Abstract
Objective
To assess the risk of recurrent preterm birth following spontaneous extreme preterm birth between 16+0 - 27+6 weeks.
Methods
A nationwide retrospective cohort study was conducted with data from the Perinatal Registry of the Netherlands. We included nulliparous women with a singleton pregnancy that ended in spontaneous preterm birth between 16+0 and 27+6 weeks of gestation without congenital anomalies or antenatal death between 2010–2014 and had a subsequent pregnancy in the 5 years following (2010–2019). The primary outcome of this study was recurrent preterm birth < 37 weeks.
Results
In total, 1011 women with linked pregnancies were included. The risk of preterm birth < 37 weeks with prior spontaneous birth between 16+0-19+6, 20+0-23+6, and 24+0-27+6 weeks was respectively 19.0 %, 29.5 % and 27.6 %. The risk of subsequent preterm birth < 24 weeks was 5.8 %, 7.2 % and 4.3 %. A short interpregnancy interval of 0–3 months was associated with increased odds for recurrent preterm birth < 32 weeks (OR 2.3 95 % CI 1.4–3.7) and preterm birth < 37 weeks (OR 1.8 95 % CI 1.2–2.6).
Conclusion
Patients with previous spontaneous preterm birth from 16 weeks GA onwards are at high risk for recurrent preterm birth and should be regarded as such in the consideration of preventive measures to prevent recurrent adverse pregnancy outcomes.
Keywords: Preterm birth, Recurrent preterm birth, Mid trimester loss, Mid pregnancy loss, Interpregnancy interval, Risk assessment
Highlights
-
•
Prior spontaneous preterm birth (sPTB) is an important risk factor for recurrent preterm birth (PTB).
-
•
Knowledge is lacking on the gestational age (GA) at prior birth from which the PTB recurrence risk is increased.
-
•
We found that previous sPTB from 16 weeks GA onwards increases recurrent PTB risk.
-
•
The lower limit of PTB should be the GA from which the recurrence risk is increased, distinguishing miscarriage from PTB.
Introduction
Preterm birth (PTB) is an important cause of neonatal morbidity and mortality. [1], [2] A great challenge in the prevention of PTB lies in the recognition of patients at risk. Previous spontaneous preterm birth (sPTB) has proven to be an important risk factor for recurrent PTB and plays part in the consideration of preventive interventions. [3], [4], [5], [6] However, knowledge is lacking on the gestational age from which the risk for recurrent PTB is increased. Thereby, patients at risk might not be recognized as such and are unjustly not considered for preventive measures.
Currently, there is a lack of consensus regarding the gestational age threshold that distinguishes miscarriage from preterm birth. The widely used WHO definition characterizes birth before 37 weeks of gestation as preterm, but does not specify a lower limit. The lower limit now varies between 20, 22 and 28 weeks for respectively the USA [7], Europe [8] and China [9]. This variation can complicate the identification of a population at risk for recurrent PTB, for instance due to disparities in how these births are recorded. Yet, especially for patients with previous birth around 20 weeks, early recognition of risk factors can contribute to timely interventions to prevent recurrence.
Therefore, in this study we will assess the recurrence risk of PTB per gestational age group, following sPTB between 16+0 - 27+6 weeks. In addition, we will assess the role of interpregnancy interval in this association. We hypothesize the risk of recurrent PTB is high following previous spontaneous PTB from 16 weeks onwards and expect that short interpregnancy intervals correlate with higher risks of recurrence.
Material & methods
Study design and population
We conducted a nationwide cohort study with data from the national Perinatal Registry of the Netherlands (PERINED). This registry contains data on pregnancy, delivery and neonatal outcome and covers 97 % of all deliveries after 16 weeks of gestation in the Netherlands. [10] The PERINED database is obtained by linkage of three registries: the midwifery registry (LVR-1), obstetrics registry (LVR-2) and the neonatology registry (LNR). Permission for usage of the PERINED data with the purpose of this study was obtained on July, 13, 2021 (approval number 21.13).
