Abstract
Periodontitis and diabetes mellitus are two prevalent chronic diseases that have been recognized to exhibit a bidirectional relationship. Individuals with diabetes are more susceptible to periodontitis, and conversely, periodontitis can exacerbate glycemic control in diabetic patients. The underlying mechanisms of this interrelationship involve complex pathways, including inflammatory responses, altered immune functions, and microbial dysbiosis. The mechanistic insights into the interrelationship between periodontitis and diabetes mellitus revolve around the role of inflammation as a common link between the two diseases. Inflammatory mediators such as cytokines, chemokines, and prostaglandins play a crucial role in the pathogenesis and progression of the diseases. The dysregulation of the immune response in diabetes can exacerbate the inflammatory response in periodontitis, leading to increased tissue destruction and bone resorption. The chronic inflammation in periodontitis can contribute to insulin resistance and impaired glycemic control in diabetic patients. Future directions in research aim to further elucidate the molecular mechanisms underlying the interrelationship between periodontitis and diabetes mellitus. Modulating the inflammatory response, restoring microbial balance, and improving glycemic control hold promise in managing both conditions simultaneously. Herein, we will provide an overview of the interrelationship of periodontitis and diabetes mellitus, and retrospect the underlying mechanisms, which may inspire investigators with further research directions.
Keywords: Periodontitis, Diabetes mellitus, Peri-implant disease, Microbial balance, Alveolar bone resorption
1. Introduction
Periodontitis and diabetes mellitus are two prevalent chronic diseases that have a complex bidirectional relationship [1,2]. The interaction between these two conditions has been widely recognized and studied due to their shared underlying mechanisms and impact on each other's progression and severity [3,4]. First and foremost, it is essential to understand the individual characteristics of periodontitis and diabetes mellitus. Periodontitis is a chronic inflammatory disease that affects the supporting structures of the teeth, leading to alveolar bone destruction and potentially tooth loss if left untreated [5]. On the other hand, diabetes mellitus (Fig. 1), including type 1, type 2, gestational, and some other types (e.g., heredity, infection-induced, and drug-induced), is a metabolic disorder characterized by elevated blood sugar levels resulting from insulin dysfunction [6,7]. Individuals with type 1 diabetes are at a higher risk of developing periodontitis compared to those without diabetes [8,9]. Studies have shown that the prevalence of periodontitis in type 1 diabetics can range from 10 % to 46 %, depending on age and disease duration [[10], [11], [12]]. Type 2 diabetes is more common and also significantly increases the risk of periodontitis. The prevalence of periodontitis in type 2 diabetics is generally higher, with estimates ranging from 30 % to 60 %, again varying with age, disease control, and other factors [13,14]. In the general population without diabetes, the prevalence of periodontitis varies widely based on age, geographic location, and other risk factors [15]. According to various studies, the prevalence of periodontitis in adults without diabetes can range from approximately 20 % to over 50 %, with severe periodontitis affecting about 5 % to 15 % of adults globally [[16], [17], [18]]. Both conditions have systemic implications and can significantly impact an individual's overall health and quality of life. The bidirectional nature of the relationship between periodontitis and diabetes mellitus is well-established [19]. Individuals with diabetes are more susceptible to periodontitis, with studies indicating a higher prevalence and severity of periodontal disease in diabetic patients compared to non-diabetic individuals [20]. Conversely, periodontitis can adversely affect glycemic control in diabetic patients, leading to complications and exacerbation of the metabolic disorder [[21], [22], [23]].
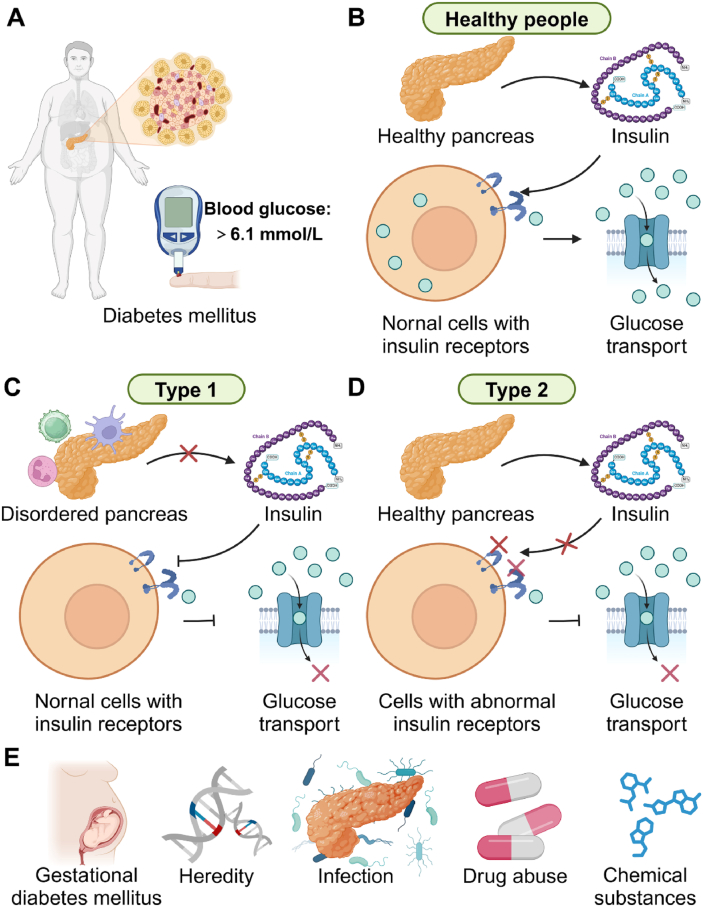
Fig. 1.
