AM, Dong Z. 2013. Signal transduction and molecular targets of selected flavonoids. Antioxidants & Redox Signaling 19: 163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttner L, Grabe V, Gablenz S, Böhme N, Appenroth KJ, Gershenzon J, Huber M. 2021. Differential localization of flavonoid glucosides in an aquatic plant implicates different functions under abiotic stress. Plant, Cell & Environment 44: 900–914. [DOI] [PubMed] [Google Scholar]
- Bottomley W, Smith H, Galston AW. 1965. A phytochrome mediated effect of light on the hydroxylation pattern of flavonoids in Pisum sativum var. ‘Alaska’. Nature 207: 1311–1312.5884659 [Google Scholar]
- Bottomley W, Smith H, Galston AW. 1966. Flavonoid complexes in Pisum sativum – III. The effect of light on the synthesis of kaempferol and quercetin complexes. Phytochemistry 5: 117–123. [Google Scholar]
- Bowles AMC, Bechtold U, Paps J. 2020. The origin of land plants is rooted in two bursts of genomic novelty. Current Biology 30: 530–536. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F et al. 2017. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304. [DOI] [PubMed] [Google Scholar]
- Brunetti C, Fini A, Sebastiani F, Gori A, Tattini M. 2018. Modulation of phytohormone signaling: a primary function of flavonoids in plant–environment interactions. Frontiers in Plant Science 9: 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti C, Sebastiani F, Tattini M. 2019. ABA, flavonols and the evolvability of land plants. Plant Science 280: 448–454. [DOI] [PubMed] [Google Scholar]
- Buer CS, Djordjevic MA. 2009. Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana . Journal of Experimental Botany 60: 751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Imin N, Djordjevic MA. 2010. Flavonoids: new roles for old molecules. Journal of Integrative Plant Biology 52: 98–111. [DOI] [PubMed] [Google Scholar]
- Buer CS, Kordbacheh F, Truong TT, Hocart CH, Djordjevic MA. 2013. Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long‐term effects on root and shoot architecture. Planta 238: 171–189. [DOI] [PubMed] [Google Scholar]
- Burchard P, Bilger W, Weissenböck G. 2000. Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV‐A and UV‐B radiation in developing rye primary leaves as assessed by ultraviolet‐induced chlorophyll fluorescence measurements. Plant, Cell & Environment 23: 1373–1380. [Google Scholar]
- Buschmann H. 2020. Into another dimension: how streptophyte algae gained morphological complexity. Journal of Experimental Botany 71: 3279–3286. [DOI] [PubMed] [Google Scholar]
- Caldwell MM. 1979. Plant life and ultraviolet radiation: some perspective in the history of the Earth's UV climate. Bioscience 29: 520–525. [Google Scholar]
- Caldwell MM, Robberecht R, Flint SD. 1983. Internal filters: prospects for UV‐acclimation in higher plants. Physiologia Plantarum 58: 445–450. [Google Scholar]
- Cannell N, Emms DM, Hetherington AJ, MacKay J, Kelly S, Dolan L, Sweetlove LG. 2020. Multiple metabolic innovations and losses are associated with major transitions in land plant evolution. Current Biology 30: 1783–1800. [DOI] [PubMed] [Google Scholar]
- Castro‐Cegrí A, Sierra S, Hidalgo‐Santiago L, Esteban‐Muñoz A, Jamilena M, Garrido D, Palma F. 2023. Postharvest treatment with abscisic acid alleviates chilling injury in zucchini fruit by regulating phenolic metabolism and non‐enzymatic antioxidant system. Antioxidants 12: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JVA, de Andrade MT, Rafael DD, Zhu F, Martins SVC, Nunes‐Nesi A, Benedito V, Fernie AR, Zsögön A. 2023. Anthocyanins and reactive oxygen species: a team of rivals regulating plant development? Plant Molecular Biology 112: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JM, Muday GK. 2021. Flavonols modulate lateral root emergence by scavenging reactive oxygen species in Arabidopsis thaliana . Journal of Biological Chemistry 296: 100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Muhlemann JK, Gayomba SR, Muday GK. 2019. RBOH‐dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chemical Research in Toxicology 32: 370–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang J, Neff MM, Xiong L. 2008. Integration of light and abscisic acid signaling during seed germination and early seedling development. Proceedings of the National Academy of Sciences, USA 105: 4495–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Xian W, Fu Y, Marin B, Keller J, Wu T, Sun W, Li X, Xu Y, Zhang Y et al. 2019. Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell 179: 1057–1067. [DOI] [PubMed] [Google Scholar]
- Chin Y‐W, Kong JY, Han S‐Y. 2013. Flavonoids as receptor tyrosine kinase FLT3 inhibitors. Bioorganic & Medicinal Chemistry Letters 23: 1768–1770. [DOI] [PubMed] [Google Scholar]
- Clarke LJ, Robinson SA. 2008. Cell wall‐bound ultraviolet‐screening compounds explain the high ultraviolet tolerance of the Antarctic moss, Ceratodon purpureus . New Phytologist 179: 776–783. [DOI] [PubMed] [Google Scholar]
- Close DC, McArthur C. 2002. Rethinking the role of many plant phenolics–protection from photodamage not herbivores? Oikos 99: 166–172. [Google Scholar]
- Cockell CS, Knowland J. 1999. Ultraviolet radiation screening compounds. Biological Reviews 74: 311–345. [DOI] [PubMed] [Google Scholar]
- Conseil G, Baubichon‐Cortay H, Dayan G, Di Pietro AD. 1998. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP‐ and steroid‐binding sites on mouse P‐glycoprotein. Proceedings of the National Academy of Science, USA 95: 9831–9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constabel PC, Yoshida K, Walker V. 2014. Diverse ecological roles of plant tannins: plant defense and beyond. In: Romani A, Lattanzio V, Quideau S, eds. Recent advances in polyphenol research. Hoboken, NJ, USA: John Wiley & Sons, 115–142. [Google Scholar]
- Cooper Driver GA. 1980. The role of flavonoids and related compounds in fern systematics. Bulletin of the Torrey Botanical Club 107: 116–127. [Google Scholar]
- Dadras A, Furst‐Jansen JMR, Darienko T, Krone D, Scholz P, Sun S, Herrfurth C, Rieseberg TP, Irisarri I, Steinkamp R. 2023a. Environmental gradients reveal stress hubs pre‐dating plant terrestrialization. Nature Plants 9: 1419–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadras A, Rieseberg TP, Zegers JMS, Irisarri I, de Vries J, de Vries S. 2023b. Accessible versatility underpins the deep evolution of plant specialized metabolism. Phytochemistry Reviews. doi: 10.1007/s11101-023-09863-2. [DOI] [Google Scholar]