We extracted the data of registered pregnancies that ended in birth following a spontaneous start of labour > 16 weeks of gestation between 2010 and 2014 (index pregnancies). We excluded pregnancies from primiparous or multiparous women, multiple pregnancies, pregnancies with birth > 28 weeks of gestation or pregnancies with an induced start of labor. Subsequently, the included pregnancies were linked with a 5 year cohort in primiparous women. This was done separately for each year with a subsequent 5-year cohort in the years 2010–2019. The 3 deterministic linkage keys were date of birth of the mother, data of previous birth/ birth date of the child and 4-digid zip code. Linkage was possible if two out of three variables were available of which maternal birth day was one of them. After linkage of prior birth and subsequent birth, prior pregnancies that were complicated with antenatal diagnosed IUFD and/or congenital anomalies were excluded from the sPTB index group in the analysis.
Outcomes
Primary outcome of this study was the recurrent risk rate of PTB before 37 weeks of gestation. Secondary outcomes included the recurrent risk of PTB < 24 and PTB < 32 weeks. The recurrent risks are reported for the total group as well as subdivided into prior sPTB between 16+0-19+6, 20+0-23+6 and 24+0-27+6 weeks of gestation.
Covariates
The interpregnancy interval (IPI) is defined as the interval between prior birth and conception date of the subsequent pregnancy using the expected due date and gestational age. Gestational age was based on crown-rump-length (CRL) during early fetal sonography. Assisted reproductive technology (ART) yes/no. Birth weight is shown in grams. Social economic status (SES) was extracted from PERINED and was determined bases on the status scores as provided by the Netherlands Institute of Social Research. Social economic status of a neighborhood was defined based on income, paid jobs and education. Low SES (most deprived) was defined as SES at the lowest quintile.
Statistical analysis
Following the linkage between nulliparous women with previous sPTB and primiparous women with an ongoing pregnancy after 16 weeks of gestation, the baseline characteristics of the first pregnancy were described and tested for the three spontaneous PTB groups of the index population; chi-square for categorical variables and anova for continues variables.
Logistic regression analysis was performed to assess the association between the gestational age at prior birth and the PTB pregnancy outcomes of the subsequent pregnancy. PTB rates are expressed per 100 births. Odds ratios (OR) and 95 % confidence intervals (C.I.) were calculated separately for the three outcome groups (PTB <24 weeks, PTB < 32 weeks and PTB <37 weeks) in the subsequent pregnancy, with prior birth between 24 + 0 – 27 + 6 weeks as the reference. Analysis was adjusted for maternal characteristics (age, ethnicity, low SES), neonatal characteristics (fetal sex) and obstetrics characteristics (interpregnancy interval).
A separate analysis was conducted to assess the association between the interpregnancy interval, gestational age at prior birth and the recurrent risk. The interpregnancy interval was subdivided into groups of 0–3, 4–6, 7–12, 13–36 and > 37 months, with 13–36 months as the reference.
Linkage and analysis were performed using SAS, version 9.4.
Results
Study population
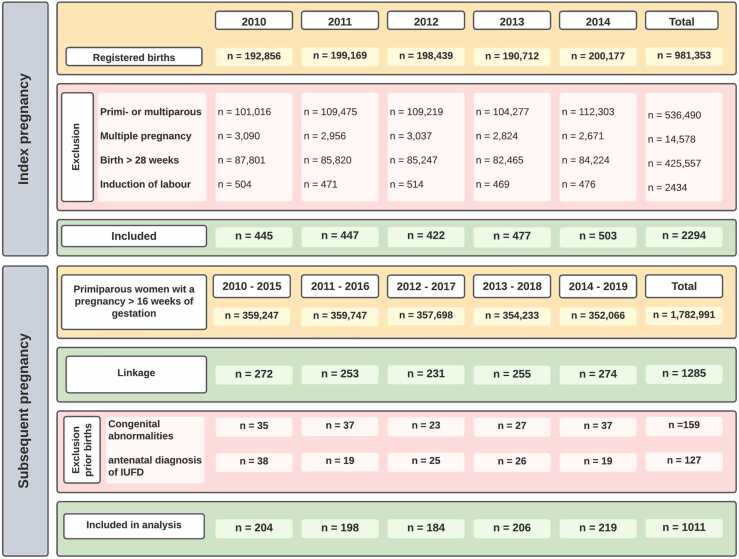
Out of 981,353 registered births in 2010–2014, we identified 4728 nulliparous women with a singleton pregnancy that ended in PTB between 16+0-27+6 weeks. From these, 2434 pregnancies were induced or ended with an elective cesarean section and were excluded. In total, 2294 prior births with a spontaneous start of labour were included for linkage (Fig. 1).