Types and occurrence mechanism of diabetes mellitus. (A) The fasting blood glucose concentration is more than 6.1 mmol/L, which may be diagnosed as diabetes mellitus. (B) For health people, the insulin is normally secreted, regularly interfacing with the receptors, finally completing blood glucose transport. (C) Type 1 diabetes is an autoimmune condition where the body's immune system attacks and destroys insulin-producing beta cells in the pancreas, leading to insulin deficiency. (D) Type 2 diabetes is a progressive condition where the body becomes resistant to the effects of insulin or doesn't produce enough insulin to maintain normal glucose levels. (E) For some idiopathic and secondary diabetes mellitus, the pregnancy, heredity, infection, drug abuse, and chemical substances would be considered as etiological factors.
The connection between periodontitis and diabetes mellitus is complex and involves various underlying mechanisms [24]. Inflammatory responses, altered immune functions, and microbial dysbiosis all play pivotal roles in linking these two conditions [24]. In particular, inflammation emerges as a common pathway through which periodontitis and diabetes mellitus influence each other [25]. The dysregulation of inflammatory mediators such as cytokines, chemokines, and prostaglandins contributes to the pathogenesis and progression of both diseases [25,26]. Moving forward, future directions in research aim to delve deeper into the molecular mechanisms underpinning the interrelationship between periodontitis and diabetes mellitus. Targeted therapies that modulate the inflammatory response, restore microbial balance, and improve glycemic control hold promise in managing both conditions simultaneously [7]. Interdisciplinary collaboration between dental and medical professionals will be crucial in providing comprehensive care and optimizing outcomes for individuals with comorbid periodontitis and diabetes mellitus [10].
The interaction between periodontitis and diabetes mellitus is a complex and multifaceted phenomenon that has important implications for clinical practice and public health. By comprehending the intricate relationship between these two chronic diseases, we can develop more effective strategies for prevention and treatment that address the underlying causes and improve outcomes for affected individuals [10,27,28]. This review sets the stage for a detailed exploration of the epidemiological evidence, mechanistic insights, and future research directions concering the interrelationship between periodontitis and diabetes mellitus, which will be further explored in subsequent sections.
2. Overview of periodontitis and diabetes mellitus
2.1. Etiology and pathogenesis of periodontitis
Periodontitis is a chronic inflammatory disease that affects the supporting structures of the teeth, including the gingiva, periodontal ligament, and alveolar bone. The etiology and pathogenesis of periodontitis are multifactorial, involving complex interactions between microbial factors, host immune responses, and environmental influences [[29], [30], [31]]. Understanding the underlying mechanisms of periodontitis is crucial for developing effective prevention and treatment strategies.
Microbial dysbiosis (Fig. 2) plays a central role in the pathogenesis of periodontitis [32]. The oral microbiota consists of a diverse community of bacteria more than 700 species, with some species (e.g., Streptococcus and Neisseria) being beneficial for oral health, while others (e.g., Porphyromonas gingivalis, Tannernella forsythia, and Treponema denticola) are pathogenic and contribute to disease development [32,33]. Dysbiotic changes in the oral microbiome, characterized by an overgrowth of pathogenic bacteria and a decrease in beneficial species, disrupt the microbial balance and promote inflammation within the periodontal tissues, which could influence the microbial community structure in other tissues (e.g., gut and intestinal tract) [34,35].
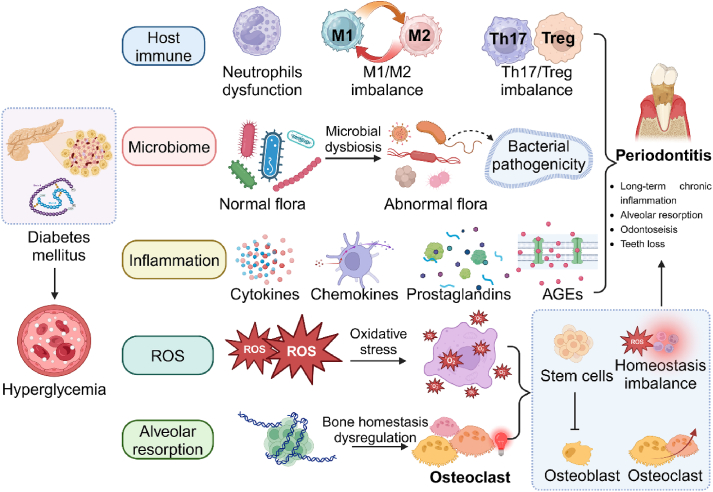
Fig. 2.
The interrelationship and association mechanism between periodontitis and diabetes mellitus. The etiology and pathogenesis of periodontitis are multifactorial, involving complex interactions between microbial factors, host immune responses, and environmental influences (e.g., location inflammation, ROS, and alveolar resorption), which can exacerbate the inflammatory response and contribute to disease progression.
The host immune response (Fig. 2) to periodontal pathogens is another key aspect of periodontitis pathogenesis [36]. In response to bacterial invasion, the host immune system mounts an inflammatory response characterized by the release of pro-inflammatory cytokines, chemokines, and other mediators [37]. Chronic inflammation leads to tissue destruction, including gingival inflammation, periodontal ligament breakdown, and alveolar bone resorption, ultimately resulting in tooth mobility and tooth loss if left untreated [38]. Previous studies have found that there existed some differences in immune cells (e.g., Treg/Th17, macrophage, and neutrophil) between the patients with and without periodontitis [[38], [39], [40]].