- Daryanavard H, Postiglione AE, Muhlemann JK, Muday GK. 2023. Flavonols modulate plant development, signaling, and stress responses. Current Opinion in Plant Biology 72: 102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KM, Jibran R, Albert NW, Zhou Y, Schwinn KE. 2021. Conservation and divergence between bryophytes and angiosperms in the biosynthesis and regulation of flavonoid production. In: Reed DJ, Perera de Freitas VA, Quideau S, eds. Recent advances in polyphenol research, vol. 7. Hoboken, NJ, USA: John Wiley & Sons, 227–263. [Google Scholar]
- Davies KM, Jibran R, Zhou Y, Albert NW, Brummell DA, Jordan BR, Bowman JL, Schwinn KE. 2020. The evolution of flavonoid biosynthesis: a bryophyte perspective. Frontiers in Plant Science 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza RFV, De Giovani WF. 2004. Antioxidant properties of complexes of flavonoids with metal ions. Redox Report 9: 97–104. [DOI] [PubMed] [Google Scholar]
- De Zelicourt A, Colcombet J, Hirt H. 2016. The role of MAPK modules and ABA during abiotic stress signaling. Trends in Plant Science 21: 677–685. [DOI] [PubMed] [Google Scholar]
- Devireddy AR, Zandalinas SI, Fichman Y, Mittler R. 2021. Integration of reactive oxygen species and hormone signaling during abiotic stress. The Plant Journal 105: 459–476. [DOI] [PubMed] [Google Scholar]
- Dietz K‐J, Vogelsang L. 2024. A general concept of quantitative abiotic stress sensing. Trends in Plant Science 29: 319–328. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Dickinson AJ. 2024. A century of studying plant secondary metabolism – from “what?” to “where, how, and why?”. Plant Physiology 195: 48–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue PCJ, Harrison CJ, Paps J, Scheider H. 2021. The evolutionary emergence of land plants. Current Biology 32: R1281–R1298. [DOI] [PubMed] [Google Scholar]
- Dos Santos Nascimento LB, Tattini M. 2022. Beyond photoprotection: the multifarious roles of flavonoids in plant terrestrialization. International Journal of Molecular Sciences 23: 5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán‐Medina Y, Ruiz‐Cortés BE, Guerrero‐Largo H, Marsch‐Martínez N. 2021. Specialized metabolism and development: an unexpected friendship. Current Opinion in Plant Biology 64: 102142. [DOI] [PubMed] [Google Scholar]
- Ehlers BK, Berg MP, Staudt M, Holmstrup M, Glasius M, Ellers J, Tomiolo S, Madsen RB, Slotsbo S, Penuelas J. 2020. Plant secondary compounds in soil and their role in belowground species interactions. Trends in Ecology & Evolution 35: 716–730. [DOI] [PubMed] [Google Scholar]
- Ehrlich PR, Raven PH. 1964. Butterflies and plants: a study in coevolution. Evolution 18: 586–608. [Google Scholar]
- Emiliani J, Grotewold E, Falcone Ferreyra ML, Casati P. 2013. Flavonols protect Arabidopsis plants against UV‐B deleterious effects. Molecular Plant 6: 1376–1379. [DOI] [PubMed] [Google Scholar]
- Erb M, Kliebenstein DJ. 2020. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiology 184: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabón G, Martinez‐Abaigar J, Tomás R, Núñez‐Olivera E. 2010. Effects of enhanced UV‐B radiation on hydroxycinnamic acid derivatives extracted from different cell compartments in the aquatic liverwort Jungermannia exsertifolia subsp. cordifolia . Physiologia Plantarum 140: 269–279. [DOI] [PubMed] [Google Scholar]
- Falcone Ferreyra ML, Serra P, Casati P. 2021. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiologia Plantarum 173: 736–749. [DOI] [PubMed] [Google Scholar]
- Feller A, Machemer K, Braun EL, Grotewold E. 2011. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. The Plant Journal 66: 94–116. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Pousinho S, Fortuna A, Falcao A, Alves G. 2015. Flavonoid compounds as reversal agents of the P‐glycoprotein‐mediated multidrug resistance: biology, chemistry and pharmacology. Phytochemistry Reviews 14: 233–272. [Google Scholar]
- Ferreres F, Figueiredo R, Bettencourt S, Corqueijeiro I, Oliveira J, Gil‐Izquerdio A, Pereira DM, Valentão P, Andrade PB, Duarte P et al. 2011. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: an H2O2 affair? Journal of Experimental Botany 62: 2841–2854. [DOI] [PubMed] [Google Scholar]
- Fini A, Brunetti C, Di Ferdinando M, Ferrini F, Tattini M. 2011. Stress‐induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling & Behavior 6: 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firn RD, Jones CG. 2000. The evolution of secondary metabolism – a unifying model. Molecular Microbiology 37: 989–994. [DOI] [PubMed] [Google Scholar]
- Fischbach RJ, Kossmann B, Panten H, Steinbrecher R, Heller W, Seidlitz HK, Sandermann H, Hertkorn N, Schnitzler J‐P. 1999. Seasonal accumulation of ultraviolet‐B screening pigments in needles of Norway spruce (Picea abies (L.) Karst.). Plant, Cell & Environment 22: 27–37. [Google Scholar]
- Flores‐Sandoval E, Nishihama R, Bowman JL. 2024. Hormonal and genetic control of pluripotency in bryophyte model systems. Current Opinion in Plant Biology 77: 102486. [DOI] [PubMed] [Google Scholar]
- Fraser DP, Sharma A, Fletcher T, Budge S, Moncrieff C, Dodd AN, Franklin K. 2017. UV‐B antagonises shade avoidance and increases levels of the flavonoid quercetin in coriander (Coriandrum sativum). Scientific Reports 7: 17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst‐Jansen JMR, de Vries S, de Vries J. 2020. Evo‐physio: on stress responses and the earliest land plants. Journal of Experimental Botany 71: 3254–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M, Galston A, Stowe B. 1962. Isolation from peas of co‐factors and inhibitors of indolyl‐3‐acetic acid oxidase. Nature 193: 456–457. [DOI] [PubMed] [Google Scholar]
- Furuya M, Thomas RG. 1964. Flavonoid complexes in Pisum sativum. II. Effects of red and far‐red light on biosynthesis of kaempferol complexes and on growth of etiolated plumules. Plant Physiology 39: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galston AW. 1969. Flavonoids and photomorphogenesis in peas. In: Harborne JB, Swain T, eds. Perspectives in phytochemistry. New York, NY, USA: Academic Press, 193–204. [Google Scholar]