Fig. 1.
Flowchart of inclusion and exclusion per year.
After linkage with a cohort of 1782,991 primiparous births, 1285 of the included 2294 women with prior sPTB could be linked with a subsequent pregnancy that ended in a birth after 16 weeks of gestation. Prior pregnancies complicated by an antenatal diagnoses of intra-uterine fetal death (IUFD) (n = 127) or fetal congenital abnormalities (n = 159) (together n = 274, numbers do not add up due to overlap in diagnosis) were excluded from the prior sPTB pregnancies after linkage. Ultimately, we were able to include the perinatal outcomes of 1011 linked pregnancies in this study. The flow chart of inclusion and exclusion per year is shown in Fig. 1.
Patient characteristics of the index pregnancy are shown in Table 1 and are shown separately for women with prior sPTB between 16+0-19+6, 20+0-23+6 and 24+0-27+6 weeks of gestation. The number of patients in each group was respectively 342, 346 and 323. The proportion of women with a western ethnicity differed significantly between groups, with the highest proportion of women with an ethnicity other than a western ethnicity in the group with birth between 16+0 and 19+6. Despite an almost equal number of male fetuses (n = 511) and female fetuses (n = 500) in the overall group, the proportion of male fetuses being born between 16+0-19+6 weeks (35.7 %) was significantly lower compared to 20+0-23+6 (59,5 %) and 24+0-27+6 (56,7 %) weeks (p < 0.001). Maternal age, ART involvement and low SES did not differ significantly between the sPTB groups.
Table 1.
Baseline characteristics of the first spontaneous PTB pregnancy in three gestational age groups.
|
16+0-19+6 |
20+0-23+6 |
24+0-27+6 |
Total |
p-value |
|||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 342) | (n = 346) | (n = 323) | (n = 1011) | ||||||
| Maternal age (years)# | 28.1 | (4.9) | 28.6 | (4.8) | 27.8 | (4.7) | 28.2 | (4.8) | 0.11 |
| Birth weight (gram)# | 192 | (148) | 479 | (276) | 889 | (201) | 574 | (353) | < 0.001 |
| Maternal Age < 25 years | 75 | 21.9 % | 77 | 22.3 % | 72 | 22.3 % | 224 | 22.2 % | 0.99 |
| Western ethnicity | 220 | 64.3 % | 248 | 71.7 % | 237 | 73.4 % | 705 | 69.7 % | 0.03 |
| ART^ | 28 | 8.2 % | 31 | 9.0 % | 22 | 6.8 % | 81 | 8.0 % | 0.58 |
| Male sex | 122 | 35.7 % | 206 | 59.5 % | 183 | 56.7 % | 511 | 50.5 % | < 0.001 |
| Deprived area (low SES) | 78 | 22.8 % | 75 | 21.7 % | 61 | 18.9 % | 214 | 21.2 % | 0.45 |