Genetic and environmental factors (Fig. 2) also contribute to the susceptibility to periodontitis [41]. Genetic polymorphisms in genes encoding immune response molecules, such as cytokines and toll-like receptors, can influence an individual's susceptibility to periodontal disease [42]. Environmental factors, such as reactive oxygen species (ROS), smoking, diabetes, and poor oral hygiene, can exacerbate the inflammatory response and contribute to disease progression [43].
The bidirectional relationship (Fig. 3A) between periodontitis and diabetes mellitus is of particular interest due to the impact of diabetes on periodontal health. Individuals with diabetes are more susceptible to periodontitis due to impaired immune function, reduced wound healing capacity, and increased levels of glucose in the gingival crevicular fluid, providing a favorable environment for bacterial growth. Poorly controlled diabetes exacerbates periodontal inflammation and tissue destruction, leading to more severe periodontal complications. Moreover, diabetes mellitus has been recognized as a risk factor for periodontitis and peri-implantitis [44,45].
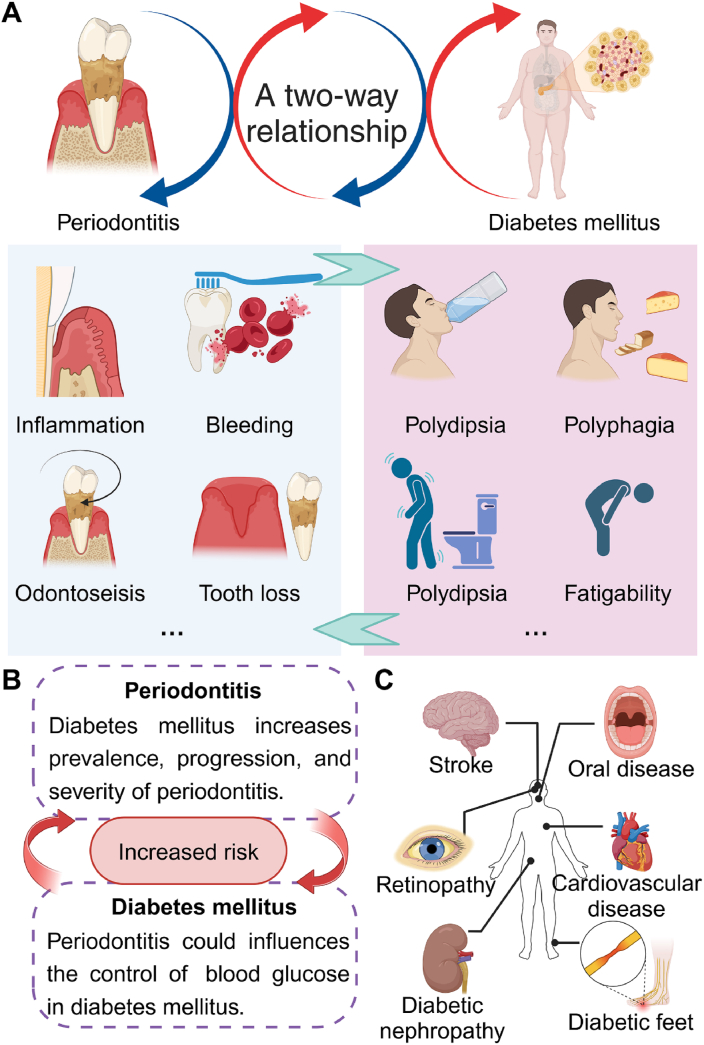
Fig. 3.
The bidirectional relationship between periodontitis and diabetes mellitus is of particular interest due to the impact of diabetes on periodontal health. (A) The relationship between periodontitis and diabetes mellitus is a two-way relationship, and the clinical manifestations of periodontitis and diabetes mellitus, respectively. (B) periodontitis and diabetes mellitus could reciprocally increase risk. Diabetes mellitus could increase the prevalence, progression, and severity of periodontitis, and periodontitis could influence the control of blood glucose in diabetes mellitus. The common complications include cardiovascular diseases, neuropathy, nephropathy, retinopathy, and foot ulcers in diabetes mellitus.
Periodontitis is a multifactorial disease with microbial, immune, genetic, and environmental factors contributing to its etiology and pathogenesis [46]. Understanding the complex interplay between these factors is essential for developing targeted therapies that modulate the host immune response (Fig. 3B), restore microbial balance, and prevent disease progression [46]. Moreover, interdisciplinary collaboration between dental and medical professionals is crucial for comprehensive care and improved outcomes for patients with periodontitis and comorbid conditions such as diabetes mellitus [47].
2.2. Types and complications of diabetes mellitus
Diabetes mellitus is a complex metabolic disorder characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both [48], in which the fasting blood glucose concentration is more than 6.1 mmol/L (Fig. 1A and B). There are several types of diabetes, with type 1 and type 2 being the most common forms [48]. Type 1 diabetes is an autoimmune condition in which the body's immune system attacks and destroys insulin-producing beta cells in the pancreas, leading to insulin deficiency (Fig. 1C). On the other hand, type 2 diabetes (Fig. 1D) is a progressive condition where the body becomes resistant to the effects of insulin or doesn't produce enough insulin to maintain normal glucose levels [48]. Moreover, the gestational diabetes, concomitantly occurring in pregnancy, would also lead to complications and even harm the health of the fetus (Fig. 1E). For some cases of idiopathic and secondary diabetes mellitus, factors such as heredity, infection, drug abuse, and chemical substances should be considered (Fig. 1E).