- Galston AW, Bonner J, Baker RS. 1950. Flavoprotein and peroxidase as constituents of the indoleacetic acid oxidase of peas. American Journal of Botany 37: 677–678. [Google Scholar]
- Gangappa SN, Botto JF. 2016. The multifaceted roles of HY5 in plant growth and development. Molecular Plant 9: 1353–1365. [DOI] [PubMed] [Google Scholar]
- Gao G, Lv Z, Zhang G, Li J, Zhang J, He C. 2021. An ABA–flavonoid relationship contributes to the differences in drought resistance between different sea buckthorn subspecies. Tree Physiology 41: 744–755. [DOI] [PubMed] [Google Scholar]
- Garagounis C, Delkis N, Papadopoulou KK. 2021. Unraveling the roles of plant specialized metabolites: using synthetic biology to design molecular biosensors. New Phytologist 231: 1338–1352. [DOI] [PubMed] [Google Scholar]
- Garcia‐Molina A, Pastor V. 2024. Systemic analysis of metabolome reconfiguration in Arabidopsis after abiotic stressors uncovers metabolites that modulate defense against pathogens. Plant Communications 5: 100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayomba SR, Muday GK. 2020. Flavonols regulate root hair development by modulating accumulation of reactive oxygen species in the root epidermis. Development 147: dev185819. [DOI] [PubMed] [Google Scholar]
- Gerhardt KE, Lampi MA, Greenberg BM. 2008. The effects of far‐red light on plant growth and flavonoid accumulation in Brassica napus in the presence of ultraviolet B radiation. Photochemistry and Photobiology 84: 1445–1454. [DOI] [PubMed] [Google Scholar]
- Ghitti E, Rolli E, Crotti E, Borin S. 2022. Flavonoids are intra‐ and inter‐kingdom modulator signals. Microorganisms 10: 2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille CE, Finnegan PM, Hayes PE, Ranathunge K, Burgess TI, de Tombeur F, Migliorini D, Dallongeville P, Glauser G, Lambers H. 2024. Facilitative and competitive interactions between mycorrhizal and non‐mycorrhizal plants in an extremely phosphorus‐impoverished environment: role of ectomycorrhizal fungi and native oomycete pathogens in shaping species coexistence. New Phytologist 242: 1630–1640. [DOI] [PubMed] [Google Scholar]
- González Moreno A, de Cózar A, Prieto P, Domínguez E, Heredia A. 2022. Radiationless mechanism of UV deactivation by cuticle phenolics in plants. Nature Communications 13: 1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori A, Brunetti C, dos Santos Nascimento LB, Marino G, Guisi L, Ferrini F, Centritto M, Fini A, Tattini M. 2021. Photoprotective role of photosynthetic and non‐photosynthetic pigments in Phillyrea latifolia: is their “antioxidant” function prominent in leaves exposed to severe summer drought? International Journal of Molecular Sciences 22: 8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay G, Constabel CP. 2019. Condensed tannins are inducible antioxidants and protect hybrid poplar against oxidative stress. Tree Physiology 39: 345–355. [DOI] [PubMed] [Google Scholar]
- Grunewald W, De Smet I, Lewis DR, Löfke C, Jansen L, Geominne G, Vanden Bossche R, Karimi M, De Rybel B, Vanholme B. 2012. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proceedings of the National Academy of Sciences, USA 109: 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Stashko MA, Puhl‐Rubio AC, Chakraborty M, Chakraborty A, Frye SV, Pearce KH, Wang X, Shears SB, Wang H. 2019. Inhibition of inositol polyphosphate kinases by quercetin and related flavonoids: a structure‐activity analysis. Journal of Medicinal Chemistry 62: 1443–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chang X, Zhang Z, Chen H, He H, Zhong B, Deng XW. 2019. Origin and evolution of core components responsible for monitoring light environment changes during plant terrestrialization. Molecular Plant 12: 847–862. [DOI] [PubMed] [Google Scholar]
- Hassan S, Mathesius U. 2012. The role of flavonoids in root–rhizosphere signalling: opportunities and challenges for improving plant–microbe interactions. Journal of Experimental Botany 63: 3429–3444. [DOI] [PubMed] [Google Scholar]
- Hayes S, Velanis CN, Jenkins GI, Franklin KA. 2014. UV‐B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proceedings of the National Academy of Sciences, USA 111: 11894–11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Van Breusegem F, Mhamdi A. 2018. Redox‐dependent control of nuclear transcription in plants. Journal of Experimental Botany 69: 3359–3372. [DOI] [PubMed] [Google Scholar]
- Heiling S, Li J, Halitschke R, Paetz C, Baldwin IT. 2022. The downside of metabolic diversity: postingestive rearrangements by specialized insects. Proceedings of the National Academy of Sciences, USA 119: e2122808119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine GF, Hernandez JM, Grotewold E. 2004. Two cysteines in plant R2R3 MYB domains participate in redox‐dependent DNA binding. Journal of Biological Chemistry 279: 37878–37885. [DOI] [PubMed] [Google Scholar]
- Henrichs S, Wang B, Fukao Y, Zhu J, Charrier L, Bailly A, Oehring SC, Linnert M, Weiward A, Endler A et al. 2012. Regulation of ABCB1/PGP1‐catalysed auxin transport by linker phosphorylation. EMBO Journal 31: 2965–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández I, Alegre A, Van Breusegem F, Munné‐Bosch S. 2009. How relevant are flavonoids as antioxidants in plants? Trends in Plant Science 14: 125–132. [DOI] [PubMed] [Google Scholar]
- Hernández‐García J, Serrano‐Mislata A, Inoue K, Vargas‐Chávez C, Esteva‐Bruna D, Arbona V, Yamaoka S, Nishihama R, Kocchi T et al. 2021. Coordination between growth and stress responses by DELLA in the liverwort Marchantia polymorpha . Current Biology 31: 3678–3686. [DOI] [PubMed] [Google Scholar]
- Hersch M, Lorrain S, de Wit M, Frankhauser C. 2012. Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis . Proceedings of the National Academy of Sciences, USA 111: 6515–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D‐X, Kumamoto T. 2010. Flavonoids as protein kinase inhibitors for cancer chemoprevention: direct binding and molecular modeling. Antioxidants & Redox Signaling 13: 691–719. [DOI] [PubMed] [Google Scholar]