| Gestational age days# | 126 | 7.6 | 154 | 8.2 | 181 | 8.4 | 153 | 24 | < 0.001 |
| Gestational age weeks# | 17+4 | 8 days | 21+4 | 8 days | 25+4 | 8 days | 21+3 | 24 days | < 0.001 |
Data shown as n (%)
# Shown as mean (SD)
^Assisted reproductive technology: IVF, ovulation induction, intra-uterine insemination & other stimulation
Outcomes
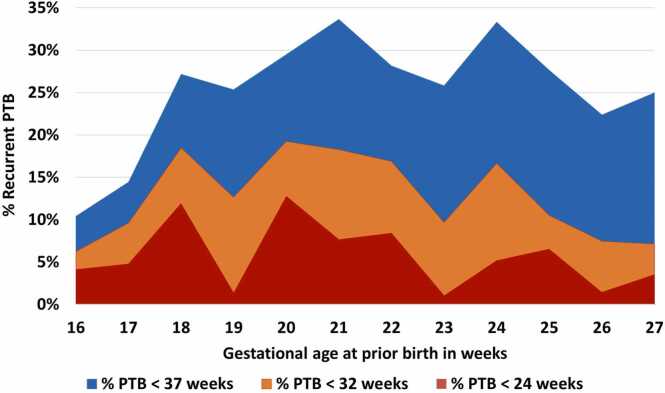
The recurrent rates for PTB < 24, < 32 and < 37 weeks with 95 %-confidence intervals are shown in Table 2. The recurrence rate of PTB < 37 weeks in women with prior birth between 16+0-19+6 was 19.0 % (95 % CI 15.0 %−23.6 %). Women with prior birth between 20+0-23+6 weeks had a recurrence rate of PTB < 37 weeks of 29.5 % (95 % CI 24.7–34.6 %) and women with prior birth between 24+0-27+6 weeks had a recurrence rate of PTB < 37 weeks of 27.6 % (95 % CI 22.8–32.8 %). For the three groups, odds for PTB < 24 and PTB < 32 weeks did not differ significantly between groups (Supplementary table 1). Fig. 2 shows the risk of recurrent preterm birth per week of gestational age groups in prior sPTB birth.
Table 2.
Subsequent PTB risk per gestational age group with 95 % CI of the point estimate.
|
PTB < 24 weeks |
PTB < 32 weeks |
PTB < 37 weeks |
||||
|---|---|---|---|---|---|---|
| GA at prior sPTB | % | 95 % CI | % | 95 % CI | % | 95 % CI |
| 16+0 −19+6 (n = 342) | 5.8 % | 3.6 %−8.9 % | 11.7 % | 8.5 %−15.6 % | 19.0 %) | 15.0 %−23.6 % |
| 20+0 −23+6 (n = 346) | 7.2 % | 4.7 %−10.5 % | 15.9 % | 12.2 %−20.2 % | 29.5 %) | 24.7 %−34.6 % |
| 24+0 −27+6 (n = 323) | 4.3 % | 2.4 %−7.2 % | 10.8 % | 7.1 %−14.1 % | 27.6 %) | 22.8 %−32.8 % |
| Total 16+0 −27+6 (n = 1011) | 5.8 % | 4.7 %−7.5 % | 12.9 % | 10.9 %−15.1 % | 25.3 %) | 22.7 %−28.1 % |
Fig. 2.
Risk of recurrent preterm birth < 37 weeks, < 32 weeks and < 24 weeks per week of gestational age at prior birth.
Interpregnancy interval The odds per interval groups are shown in Table 3. Compared to the reference group with an interpregnancy interval of 13–36 months, an interval of 0–3 months was found to be associated with significantly increased odds for PTB < 32 weeks (OR 2.3 95 % CI 1.4–3.7) and PTB < 37 weeks (OR 1.8 95 % CI 1.2–2.6). Compared to women with prior birth at a viable gestational age > 24 weeks, women with previous pre-viable birth between 16+0-19+6, 20+0-23+6 weeks were more likely to have a short interpregnancy interval < 3 months (14.2 % vs respectively 19.6 % and 28.0 %). (supplementary table 2).
Table 3.