Complications of diabetes mellitus can affect various organs and systems in the body, potentially leading to serious health issues if left unmanaged [49]. Some common complications include cardiovascular diseases, neuropathy, nephropathy, retinopathy, and foot ulcers (Fig. 3C). Chronic hyperglycemia in diabetes can damage blood vessels and nerves, leading to cardiovascular complications such as heart disease, stroke, and peripheral arterial disease [49]. Neuropathy can result in numbness, tingling, or pain in the extremities, while nephropathy can cause kidney damage and eventually kidney failure [49]. Retinopathy is a common complication of diabetes affecting the eyes, leading to vision impairment and blindness if left untreated. Regular eye examinations are crucial for early detection and management of diabetic retinopathy. Foot ulcers are another significant complication of diabetes, often due to poor circulation and nerve damage in the feet. Proper foot care, including regular inspection, hygiene, and appropriate footwear, is essential to prevent foot ulcers and infections in diabetic patients [49].
The management of diabetes mellitus involves a multidisciplinary approach that includes lifestyle modifications, pharmacological interventions, and monitoring of blood glucose levels [50] (Fig. 4). Diet and exercise play a crucial role in managing diabetes by controlling blood sugar levels and reducing the risk of complications [50]. Medications such as insulin, oral hypoglycemic agents, and other anti-diabetic drugs are prescribed based on the type and severity of diabetes [51]. Regular monitoring of blood glucose levels, HbA1c levels, blood pressure, and cholesterol levels is essential to assess the effectiveness of diabetes management and adjust treatment strategies accordingly [52]. Patient education on self-care practices, medication adherence, and recognizing early signs of complications is vital for optimal diabetes control and improved quality of life.
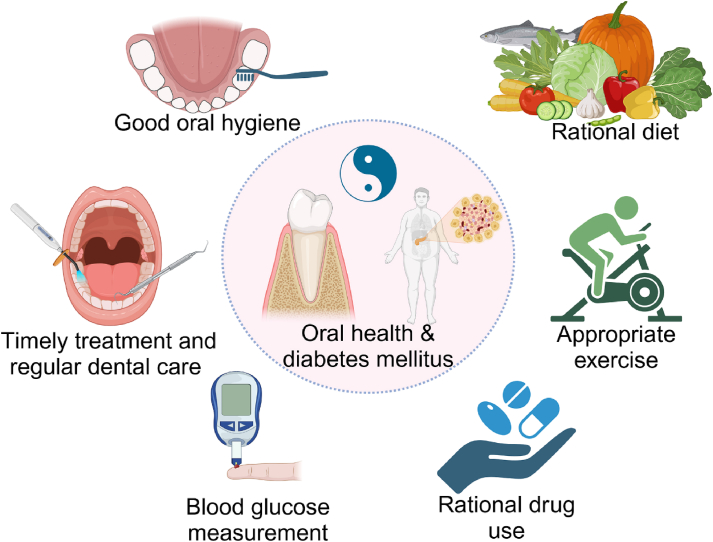
Fig. 4.
Management of diabetes mellitus and oral health involves a multidisciplinary approach that includes lifestyle modifications (e.g., diet and exercise), pharmacological interventions, and monitoring of blood glucose levels. Moreover, the good oral hygiene, timely treatment, and regular dental care are also important for patients with diabetes mellitus to avoid the occurrence of periodontal diseases.
Diabetes mellitus is a complex metabolic disorder with various types and complications that can significantly impact the health and well-being of individuals. Effective management of diabetes requires a comprehensive approach that addresses both the medical and lifestyle aspects of the condition [52]. By maintaining good glycemic control, adhering to treatment regimens, and adopting healthy habits, individuals with diabetes can reduce the risk of complications and enhance their overall quality of life [52].
3. Epidemiological evidence on the interrelationship
Epidemiological evidence plays a crucial role in understanding the interrelationship between periodontitis and diabetes mellitus, which is necessary for the diagnosis and early intervention of the patients suffering from the two chronic diseases [53]. In this section, we will delve into the prevalence of periodontitis in diabetic patients to highlight the impact of these two chronic diseases on each other, and the mutual effects may guide the clinical treatment of such patients [54].
3.1. Prevalence of periodontitis in diabetic patients
Epidemiological studies have consistently shown a higher prevalence and severity of periodontitis in individuals with diabetes compared to non-diabetic individuals [23]. The bidirectional relationship between periodontitis and diabetes mellitus is well-established, with each condition exacerbating the progression and severity of the other. Some studies reported that some young subjects who suffered from unexpected periodontitis showed higher risk in type 1 diabetes mellitus [10,55,56]. Patients with poor control of diabetes also exhibited more rapid and serious progress of attachment and alveolar bone loss compared to those with well-controlled diabetes.
3.2. Risk factors for periodontitis in diabetic patients
Several risk factors contribute to the increased susceptibility of diabetic patients to periodontitis. Poor glycemic control, prolonged hyperglycemia, and altered immune responses in diabetes create an environment conducive to the development and progression of periodontal disease [57]. Additionally, systemic inflammation and impaired wound healing in diabetic individuals further exacerbate the inflammatory response in periodontitis [58]. Previous studies have found that the high glucose levels in gingival crevicular fluid can disturb the microbial balance, and the proportion of pathogenic bacteria was increased, such as aggregatibacter actinomycetem-comitans (Aa) and porphyromonas gingivalis (Pg) [58]. For the influences of diabetes mellitus to the host immune, the function of immune cells has been proven to be subject to change (Fig. 2), such as the dysfunction of neutrophils, polarization imbalance of macrophages, and disorder of T cells in differentiation and function [58,59]. The immune dysregulation could exacerbate the progression of periodontitis. Inflammation is also regarded as a vital risk factor between periodontitis and diabetes mellitus [59]. For diabetic patients, when the inflammation happened, it would be even worse than that without diabetes mellitus. The more intense inflammatory response in diabetic patients would be related to the immune dysregulation, and then some inflammatory products would further intensify the immune response or prolong the immune response course, such as the advanced glycosylation end products (AGEs) [60].