- Hrazdina G, Marx GA, Hoch HC. 1982. Distribution of secondary plant metabolites and their biosynthetic enzymes in pea (Pisum sativum L.) leaves: anthocyanins and flavonol glycosides. Plant Physiology 70: 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Wu Z, Robert C, Erb M. 2021. Soil chemistry determines whether defensive plant secondary metabolites promote or suppress herbivore growth. Proceedings of the National Academy of Sciences, USA 118: e2109602118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E. 2018. Direct convergence of light and auxin signaling pathways in Arabidopsis. Molecular Plant 11: 515–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler P, Fishback R, Heller W, Jungblut TP, Reuber S, Schmitz R, Veit M, Weissenböck G, Schnitzler J‐P. 1998. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. Journal of Experimental Botany 49: 953–965. [Google Scholar]
- Imran QM, Hussain A, Lee S‐U, Mun B‐G, Falak N, Loake GJ, Yun B‐W. 2018. Transcriptome profile of NO‐induced Arabidopsis transcription factor genes suggests their putative regulatory role in multiple biological processes. Scientific Reports 8: 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguiree MM, Scopel AL, Baldwin IT, Ballaré CL. 2003. Convergent responses to stress. Solar ultraviolet‐B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field‐grown plants of Nicotiana longiflora . Plant Physiology 132: 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhaki K. 2002. Emodin – a secondary metabolite with multiple ecological functions in higher plants. New Phytologist 155: 205–217. [Google Scholar]
- Jacobs M, Rubery PH. 1988. Naturally occurring auxin transport regulators. Science 241: 346–349. [DOI] [PubMed] [Google Scholar]
- Jammes F, Song C, Shin D, Kwak JM. 2009. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS‐mediated ABA signaling. Proceedings of the National Academy of Sciences, USA 106: 20520–20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK. 2002. Ultraviolet‐B radiation effects on plants: induction of morphogenic responses. Physiologia Plantarum 116: 423–429. [Google Scholar]
- Jenkins GI. 2009. Signal transduction in responses to UV‐B radiation. Annual Review of Plant Biology 60: 407–431. [DOI] [PubMed] [Google Scholar]
- Jiang C‐K, Rao G‐Y. 2020. Insights into the diversification and evolution of R2R3‐MYB transcription factors in plants. Plant Physiology 183: 637–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG, Firn RD. 1991. On the evolution of plant secondary diversity. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 333: 273–280. [Google Scholar]
- Keeling CI, Dullat HK, Yuen M, Ralph SG, Jancsik S, Bohlmann J. 2010. Identification and functional characterization of monofunctional ent‐copalyl diphosphate and ent‐kaurene synthases in white spruce reveal different patterns for diterpene synthase evolution for primary and secondary metabolism in gymnosperms. Plant Physiology 152: 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketudat Cairns JR, Mahong B, Baiya S, Jeon J‐S. 2015. β‐Glucosidases: multitasking, moonlighting or simply misunderstood? Plant Science 241: 246–259. [DOI] [PubMed] [Google Scholar]
- Kim RJ, Lee SB, Pandey G, Suh MC. 2022. Functional conservation of an AP2/ERF transcription factor in cuticle formation suggests an important role in the terrestrialization of early land plants. Journal of Experimental Botany 73: 7450–7466. [DOI] [PubMed] [Google Scholar]
- Kitamura S. 2006. Transport of flavonoids: from cytosolic synthesis to vacuolar accumulation. In: Grotewold E, ed. The science of flavonoids. New York, NY, USA: Springer, 123–146. [Google Scholar]
- Kliebenstein DJ. 2013. Making new molecules – evolution of structures for novel metabolites in plants. Current Opinion in Plant Biology 16: 112–117. [DOI] [PubMed] [Google Scholar]
- Kolb CA, Käser MA, Kopecký J, Zotz G, Riederer M, Pfündel EF. 2001. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiology 127: 863–875. [PMC free article] [PubMed] [Google Scholar]
- Komatsu K, Suzuki N, Kuwamura M, Nishikawa Y, Nakatami M, Ohtawa H, Takezawa D, Seki M, Tanaka M, Taji T et al. 2013. Group A PP2Cs evolved in land plants as key regulators of intrinsic desiccation tolerance. Nature Communications 4: 2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriechbaumer V, Botchway SW, Hawes C. 2017. Localization and interactions between Arabidopsis auxin biosynthetic enzymes in the TAA/YUC‐dependent pathway. Journal of Experimental Botany 68: 4195–4207. [DOI] [PubMed] [Google Scholar]
- Kumar GA, Kumar S, Bhardwaj R, Swapnil P, Meena M, Seth CS, Yadav A. 2024. Recent advancements in multifaceted roles of flavonoids in plant–rhizomicrobiome interactions. Frontiers in Plant Science 14: 1297706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa J, Schull TE, Smalle JA. 2023. Friends in arms: flavonoids and the auxin/cytokinin balance in terrestrialization. Plants 23: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry LC, Chapple CCS, Last R. 1995. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet‐b injury and oxidative damage. Plant Physiology 109: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JD, Moran EV, Hart SC. 2019. Fight or flight? Potential tradeoffs between drought defense and reproduction in conifers. Tree Physiology 39: 1071–1085. [DOI] [PubMed] [Google Scholar]
- Le Roy J, Huss B, Creach A, Hawkins S, Neutelings G. 2016. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Frontiers in Plant Science 7: 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne A. 2008. Robustness: confronting lessons from physics and biology. Biological Reviews 83: 509–532. [DOI] [PubMed] [Google Scholar]
- Levin DA. 1971. Plant phenolics: an ecological perspective. The American Naturalist 105: 157–181. [Google Scholar]
- Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BSJ, Muday GK. 2011. Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiology 156: 144–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D‐D, Ni R, Wang P‐P, Shuang X, Wang P‐Y, Zhu T‐T, Sun C‐J, Liu C‐J, Lou H‐X, Cheng A‐X. 2020. Molecular basis for chemical evolution of flavones to flavonols and anthocyanins in land plants. Plant Physiology 184: 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Grotewold E, Dudareva N. 2024. Enough is enough: feedback control of specialized metabolism. Trends in Plant Science 29: 514–523. [DOI] [PubMed] [Google Scholar]
- Lillo C, Lea US, Ruoff P. 2008. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant, Cell & Environment 31: 587–601. [DOI] [PubMed] [Google Scholar]