Risk of PTB in subsequent birth per interpregnancy interval.
|
Recurrent PTB |
PTB < 24 |
PTB < 32 |
PTB < 37 |
||||
|---|---|---|---|---|---|---|---|
| Interpregnancy interval | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |
| 0 −3 months | 1.5 | 0.76 - 2.9 | 2.3 | 1.4 - 3.7 | 1.8 | 1.2 – 2.6 | |
| 4 −6 months | 1.0 | 0.45 - 2.2 | 1.2 | 0.66 - 2.1 | 1.3 | 0.83 – 1.9 | |
| 7 −12 months | 0.89 | 0.39 - 2.0 | 1.0 | 0.58 - 1.9 | 0.89 | 0.57 – 1.4 | |
| 13 −36 months | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | |
| > 36 months | 0.39 | 0.09 - 1.7 | 0.89 | 0.40 – 2.0 | 0.54 | 0.28 – 1.1 | |
Discussion
The aim of this study was to assess the risk of recurrent preterm birth following spontaneous preterm birth between 16+0-27+6 weeks. We found that, at all gestational ages, patients with previous sPTB from 16 weeks onwards are at high risk for recurrent preterm birth.
A short interpregnancy interval of 0–3 months was associated with an increased risk of subsequent preterm birth < 37 and < 32 weeks. Short intervals of 0–3 months were more frequent in patients with prior sPTB < 24 weeks. Since in the Netherlands, no active support is offered to neonates born before 24 weeks, these births are most likely to end in perinatal death. Therefore, parents might pursue a subsequent pregnancy shortly after the immature birth.
Multiple studies assessed the association between obstetric history and the risk for subsequent PTB and found elevated risks of PTB following recurrent miscarriage or prior PTB < 37 weeks. [5], [11], [12], [13], [14], [15], [16], [17], [18] Limited research is available on the subsequent risk after birth between 16–24 weeks and a cohort comparison is complicated by international differences in terminology and registration. One study by Goldenberg et al. (‘93) observed a PTB rate of 39 % in women who had given birth between 13 and 24 weeks, which increased to 62 % if the first birth was between 19 and 22 weeks. [12] Edlow et. al. (’07) found that women with prior loss between 14 and 24 weeks were 10.8 times more likely to experience recurrent second-trimester loss or PTB compared to those with previous full-term delivery. [13] A third study from Denmark (’17) reported a recurrence rate of 7.3 % following birth between 16 and 28 weeks, but this rate varied significantly depending on the characteristics of the previous birth (fetal anomaly, multiple gestation, or intrauterine fetal demise), complicating a comparison with our findings. [11].
Women with prior sPTB between 16+0-19+6 weeks had a recurrent risk for PTB < 32 and < 37 weeks of respectively 11.7 % and 19.0 %, which is high compared to respectively 1.0 % and 5.5 % in a general Dutch population of multiparous women with singleton and multiple pregnancies in 2021 (www.peristat.nl). The odds for recurrent PTB < 24 and < 32 weeks did not differ between groups. This emphasizes that prior birth between 16+0-19+6 weeks deserves consideration in the risk assessment for subsequent PTB. Labelling spontaneous birth at this gestational age range as a miscarriage, may underestimate the risk for subsequent PTB. Ideally, the threshold that distinguishes between miscarriage and PTB (e.g. the lower limit of PTB), is the gestational age at which the recurrence risk is increased. Thereby, when referring to obstetric history, terminology can be used that adequately acknowledges the increased risk. Therefore, spontaneous birth from 16 weeks onwards should be classified as sPTB instead of miscarriage or mid-trimester loss, to enhance the recognition, approach and preventive treatment of patients at risk.
The high recurrent risk after births at low gestational ages raises questions whether the subsequent risk may also be increased after birth at gestational ages below 16 weeks. Accurate national registration is vital to assess PTB risk following births below 16 weeks. All pregnant women in the Netherlands are advised to contact a midwife or general practitioner before 10 weeks of pregnancy, allowing for precise gestational age determination via ultrasound. Registering these pregnancy outcomes will help PTB risk evaluation. If an increased PTB risk is found, further research is needed to assess whether and which preventive measures improve subsequent pregnancy outcomes.
This study used data from a large perinatal registry in the Netherlands, covering 97 % of births. [10] The large sample size with data from multiple consecutive years enabled a detailed assessment on subsequent PTB risk by gestational age and allowed for analysis on the interpregnancy interval. However, due to non-mandatory registration for births until 24 weeks, underrepresentation is likely for prior births between 16–24 weeks and also for the recurrence risk in that range.