3.3. Mechanisms underlying the increased prevalence of periodontitis in diabetic patients
The pathophysiological mechanisms linking diabetes and periodontitis is a complex interaction of inflammatory mediators, immune responses, and microbial dysbiosis. Diabetic individuals have higher levels of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), which contribute to the chronic inflammatory state observed in periodontitis [61]. Moreover, the dysregulation of immune functions in diabetes compromises the host's ability to combat periodontal pathogens, leading to increased tissue destruction and bone loss.
3.4. Clinical implications and management strategies
The high prevalence of periodontitis in diabetic patients underscores the importance of comprehensive dental care in managing diabetes [36,38]. Dental professionals play a critical role in early detection and treatment of periodontal disease in diabetic individuals to prevent complications and improve overall health outcomes. Integrated approaches that focus on controlling glycemic levels, optimizing oral hygiene practices, and addressing systemic inflammation are essential in managing the interrelationship between periodontitis and diabetes mellitus [32].
In conclusion, the epidemiological evidence supports the bidirectional relationship between periodontitis and diabetes mellitus, highlighting the need for interdisciplinary collaboration and targeted interventions to improve outcomes for individuals with comorbid conditions [3]. By addressing the prevalence of periodontitis in diabetic patients and understanding the underlying mechanisms, healthcare providers can develop effective strategies for the prevention and management of these chronic diseases [19,48].
4. Impact of periodontitis on glycemic control
Epidemiological studies have highlighted the significant impact of periodontitis on glycemic control in individuals with diabetes mellitus [1]. The bidirectional relationship between periodontitis and diabetes mellitus underscores the importance of understanding how periodontal disease can influence the management of diabetes through its effects on glycemic control [59].
Periodontitis, as a chronic inflammatory condition, can exacerbate glycemic control in diabetic patients through various mechanisms [36]. One key mechanism is the release of inflammatory mediators by periodontal tissues in response to bacterial infection [21]. These inflammatory mediators, including cytokines such as IL-1β and TNF-α, can contribute to insulin resistance and impaired glucose utilization in diabetic individuals [10]. The chronic inflammation associated with periodontitis can lead to systemic inflammation, further exacerbating insulin resistance and hindering glycemic control. Moreover, periodontal pathogens and their byproducts can enter the bloodstream through the inflamed periodontal tissues, triggering a cascade of inflammatory responses in distant organs, including the pancreas. This systemic inflammation can disrupt pancreatic function, impairing insulin secretion and exacerbating hyperglycemia in diabetic individuals [51]. The dysbiosis of the oral microbiota in periodontitis can also contribute to systemic inflammation and metabolic disturbances, further compromising glycemic control in diabetes [55].
Furthermore, the chronic nature of periodontitis can lead to persistent low-grade inflammation, which has been linked to the development of insulin resistance and metabolic dysfunction [31]. The presence of periodontal pockets and deep periodontal pockets can serve as reservoirs for pathogenic bacteria, perpetuating the inflammatory response and systemic dissemination of bacterial products that can impact glycemic control in diabetic individuals [6]. Studies have shown that effective periodontal treatment can improve glycemic control in diabetic patients by reducing the inflammatory burden associated with periodontitis. Periodontal therapy, including scaling and root planning, can decrease the levels of inflammatory mediators in the periodontal tissues, leading to a reduction in systemic inflammation and improved insulin sensitivity [61]. Improved periodontal health can also enhance the response to antidiabetic medications and lifestyle interventions, contributing to better glycemic control in individuals with diabetes.
In conclusion, the impact of periodontitis on glycemic control in individuals with diabetes mellitus underscores the need for comprehensive management strategies that address both oral health and systemic health [35]. By recognizing the role of periodontal disease in exacerbating insulin resistance and metabolic dysfunction, healthcare providers can implement integrated care plans that target both conditions simultaneously [53]. Future research should continue to investigate the mechanisms underlying the interplay between periodontitis and diabetes mellitus to optimize treatment approaches and improve outcomes for individuals with comorbid conditions.
5. Mechanistic insights and future directions
The interrelationship between periodontitis and diabetes mellitus is a complex phenomenon that involves intricate mechanistic pathways [24]. Understanding the underlying mechanisms is crucial for developing targeted therapies to manage both conditions simultaneously. In this chapter, we explore the mechanistic insights and future research directions to better understand the interplay between periodontitis and diabetes mellitus.
5.1. Inflammatory responses
In both periodontitis and diabetes mellitus, inflammatory responses play a pivotal role in disease pathogenesis and progression. In periodontitis, microbial dysbiosis triggers an inflammatory cascade, leading to tissue destruction and bone resorption [32]. The release of inflammatory mediators such as cytokines, chemokines, and prostaglandins further exacerbates the inflammatory response, contributing to periodontal complications [33]. In diabetes, chronic inflammation resulting from dysregulated immune responses can lead to systemic complications, including impaired wound healing and increased susceptibility to infections [60]. The crosstalk between periodontal inflammation and systemic inflammation exacerbates the severity of both diseases, creating a vicious cycle that hampers optimal disease management.