- Lim YJ, Kwon S‐J, Eom SH. 2023. Red and blue light‐specific metabolic changes in soybean seedlings. Frontiers in Plant Science 14: 1128001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Fang S, Cong B, Li T, Yi D, Zhang Z, Zhao L, Zhang P. 2022. The Antarctic moss Pohlia nutans genome provides insights into the evolution of bryophytes and the adaptation to extreme terrestrial habitats. Frontiers in Plant Science 13: 920138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S‐CC, Nicholson RL. 1998. Reduction of light‐induced anthocyanin accumulation in inoculated sorghum mesocotyls. Plant Physiology 116: 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann E, Hahlbrock K. 2002. Crosstalk among stress responses in plants: pathogen defense overrides UV protection through an inversely regulated ACE/ACE type of light‐responsive gene promoter unit. Proceedings of the National Academy of Sciences, USA 99: 2428–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoya E, Block A, Lois R, Hahlbrock K, Scheel D. 1991. Transcriptional repression of light‐induced flavonoid synthesis by elicitor treatment of cultured parsley cells. The Plant Journal 1: 227–234. [Google Scholar]
- Lu P, Sang W‐E, Ma K‐P. 2008. Differential responses of the activities of antioxidant enzymes to thermal stresses between two invasive Eupatorium species in China. Journal of Integrative Plant Biology 50: 393–401. [DOI] [PubMed] [Google Scholar]
- Maeda HA. 2019. Harnessing evolutionary diversification of primary metabolism for plant synthetic biology. Journal of Biological Chemistry 294: 16549–16566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham KK, Greenham K. 2021. Abiotic stress through time. New Phytologist 231: 40–46. [DOI] [PubMed] [Google Scholar]
- Markham KR, Ryan KG, Bloor SJ, Mitchell KA. 1998. An increase in the luteolin: apigenin ratio in Marchantia polymorpha on UV‐B enhancement. Phytochemistry 48: 791–794. [Google Scholar]
- Martin E, Postiglione AE, Muday GK. 2022. Reactive oxygen species function as signaling molecules in controlling plant development and hormonal responses. Current Opinion in Plant Biology 69: 102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FM, van der Heijden MGA. 2024. The mycorrhizal symbiosis: research frontiers in genomics, ecology, and agricultural application. New Phytologist 242: 1486–1506. [DOI] [PubMed] [Google Scholar]
- Martínez‐Abaigar J, Núñez‐Olivera E. 2022. Bryophyte ultraviolet‐omics: from genes to the environment. Journal of Experimental Botany 73: 4412–4426. [DOI] [PubMed] [Google Scholar]
- Mathesius U. 2001. Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. Journal of Experimental Botany 52: 419–426. [DOI] [PubMed] [Google Scholar]
- Mathesius U. 2018. Flavonoid functions in plants and their interactions with other organisms. Plants 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL. 2000. Functional significance and induction by solar radiation of ultraviolet‐absorbing sunscreens in field‐grown soybean crops. Plant Physiology 122: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor NI, Voß U, Ware A, Janes G, Barrack D, Bishopp A, Bennett MJ, Geisler M, Wells DR, Band LR. 2022. Systems approaches reveal that ABCB and PIN proteins mediate co‐dependent auxin efflux. Plant Cell 34: 2309–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F et al. 2007. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130: 1044–1056. [DOI] [PubMed] [Google Scholar]
- Milo R, Last RL. 2012. Achieving diversity in the face of constraints: lessons from metabolism. Science 336: 1663–1667. [DOI] [PubMed] [Google Scholar]
- Mohana S, Ganesan M, Agilan B, Karthikeyan R, Srithar G, Beaulah MR, Ananthakrishnan D, Velmurugan D, Prasad NR, Ambudkar SV. 2016. Screening dietary flavonoids for the reversal of P‐glycoprotein‐mediated multidrug resistance in cancer. Molecular BioSystems 12: 2458–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monforte L, Soriano G, Núñez‐Olivera E, Martínez‐Abaigar J. 2018. Cell compartmentation of ultraviolet‐absorbing compounds: an underexplored tool related to bryophyte ecology, phylogeny and evolution. Functional Ecology 32: 882–893. [Google Scholar]
- Moody LA, Kelly S, Clayton R, Weeks Z, Emms DM, Langdale JA. 2021. NO GAMETOPHORES 2 is a novel regulator of the 2D to 3D growth transition in the moss Physcomitrella patens . Current Biology 31: 555–563. [DOI] [PubMed] [Google Scholar]
- Moore CE, Meachan‐Hensold K, Lemonnier P, Slattery RA, Benjamin C, Bernacchi CJ, Lawson T, Cavanagh AP. 2021. The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems. Journal of Experimental Botany 72: 2822–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PW, Joham HE, Amin JV, Amin JW. 1966. Effect of manganese toxicity on the indoleacetic acid oxidase system of cotton. Plant Physiology 41: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J, Skůpa P, Bailly A, Hoyerová K, Krecek P, Bielach A, Petrásek J, Zhang J, Gaykova V, Stierhof YD et al. 2009. Subcellular homeostasis of phytohormone auxin is mediated by the ER‐localized PIN5 transporter. Nature 459: 1136–1140. [DOI] [PubMed] [Google Scholar]
- Muhlemann JK, Younts TLB, Muday GK. 2018. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high‐temperature stress. Proceedings of the National Academy of Sciences, USA 115: E11188–E11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutwil M. 2020. Computational approaches to unravel the pathways and evolution of specialized metabolism. Current Opinion in Plant Biology 55: 38–46. [DOI] [PubMed] [Google Scholar]
- Nakabayashi R, Yonekura‐Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K et al. 2015. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. The Plant Journal 77: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugart S, Fiol M, Schreiner M, Rohn S, Zrenner R, Jroh LW, Krunbein A. 2014. Interaction of moderate UV‐B exposure and temperature on the formation of structurally different flavonol glycosides and hydroxycinnamic acid derivatives in kale (Brassica oleracea var. sabellica). Journal of Agricultural and Food Chemistry 62: 4054–4062. [DOI] [PubMed] [Google Scholar]
- Neugart S, Schreiner M. 2018. UVB and UVA as eustressors in horticultural and agricultural crops. Scientia Horticulturae 234: 370–381. [Google Scholar]
- Ng JLP, Welvaert A, Wen J, Chen R, Mathesius U. 2020. The Medicago truncatula PIN2 auxin transporter mediates basipetal auxin transport but is not necessary for nodulation. Journal of Experimental Botany 71: 1562–1573. [DOI] [PubMed] [Google Scholar]