In the index pregnancy selection, we excluded induced births, focusing on spontaneous and unknown start of labor. Excluding pregnancies complicated by congenital abnormalities or IUFD in the index pregnancy, likely removed inaccurately registered induced deliveries as well. However, we cannot rule out the possibility that the index pregnancy cohort might still include induced births, thereby potentially underestimating the risk of subsequent PTB after sPTB. In our subsequent cohort, all pregnancies were included(e.g., we did not exclude pregnancies with multiples, congenital abnormalities, IUFD). Therefore, the risk is most likely lower for uncomplicated singletons. Still, our PTB rates remain notably high when compared to national PTB rates.
No data were available regarding the use of preventive measures in the subsequent pregnancy. In the Netherlands, patients with previous sPTB < 34 weeks of gestation are typically offered preventive progesterone treatment, serial cervical length screening and potentially receive other interventions to effectively reduce PTB risk such as a cervical cerclage [19]. [20] Therefore, it is plausible that a significant portion of our study population received preventive treatment in the subsequent pregnancy, which could underestimate the actual risk faced by patients. However, there may be limited awareness regarding the increased risk following PTB around 16 weeks, resulting in fewer or no preventive measures and therefore providing a representative risk estimate for this subgroup.
Out of 2294 women with prior sPTB, we successfully linked 1285 nulliparous women to a subsequent pregnancy in a primiparous cohort. No linkage could be established in 1009 women, possibly due to insufficient matching variables. For example, if the birth record of the subsequent pregnancy did not include the date of the prior birth and if ZIP code changed over time, there would insufficient matching variables to establish a linkage. Other reasons could include no subsequent pregnancy within the 5-year timeframe, cases where the only pregnancy within the 5-year timeframe resulted in a miscarriage or termination before 16 weeks, or misreported subsequent births as nulliparous births. Also, 172 patients from the index cohort were excluded due to the antenatal diagnosis of IUFD, which might involve cases of IUFD due to fetal distress from extreme preterm labor. Given its likelier occurrence before 24 weeks, the group of patients with prior birth between 16–24 weeks may not be entirely represented.
We recommend that the lower limit of PTB, distinguishing miscarriage from PTB, should be the gestational age from which the recurrence risk is increased to facilitate recognition and allow for preventive measures. Since patients with previous spontaneous PTB from 16 weeks onwards are at high risk for recurrent PTB, they should be considered as such using the appropriate terminology. A short interpregnancy interval of 0–3 months was associated with significantly increased odds of subsequent PTB and should be discouraged.
Contributions
All contributors to this manuscript are listed as authors. MAB and EIK proposed the research idea; MAB, EIK, AR and ALG were responsible for the design of the study. AR was responsible for retrieving the data from PERINED for the purposes of this study and analysis of retrieved data; ALG drafted the manuscript; AR, EIK, BMK, EP, MAO and MAB reviewed all versions of the article and contributed to the interpretation of the results. All authors were sent the paper as prepared for submission and approved the final version.
Authors' Data Sharing Statement
-
1.
Will individual participant data be available (including data dictionaries): No, data was used from the national Perinatal Registry of the Netherlands (PERINED). Permission for usage of the PERINED data with the purpose of this study was obtained on July, 13, 2021 (approval number 21.13).
-
2.
What data in particular will be shared? Not applicable.
-
3.
What other documents will be available? Not applicable.
-
4.
When will data be available (start and end dates)? Not applicable.