5.2. Altered immune functions
The dysregulation of immune functions in diabetes can impact the immune response to periodontal pathogens, exacerbating tissue damage and bone loss in periodontitis [34]. Diabetes-induced immune dysfunction compromises the host's ability to combat oral infections, leading to persistent inflammation and delayed healing in periodontal tissues [43]. Understanding the immune modulations in comorbid periodontitis and diabetes is essential for developing immune-targeted therapies to restore immune homeostasis and mitigate disease progression.
5.3. Microbial dysbiosis
Periodontitis is characterized by a dysbiotic shift in the oral microbiome, with pathogenic bacteria driving inflammatory processes and tissue destruction [62]. The altered microbial composition in periodontitis can impact systemic health, including glycemic control in diabetic individuals [42]. The translocation of oral bacteria into the systemic circulation can elicit immune responses and contribute to low-grade systemic inflammation, exacerbating insulin resistance and metabolic dysfunction in diabetes [63].
Here's a detailed discussion on the potential changes in the oral flora post-treatment and the differences observed between normal conditions and pre- or post-treatment states [64]. Treatment typically involves mechanical removal of plaque and tartar, along with antibiotics if necessary. This can lead to a significant reduction in pathogenic bacteria such as Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola [[65], [66], [67], [68]]. While osteoporosis itself does not directly alter the oral flora, the treatments (like bisphosphonates) might have indirect effects [69]. However, managing periodontitis can help in maintaining a healthier oral environment. Effective periodontal therapy can promote the growth of beneficial bacteria like Streptococcus sanguinis and Actinomyces naeslundii, which are associated with gingival health. Treatment can help in restoring the normal pH balance in the mouth, which is less conducive to the growth of acidophilic bacteria that contribute to dental decay and periodontal disease.
The oral cavity harbors a diverse microbiota, including both commensal and pathogenic bacteria in a balanced state. The pH is relatively neutral, and the gingival sulcus is shallow with minimal inflammation. There is an overgrowth of pathogenic bacteria, leading to a dysbiosis [70]. The pH might be more acidic due to increased production of organic acids by these bacteria. Although osteoporosis does not directly alter the oral flora, patients might have increased susceptibility to periodontal disease due to reduced bone density and possibly altered immune responses. Post-treatment, there should be a significant reduction in pathogenic bacteria, and the oral flora starts to resemble a healthier state. The pH should normalize, and gingival inflammation should decrease. Continued management of periodontal health can help maintain a balanced oral flora, even though osteoporosis treatments might have some side effects on the oral cavity (e.g., bisphosphonate-related osteonecrosis of the jaw).
5.4. Future research directions
Future research endeavors aim to unravel the molecular mechanisms that underpin the interrelationship between periodontitis and diabetes mellitus [49]. Novel therapeutic strategies targeting specific inflammatory pathways, restoring microbial balance, and improving glycemic control hold promise in managing both conditions effectively [20]. Additionally, exploring the role of the oral-gut axis in mediating the crosstalk between periodontitis and diabetes opens new avenues for understanding disease pathogenesis and developing personalized treatment approaches [28].
Interdisciplinary collaborations between dental and medical professionals are essential for comprehensive patient care and improved clinical outcomes in individuals with comorbid periodontitis and diabetes mellitus [45]. By integrating epidemiological data, mechanistic insights, and innovative research approaches, we can advance our understanding of the complex interplay between periodontitis and diabetes, paving the way for novel therapeutic interventions and improved quality of life for affected individuals [41,54].
6. Conclusion
In this work, we have summarized the interrelationship and the association mechanisms between periodontitis and diabetes mellitus, as well as the understanding of the interaction effects of the both chronic diseases. The underlying mechanism would inspire more researchers conduct further exploration of the combination therapies for periodontitis and diabetes mellitus, and block up the interrelationship and association of them. The interrelationship between periodontitis and diabetes mellitus is a complex and multifaceted phenomenon, and understanding the interplay between these two chronic diseases is crucial for developing effective prevention and treatment strategies that address the underlying mechanisms and improve outcomes for affected individuals. Periodontitis and diabetes affect each other, but whether there is a causal link between the two needs to be further clarified by high-quality cohort studies. In view of the close association between periodontitis and diabetes, patients' visit to the stomatology department provides a possible way to screen for diabetes. Dental medical workers should pay attention to the diagnosis and treatment of patients with periodontitis and diabetes, provide oral health education and perfect periodontal treatment plan for diabetes patients in time, and fully consider the specific situation of blood sugar control and the overall health status of patients during periodontal treatment. In clinical work, oral doctors and internal medicine doctors should fully understand the close relationship between the two, and pay attention to the control of another disease while treating one, which may improve the control effect of the disease.
Author contributions
Yongqiang Yang and Xia Sun contributed to review of the literature and manuscript writing. Yucheng Yang and Yingchun Qie designed the concept of the review and offered scientific editing. All authors were responsible for the designed critical revision of the manuscript. All authors have seen and approved the manuscript during the sub-mission process. All authors have accepted responsibility for the entire content of this manuscript and approved its final version and submission.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
All figures in this work were created by Biorender.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Yongqiang Yang, Email: 15953606977@163.com.
Xia Sun, Email: xiami102722@163.com.
Yucheng Yang, Email: yangyucheng2003@163.com.
Yingchun Qie, Email: qyc198512@126.com.