- Palit S, Bhide AJ, Mohanasundaram B, Pala M, Banerjee AK. 2024. Peptides from conserved tandem direct repeats of SHORT‐LEAF regulate gametophore development in moss P. patens . Plant Physiology 194: 434–455. [DOI] [PubMed] [Google Scholar]
- Peer WA, Cheng Y, Murphy AS. 2013. Evidence of oxidative attenuation of auxin signalling. Journal of Experimental Botany 64: 2629–2639. [DOI] [PubMed] [Google Scholar]
- Peer WA, Murphy AS. 2006. Flavonoids as signal molecules. Targets of flavonoid action. In: Grotewold E, ed. The science of flavonoids. New York, NY, USA: Springer, 239–273. [Google Scholar]
- Peer WA, Murphy AS. 2007. Flavonoids and auxin transport: modulators or regulators? Trends in Plant Science 12: 556–563. [DOI] [PubMed] [Google Scholar]
- Peláez‐Vico MÁ, Fichman Y, Zandalinas SI, Van Breusegem F, Karpiński SM, Mittler R. 2022. ROS and redox regulation of cell‐to‐cell and systemic signaling in plants during stress. Free Radical Biology & Medicine 193: 354–362. [DOI] [PubMed] [Google Scholar]
- Peltzer D, Polle A. 2001. Diurnal fluctuations of antioxidative systems in leaves of field‐grown beech trees (Fagus sylvatica): responses to light and temperature. Physiologia Plantarum 111: 158–164. [Google Scholar]
- Podolec R, Demarsy E, Ulm R. 2021. Perception and signaling of ultraviolet‐B radiation in plants. Annual Review of Plant Biology 72: 793–822. [DOI] [PubMed] [Google Scholar]
- Pollastri S, Tattini M. 2011. Flavonoids: old compounds for old roles. Annals of Botany 108: 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster J, Dithmar H, Buremeister R, Friedemann G, Feucht W. 2006. Flavonoids in plant nuclei: detection by laser microdissection and pressure catapulting (LMPC), in vivo staining, and uv–visible spectroscopic titration. Physiologia Plantarum 128: 163–174. [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK. 2007. Stress‐induced morphogenic responses: growing out of trouble? Trends in Plant Science 12: 98–105. [DOI] [PubMed] [Google Scholar]
- Pratyusha DS, Sarada DVL. 2022. MYB transcription factors – master regulators of phenylpropanoid biosynthesis and diverse developmental and stress responses. Plant Cell Reports 41: 2245–2260. [DOI] [PubMed] [Google Scholar]
- Rausher MD. 2001. Co‐evolution and plant resistance to natural enemies. Nature 411: 857–864. [DOI] [PubMed] [Google Scholar]
- Renault H, Alber A, Horst NA, Basilio Lopes A, Fich EA, Krieghauser L, Wiedemann G, Ullmann P, Herrgott L, Erhardt M et al. 2017. A phenol‐enriched cuticle is ancestral to lignin evolution in land plants. Nature Communications 8: 14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA. 2018. Great moments in evolution: the conquest of land by plants. Current Opinion in Plant Biology 42: 49–54. [DOI] [PubMed] [Google Scholar]
- Rensing SA. 2020. How plants conquered land. Cell 181: 964–966. [DOI] [PubMed] [Google Scholar]
- Rice‐Evans CA, Miller NJ, Papanga G. 1996. Structure‐antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology & Medicine 20: 933–956. [DOI] [PubMed] [Google Scholar]
- Richter AS, Nägele T, Grimm B, Kaufmann K, Schroda M, Leister D, Kleine T. 2023. Retrograde signaling in plants: a critical review focusing on the GUN pathway and beyond. Plant Communications 9: 1005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter AS, Tohge T, Fernie AR, Grimm B. 2020. The genomes uncoupled dependent signalling pathway coordinates plastid biogenesis with the synthesis of anthocyanins. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 375: 20190403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg TM, Dadras A, Fürst‐Jansen JMR, Ashok AD, Darienko T, de Vries S, Irisarri I, de Vries J. 2021. Crossroads in the evolution of plant specialized metabolism. Seminars in Cell & Developmental Biology 134: 37–58. [DOI] [PubMed] [Google Scholar]
- Robberecht R, Caldwell MM. 1978. Leaf epidermal transmittance of ultraviolet radiation and its implications for plant sensitivity to ultraviolet‐radiation induced injury. Oecologia 32: 277–287. [DOI] [PubMed] [Google Scholar]
- Robson TM, Klem K, Urban O, Jansen MAK. 2015. Re‐interpreting plant morphological responses to UV‐B radiation. Plant, Cell & Environment 38: 856–866. [DOI] [PubMed] [Google Scholar]
- Roepke J, Bozzo GG. 2015. Arabidopsis thaliana β‐glucosidase BGLU15 attacks flavonol 3‐O‐β‐glucoside‐7‐O‐α‐rhamnosides. Phytochemistry 109: 14–24. [DOI] [PubMed] [Google Scholar]
- Rousseaux MC, Julkunen‐Tiitto R, Searles PS, Scopel AL, Ballaré CL. 2004. Solar UV‐B radiation affects leaf quality and insect herbivory in the southern beech tree Nothofagus Antarctica . Oecologia 138: 505–512. [DOI] [PubMed] [Google Scholar]
- Rozema J, Björn OL, Bornman JF, Gaberscik A, Häder D‐P, Trost T, Germ M, Klisch M, Gröniger A, Sinha RP et al. 2002. The role of UV‐B radiation in aquatic and terrestrial ecosystems – an experimental and functional analysis of the evolution of UV‐absorbing compounds. Journal of Photochemistry and Photobiology B 66: 2–12. [DOI] [PubMed] [Google Scholar]
- Rozema J, van de Staaij J, Björn LO, Caldwell M. 1997. UV‐B as an environmental factor in plant life: stress and regulation. Trends in Ecology & Evolution 12: 22–28. [DOI] [PubMed] [Google Scholar]
- Ryan KG, Swinny EE, Winefield C, Markham KE. 2001. Flavonoids and UV photoprotection in Arabidopsis mutants. Zeitschrift für Naturforschung B 56c: 745–754. [DOI] [PubMed] [Google Scholar]
- Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M. 2021. Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen J‐P, Karonen M. 2011. Chemical ecology of tannins and other phenolics: we need a change in approach. Functional Ecology 25: 325–338. [Google Scholar]
- Saracini E, Tattini M, Traversi ML, Vincieri FF, Pinelli P. 2005. Simultaneous LC‐DAD and LC‐MS determination of ellagitannins, flavonoid glycosides, and acyl‐glycosyl flavonoids in Cistus salvifolius L. leaves. Chromatographia 62: 245–249. [Google Scholar]
- Schneider GF, Coley PD, Younkin GC, Forrister DL, Mills AG, Kursar TA. 2019. Phenolics lie at the centre of functional versatility in the responses of two phytochemically diverse tropical trees to canopy thinning. Journal of Experimental Botany 20: 5853–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler J‐P, Jungblut TP, Heller W, Kofferlein M, Hutzler P, Heinzmann U, Schmelzer E, Ernst D, Langebertels C, Sandermann H Jr. 1996. Tissue localization of u.v.‐B‐screening pigments and of chalcone synthase mRNA in needles of Scots pine seedlings. New Phytologist 132: 247–258. [Google Scholar]