-
5.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article’.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.eurox.2024.100356.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371 doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purisch S.E., Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017;41(7):387–391. doi: 10.1053/j.semperi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Esplin M.S., O’Brien E., Fraser A., et al. Estimating recurrence of spontaneous preterm delivery. Obstet Gynecol. 2008;112(3):516–523. doi: 10.1097/AOG.0b013e318184181a. [DOI] [PubMed] [Google Scholar]
- 4.Bloom S.L., Yost N.P., McIntire D.D., Leveno K.J. Recurrence of preterm birth in singleton and twin pregnancies. Obstet Gynecol. 2001;98(3):379–385. doi: 10.1016/S0029-7844(01)01466-1. [DOI] [PubMed] [Google Scholar]
- 5.Phillips C., Velji Z., Hanly C., Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7(6):1–7. doi: 10.1136/bmjopen-2016-015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Care A., Nevitt S.J., Medley N., et al. Interventions to prevent spontaneous preterm birth in women with singleton pregnancy who are at high risk: systematic review and network meta-analysis. BMJ. 2022;376:1–12. doi: 10.1136/bmj-2021-064547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prager S.M., Micks E.M., Dalton, V.K.M. Pregnancy loss (miscarriage): Terminology, risk factors, and etiology. In: UpToDate. Literature.; 2022.
- 8.Di Renzo G.C., Cabero Roura L., Facchinetti F., et al. Preterm labor and birth management: recommendations from the European association of perinatal medicine. J Matern Neonatal Med. 2017;30(17):2011–2030. doi: 10.1080/14767058.2017.1323860. [DOI] [PubMed] [Google Scholar]
- 9.Song Q., Chen J., Zhou Y., Li Z., Li H., Liu J. Preterm delivery rate in China: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2022;22(1):1–9. doi: 10.1186/s12884-022-04713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PERINED. Perinatale Zorg in Nederland Anno 2020: Duiding Door Landelijke Perinatale Audit En Registratie. Utrecht; 2020.
- 11.Sneider K., Christiansen O.B., Sundtoft I.B., Langhoff-Roos J. Recurrence of second trimester miscarriage and extreme preterm delivery at 16–27 weeks of gestation with a focus on cervical insufficiency and prophylactic cerclage. Acta Obstet Gynecol Scand. 2016;95(12):1383–1390. doi: 10.1111/aogs.13027. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg R.L., Mayberry S.K., Copper R.L., Dubard M.B., Hauth J.C. Pregnancy outcome following a second-trimester loss. Obstet Gynaecol. 1993;81(3):444–446. [PubMed] [Google Scholar]
- 13.Edlow A.G., Srinivas S.K., Elovitz M.A. Second-trimester loss and subsequent pregnancy outcomes: what is the real risk? Am J Obstet Gynecol. 2007;197(6):581. doi: 10.1016/j.ajog.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Linehan L.A., Morris A.G., Meaney S., O’Donoghue K. Subsequent pregnancy outcomes following second trimester miscarriage—a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2019;237:198–203. doi: 10.1016/j.ejogrb.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Oliver-Williams C., Fleming M., Wood A.M., Smith G.C.S. Previous miscarriage and the subsequent risk of preterm birth in Scotland, 1980-2008: a historical cohort study. BJOG Int J Obstet Gynaecol. 2015;122(11):1525–1534. doi: 10.1111/1471-0528.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey M.A., Flood K., Burke N., et al. Perinatal outcomes of women with a prior history of unexplained recurrent miscarriage. J Matern Neonatal Med. 2015;28(5):522–525. doi: 10.3109/14767058.2014.923394. [DOI] [PubMed] [Google Scholar]
- 17.Field K., Murphy D.J. Perinatal outcomes in a subsequent pregnancy among women who have experienced recurrent miscarriage: a retrospective cohort study. Hum Reprod. 2015;30(5):1239–1245. doi: 10.1093/humrep/dev044. [DOI] [PubMed] [Google Scholar]
- 18.Wu C.Q., Nichols K., Carwana M., Cormier N.M.C. Preterm birth after recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2022;117(4):811–819. doi: 10.1016/j.fertnstert.2022.01.004. 〈https://pubmed.ncbi.nlm.nih.gov/35131102/〉 [DOI] [PubMed] [Google Scholar]
- 19.Berghella V., Rafael T.J., Szychowski J.M., Rust O.A., Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol. 2011;117(3):663–671. doi: 10.1097/AOG.0b013e31820ca847. [DOI] [PubMed] [Google Scholar]
- 20.Nederlandse Vereniging voor Obsterie en Gynaecologie. Preventie Recidief Spontane Vroeggeboorte Versie 1.0.; 2007.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material