References
- 1.Wu C.Z., Yuan Y.H., Liu H.H., Li S.S., Zhang B.W., Chen W., et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20(1):204. doi: 10.1186/s12903-020-01180-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudiyirickal M.G., Pappachan J.M. Periodontitis: an often-neglected complication of diabetes. World J Diabetes. 2024;15(3):318–325. doi: 10.4239/wjd.v15.i3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson T.C., Clarkson J.E., Worthington H.V., MacDonald L., Weldon J.C., Needleman I., et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2022;4(4) doi: 10.1002/14651858.CD004714.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akazawa H. Periodontitis and Diabetes Mellitus: Be true to your teeth. Int Heart J. 2018;59(4):680–682. doi: 10.1536/ihj.18-410. [DOI] [PubMed] [Google Scholar]
- 5.Bitencourt F.V., Nascimento G.G., Costa S.A., Andersen A., Sandbæk A., Leite F.R.M. Co-Occurrence of periodontitis and diabetes-related complications. J Dent Res. 2023;102(10):1088–1097. doi: 10.1177/00220345231179897. [DOI] [PubMed] [Google Scholar]
- 6.Zhao M., Xie Y., Gao W., Li C., Ye Q., Li Y. Diabetes mellitus promotes susceptibility to periodontitis-novel insight into the molecular mechanisms. Front Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1192625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendek M.J., Canedo-Marroquín G., Realini O., Retamal I.N., Hernández M., Hoare A., et al. Periodontitis and gestational diabetes mellitus: a potential inflammatory vicious cycle. Int J Mol Sci. 2021;22(21) doi: 10.3390/ijms222111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triebl Z., Bencze B., Bányai D., Rózsa N., Hermann P., Végh D. Poor glycemic control impairs oral health in children with type 1 diabetes mellitus - a systematic review and meta-analysis. BMC Oral Health. 2024;24(1):748. doi: 10.1186/s12903-024-04516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duda-Sobczak A., Zozulinska-Ziolkiewicz D., Wyganowska-Swiatkowska M. Type 1 diabetes and periodontal health. Clin Therapeut. 2018;40(6):823–827. doi: 10.1016/j.clinthera.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Preshaw P.M., Alba A.L., Herrera D., Jepsen S., Konstantinidis A., Makrilakis K., et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graves D.T., Ding Z., Yang Y. The impact of diabetes on periodontal diseases. Periodontol 2000. 2020;82(1):214–224. doi: 10.1111/prd.12318. [DOI] [PubMed] [Google Scholar]
- 12.Reddy M., Gopalkrishna P. Type 1 diabetes and periodontal disease: a literature review, Canadian journal of dental hygiene : CJDH = Journal canadien de l'hygiene dentaire : JCHD. 2022;56(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Cao X., Huo P., Li W., Li P., He L., Meng H. Interactions among moderate/severe periodontitis, ADIPOQ-rs1501299, and LEPR-rs1137100 polymorphisms on the risk of type 2 diabetes in a Chinese population. Arch Oral Biol. 2019;103:26–32. doi: 10.1016/j.archoralbio.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Pavankumar S., Yellarthi P.K., Jn S., Boyapati R., Damera T.K., N.V.K. G Evaluation of periodontal status in women with polycystic ovary syndrome versus healthy women: a cross-sectional study. J Yeungnam Med Sci. 2023;40(Suppl):S17–s22. doi: 10.12701/jyms.2023.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun G., Wu K., Hu T., Bao C., Zhong Y., Yin W. Analysis of the willingness of community residents of Chengdu city to participate in various modes of family dental services and the factors influencing their decision. Hua xi kou qiang yi xue za zhi = Huaxi kouqiang yixue zazhi = West China J Stomat. 2022;40(1):80–85. doi: 10.7518/hxkq.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao J., Jing W., Si Y., Feng X., Tai B., Hu D., et al. The prevalence and severity of periodontal disease in mainland China: data from the Fourth National Oral Health Survey (2015-2016) J Clin Periodontol. 2021;48(2):168–179. doi: 10.1111/jcpe.13396. [DOI] [PubMed] [Google Scholar]
- 17.Sun H., Du M., Tai B., Chang S., Wang Y., Jiang H. Prevalence and associated factors of periodontal conditions among 55- to 74-year-old adults in China: results from the 4th National Oral Health Survey. Clin Oral Invest. 2020;24(12):4403–4412. doi: 10.1007/s00784-020-03306-4. [DOI] [PubMed] [Google Scholar]
- 18.Sun H.Y., Jiang H., Du M.Q., Wang X., Feng X.P., Hu Y., et al. The prevalence and associated factors of periodontal disease among 35 to 44-year-old Chinese adults in the 4th National Oral Health Survey. Chin J Dent Res. 2018;21(4):241–247. doi: 10.3290/j.cjdr.a41082. [DOI] [PubMed] [Google Scholar]
- 19.Nibali L., Gkranias N., Mainas G., Di Pino A. Periodontitis and implant complications in diabetes. Periodontol 2000. 2022;90(1):88–105. doi: 10.1111/prd.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Aiuto F., Gkranias N., Bhowruth D., Khan T., Orlandi M., Suvan J., et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial, the lancet. Diab Endocrinol. 2018;6(12):954–965. doi: 10.1016/S2213-8587(18)30038-X. [DOI] [PubMed] [Google Scholar]
- 21.Cruz D.F.D., Duarte P.M., Figueiredo L.C., da Silva H.D.P., Retamal-Valdes B., Feres M., et al. Metronidazole and amoxicillin for patients with periodontitis and diabetes mellitus: 5-year secondary analysis of a randomized controlled trial. J Periodontol. 2021;92(4):479–487. doi: 10.1002/JPER.20-0196. [DOI] [PubMed] [Google Scholar]