- Schreiber M, Rensing SA, Gould SB. 2022. The greening ashore. Trends in Plant Science 27: 847–857. [DOI] [PubMed] [Google Scholar]
- Segarra‐Medina C, Alseekh S, Fernie AR, Rambla JL, Pérez‐Clemente RM, Gómez‐Cádenas A, Zandalinas SI. 2023. Abscisic acid promotes plant acclimation to the combination of salinity and high light stress. Plant Physiology and Biochemistry 203: 108008. [DOI] [PubMed] [Google Scholar]
- Serrano M, Kanehara K, Torres M, Yamada K, Tintor N, Kombrink E, Schulze‐Lefeer P, Saijo Y. 2012. Repression of sucrose/ultraviolet B light‐induced flavonoid accumulation in microbe‐associated molecular pattern‐triggered immunity in Arabidopsis. Plant Physiology 158: 408–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitan N, Yazaki K. 2020. Dynamism of vacuoles toward survival strategy in plants. Biochimica et Biophysica Acta – Biomembranes 1862: 183127. [DOI] [PubMed] [Google Scholar]
- Siipola SM, Kotilanen T, Sipari N, Morales LO, Lindfors AV, Robson TM, Aphalo PG. 2016. Epidermal UV‐A absorbance and whole‐leaf flavonoid composition in pea respond more to solar blue light than to solar UV radiation. Plant, Cell & Environment 38: 941–952. [DOI] [PubMed] [Google Scholar]
- Singh G, Agrawal H, Bednarek P. 2023. Specialized metabolites as versatile tools in shaping plant–microbe associations. Molecular Plant 16: 122–144. [DOI] [PubMed] [Google Scholar]
- Singh P, Arif Y, Bajguz A, Hayat S. 2021. The role of quercetin in plants. Plant Physiology and Biochemistry 166: 10–19. [DOI] [PubMed] [Google Scholar]
- Skokan R, Medvecka E, Viane T, Vosolsobê S, Zwiewka M, Muller K, Skupa P, Karadi M, Zhang Y, Janacek DP et al. 2019. PIN‐driven auxin transport emerged early in streptophyte evolution. Nature Communications 5: 1114–1119. [DOI] [PubMed] [Google Scholar]
- Soengas P, Rodríguez VM, Velasco P, Cartea ME. 2018. Effect of temperature stress on antioxidant defenses in Brassica oleracea . ACS Omega 3: 5237–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Kong L, Yang J, Wang X, Zhao Z, Zhang Y, Xu C, Fan C, Luo K. 2024. The IAA17.1/HSFA5a module enhances salt tolerance in Populus tomentosa by regulating flavonol biosynthesis and ROS levels in lateral roots. New Phytologist 241: 592–606. [DOI] [PubMed] [Google Scholar]
- Song Z, Lai X, Chen H, Wang L, Pang X, Hao Y, Lu W, Chen W, Zhu X, Li X. 2022. Role of MaABI5‐like in abscisic acid‐induced cold tolerance of ‘Fenjiao’ banana fruit. Horticulture Research 9: uhac130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano G, Clix C, Heilmann M, Núñez‐Olivera E, Martínez‐Abaigar J, Jenkins GI. 2018. Evolutionary conservation of structure and function of the UVR8 photoreceptor from the liverwort Marchantia polymorpha and the moss Physcomitrella patens . New Phytologist 217: 151–162. [DOI] [PubMed] [Google Scholar]
- Stafford HA. 1965. Flavonoids and related phenolic compounds produced in the first internode of Sorghum vulgare Pers. in darkness and in light. Plant Physiology 40: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford HA. 1991. Flavonoid evolution: an enzymic approach. Plant Physiology 96: 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steemans P, Le Hérissé A, Melvin J, Miller MA, Paris F, Verniers J, Wellman CH. 2009. Origin and radiation of the earliest vascular land plants. Science 324: 353. [DOI] [PubMed] [Google Scholar]
- Steppuhun A, Baldwin IT. 2008. Induced defenses and the cost‐benefit paradigm. In: Schaller A, ed. Induced plant resistance to herbivory. New York, NY, USA: Springer Science, 61–87. [Google Scholar]
- Sugimoto K, Zager JJ, St Aubin B, Lange BM, Howe GA. 2022. Flavonoid deficiency disrupts redox homeostasis and terpenoid biosynthesis in glandular trichomes of tomato. Plant Physiology 188: 1450–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain T. 1975. Evolution of flavonoid compounds. In: Harborne JB, Mabry TJ, Mabry H, eds. The flavonoids. Boston, MA, USA: Springer, 1096–1129. [Google Scholar]
- Swain T. 1977. Secondary compounds as protective agents. Annual Review of Plant Physiology 28: 479–501. [Google Scholar]
- Tang H, Lu K‐J, Zhang Y, Cheng Y‐L, Tu S‐L, Friml J. 2024. Divergence of trafficking and polarization mechanisms for PIN auxin transporters during land plant evolution. Plant Communications 5: 100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G. 2004. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytologist 163: 547–561. [DOI] [PubMed] [Google Scholar]
- Tattini M, Gravano E, Pinelli P, Mulinacci N, Romani A. 2000. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytologist 148: 69–77. [DOI] [PubMed] [Google Scholar]
- Tattini M, Loreto F, Fini A, Guidi L, Brunetti C, Velikova V, Gori A, Ferrini F. 2015. Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought‐stressed Platanus × acerifolia plants during Mediterranean summers. New Phytologist 207: 613–626. [DOI] [PubMed] [Google Scholar]
- Tattini M, Matteini P, Saracini E, Traversi ML, Giordano C, Agati G. 2007. Morphology and biochemistry of non‐glandular trichomes in Cistus salvifolius L. leaves growing in extreme habitats of the Mediterranean basin. Plant Biology 9: 411–419. [DOI] [PubMed] [Google Scholar]
- Taulavuori K, Pyysalo A, Taulavuori E, Julkunen‐Tiitto R. 2018. Responses of phenolic acid and flavonoid synthesis to blue and blue‐violet light depends on plant species. Environmental and Experimental Botany 150: 183–187. [Google Scholar]
- Taylor LP, Grotewold E. 2005. Flavonoids as developmental regulators. Current Opinion in Plant Biology 8: 317–323. [DOI] [PubMed] [Google Scholar]
- Teale WD, Pasternal T, Dal Bosco C, Dovzhenko A, Kratzat K, Bildl E, Schwörer M, Falk T, Ruperti B, Schaefer JV. 2020. Flavonol‐mediated stabilization of PIN efflux complexes regulates polar auxin transport. EMBO Journal 40: e104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Pei Y, Huang W, Ding J, Siemann E. 2021. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. The ISME Journal 15: 1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohge T, Perez de Souza L, Fernie AR. 2018. On the natural diversity of phenylacylated‐flavonoid and their in planta function under conditions of stress. Phytochemistry Reviews 17: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohge T, Wendenburg R, Ishihara H, Nakabayashi R, Watanabe M, Sulpice R, Hoefgen R, Takayama H, Saito K, Stitt M et al. 2016. Characterization of a recently evolved flavonol‐phenylacyltransferase gene provides signatures of natural light selection in Brassicaceae . Nature Communications 7: 12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp EA, Zhuang Y, Schreiber M, Stone H, Berardi EA. 2018. Evolutionary and ecological drivers of plant flavonoids across a large latitudinal gradient. Molecular Phylogenetics and Evolution 128: 147–161. [DOI] [PubMed] [Google Scholar]
- Uehara A, Shimoda K, Murai Y, Ivashina T. 2018. Flavonoid aglycones and glycosides from the leaves of some Japanese Artemisia species. Natural Products Communications 13: 551–554. [Google Scholar]
- Ung KL, Winkler M, Schulz L, Kolb M, Janacek DP, Dedic E, Stokes DL, Hammes UZ, Pedersen BP. 2022. Structures and mechanism of the plant PIN‐FORMED auxin transporter. Nature 609: 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas‐Molina J, Molina‐Hidalgo FJ, Clicque E, Goosens A. 2021. Why and how to dig into plant metabolite‐protein interactions. Trends in Plant Science 26: 472–483. [DOI] [PubMed] [Google Scholar]
- Viaene T, Landberg K, Thelander M, Medvecka E, Pederson E, Feraru E, Cooper ED, Karimi M, Delwiche CF, Ljung K et al. 2014. Directional auxin transport mechanisms in early diverging land plants. Current Biology 24: 2786–2791. [DOI] [PubMed] [Google Scholar]
- de Vries J, de Vries S, Slamovits CH, Rose LA, Archibald JM. 2017. How embryophytic is the biosynthesis of phenylpropanoids and their derivatives in Streptophyte algae? Plant & Cell Physiology 58: 934–945. [DOI] [PubMed] [Google Scholar]
- de Vries S, Furst‐Jansen JMR, Irisarri I, Ashok AD, Ischebeck T, Feussner K, Abreu IN, Petersen M, Feussner I, de Vries J. 2021. The evolution of the phenylpropanoid pathway entailed pronounced radiations and divergences of enzyme families. The Plant Journal 107: 975–1002. [DOI] [PubMed] [Google Scholar]
- Wagner A. 2011. The molecular origins of evolutionary innovations. Trends in Genetics 27: 397–410. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu Y, Li S‐S, Han G‐Z. 2015. Insights into the origin and evolution of the plant hormone signaling machinery. Plant Physiology 167: 872–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Liu W, Yu L, Guo Z, Chen Z, Jiang S, Xu H, Fang H, Wang Y, Zhang Z et al. 2020. HEAT SHOCK FACTOR A8a modulates flavonoid synthesis and drought tolerance. Plant Physiology 184: 1273–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Alseekh S, Fernie AR, Luo J. 2019. The structure and function of major plant metabolite modifications. Molecular Plant 12: 899–919. [DOI] [PubMed] [Google Scholar]
- Watkins JM, Chapman JM, Muday GK. 2017. Abscisic acid‐induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiology 175: 1807–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JM, Hechler PJ, Muday GK. 2014. Ethylene‐induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiology 164: 1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann E. 1976. Specific ultraviolet effects in plant morphogenesis. Photochemistry and Photobiology 24: 659–660. [DOI] [PubMed] [Google Scholar]
- Wen W, Alseekh S, Fernie AR. 2020. Conservation and diversification of flavonoid metabolism in the plant kingdom. Current Opinion in Plant Biology 55: 100–108. [DOI] [PubMed] [Google Scholar]
- Weng J‐K, Lynch JH, Matos JO, Dudareva N. 2021. Adaptive mechanisms of plant specialized metabolism connecting chemistry to function. Nature Chemical Biology 17: 1037–1045. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Spencer JPE, Rice‐Evans CA. 2004. Flavonoids: antioxidants or signalling molecules? Free Radical Biology & Medicine 36: 838–849. [DOI] [PubMed] [Google Scholar]
- Williamson G. 2002. The use of flavonoid aglycones in in vitro systems to test biological activities: based on bioavailability data, is this a valid approach? Phytochemistry Reviews 1: 215–222. [Google Scholar]
- Wink M. 1999. Functions of plant secondary metabolites and their exploitation in biotechnology. Annual plant reviews, vol. 3. Sheffield, UK: Sheffield Academic Press. [Google Scholar]
- Wolf L, Rizzini L, Stracke R, Ulm R, Rensing SA. 2010. The molecular and physiological responses of Physcomitrella patens to ultraviolet‐B radiation. Plant Physiology 153: 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber E, Dörr M, Christ M. 2011. Flavonoid aglycones from the leaf and stem exudates of some Geraniaceae species. Natural Products Communications 6: 15–16. [PubMed] [Google Scholar]
- Xu B, Taylor L, Pucker B, Feng T, Glover BJ, Brockington SF. 2021. The land plant‐specific MIXTA‐MYB lineage is implicated in the early evolution of the plant cuticle and the colonization of land. New Phytologist 229: 2324–2338. [DOI] [PubMed] [Google Scholar]
- Xu W, Dubos C, Lepiniec L. 2015. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends in Plant Science 20: 176–185. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Ikebara N. 1997. Flavonoid‐peroxidase reaction as a detoxification mechanism of plant cells against H2O2 . Plant Physiology 115: 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura‐Sakakibara K, Higashi Y, Nakabayashi R. 2019. The origin and evolution of plant flavonoid metabolism. Frontiers in Plant Science 10: 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Jian C, Huang C, Wen D, Lu J, Chen S, Zhang T, Shi Y, Xue J, Ma W et al. 2018. The light‐induced transcription factor FtMYB116 promotes accumulation of rutin in Fagopyrum tataricum . Plant, Cell & Environment 42: 1340–1351. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lin JL, Harris C, Peer WA. 2016. DAO1 catalyzes temporal and tissue‐specific oxidative inactivation of auxin in Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 113: 11010–11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Peer WA. 2017. Auxin homeostasis: the DAO of catabolism. Journal of Experimental Botany 68: 3145–3154. [DOI] [PubMed] [Google Scholar]
- Zhang J, Subramanian S, Stacey G, Yu O. 2009. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti . The Plant Journal 57: 171–183. [DOI] [PubMed] [Google Scholar]