- 22.Liccardo D., Cannavo A., Spagnuolo G., Ferrara N., Cittadini A., Rengo C., et al. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. 2019;20(6) doi: 10.3390/ijms20061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kocher T., König J., Borgnakke W.S., Pink C., Meisel P. Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontol 2000. 2018;78(1):59–97. doi: 10.1111/prd.12235. [DOI] [PubMed] [Google Scholar]
- 24.Ghallab N.A., Amr E.M., Shaker O.G. Expression of leptin and visfatin in gingival tissues of chronic periodontitis with and without type 2 diabetes mellitus: a study using enzyme-linked immunosorbent assay and real-time polymerase chain reaction. J Periodontol. 2015;86(7):882–889. doi: 10.1902/jop.2015.140434. [DOI] [PubMed] [Google Scholar]
- 25.Sun W.L., Chen L.L., Zhang S.Z., Wu Y.M., Ren Y.Z., Qin G.M. Inflammatory cytokines, adiponectin, insulin resistance and metabolic control after periodontal intervention in patients with type 2 diabetes and chronic periodontitis. Intern Med. 2011;50(15):1569–1574. doi: 10.2169/internalmedicine.50.5166. [DOI] [PubMed] [Google Scholar]
- 26.Sun W.L., Chen L.L., Zhang S.Z., Ren Y.Z., Qin G.M. Changes of adiponectin and inflammatory cytokines after periodontal intervention in type 2 diabetes patients with periodontitis. Arch Oral Biol. 2010;55(12):970–974. doi: 10.1016/j.archoralbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Baeza M., Morales A., Cisterna C., Cavalla F., Jara G., Isamitt Y., et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci : Revista FOB. 2020;28 doi: 10.1590/1678-7757-2019-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauri-Obradors E., Merlos A., Estrugo-Devesa A., Jané-Salas E., López-López J., Viñas M. Benefits of non-surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: a randomized controlled trial. J Clin Periodontol. 2018;45(3):345–353. doi: 10.1111/jcpe.12858. [DOI] [PubMed] [Google Scholar]
- 29.Corbella S., Calciolari E., Donos N., Alberti A., Ercal P., Francetti L. Laser treatments as an adjunct to non-surgical periodontal therapy in subjects with periodontitis and type 2 diabetes mellitus: a systematic review and meta-analysis. Clin Oral Invest. 2023;27(4):1311–1327. doi: 10.1007/s00784-023-04873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva-Junior P.G.B., Abreu L.G., Costa F.O., Cota L.O.M., Esteves-Lima R.P. The effect of antimicrobial photodynamic therapy adjunct to non-surgical periodontal therapy on the treatment of periodontitis in individuals with type 2 diabetes mellitus: a systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2023;42 doi: 10.1016/j.pdpdt.2023.103573. [DOI] [PubMed] [Google Scholar]
- 31.Beck J.D., Papapanou P.N., Philips K.H., Offenbacher S. Periodontal medicine: 100 Years of progress. J Dent Res. 2019;98(10):1053–1062. doi: 10.1177/0022034519846113. [DOI] [PubMed] [Google Scholar]
- 32.Graves D.T., Corrêa J.D., Silva T.A. The oral microbiota is modified by systemic diseases. J Dent Res. 2019;98(2):148–156. doi: 10.1177/0022034518805739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Y., Kim H.J., Song J.M., Kang J., Lee H., Park H.R., et al. Differential microbiota network in gingival tissues between periodontitis and periodontitis with diabetes. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.1061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J., Lu H., Wu H., Huang S., Chen L., Gui Q., et al. Periodontitis in elderly patients with type 2 diabetes mellitus: impact on gut microbiota and systemic inflammation. Aging. 2020;12(24):25956–25980. doi: 10.18632/aging.202174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren Y., Hao L., Liu J., Wang P., Ding Q., Chen C., et al. Alterations in the gut microbiota in pregnant women with pregestational type 2 diabetes mellitus. mSystems. 2023;8(2) doi: 10.1128/msystems.01146-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunte K., Beikler T. Th17 cells and the IL-23/IL-17 Axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20(14) doi: 10.3390/ijms20143394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirih F.Q., Monajemzadeh S., Singh N., Sinacola R.S., Shin J.M., Chen T., et al. Association between metabolic syndrome and periodontitis: the role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol 2000. 2021;87(1):50–75. doi: 10.1111/prd.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang L., Zhang J., Fang M., Qin Y., Huang Y., Tao R. Analysis of subgingival micro-organisms based on multi-omics and Treg/Th17 balance in type 2 diabetes with/without periodontitis. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.939608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi B., Lux R., Klokkevold P., Chang M., Barnard E., Haake S., et al. The subgingival microbiome associated with periodontitis in type 2 diabetes mellitus. ISME J. 2020;14(2):519–530. doi: 10.1038/s41396-019-0544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abusleme L., Hoare A., Hong B.Y., Diaz P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontol 2000. 2021;86(1):57–78. doi: 10.1111/prd.12362. [DOI] [PubMed] [Google Scholar]
- 41.Kinane D.F., Stathopoulou P.G., Papapanou P.N. Periodontal diseases. Nat Rev Dis Prim. 2017;3 doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 42.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 43.Larsson L., Kavanagh N.M., Nguyen T.V.N., Castilho R.M., Berglundh T., Giannobile W.V. Influence of epigenetics on periodontitis and peri-implantitis pathogenesis. Periodontol 2000. 2022;90(1):125–137. doi: 10.1111/prd.12453. [DOI] [PubMed] [Google Scholar]
- 44.Darby I. Risk factors for periodontitis & peri-implantitis. Periodontol 2000. 2022;90(1):9–12. doi: 10.1111/prd.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanz M., Ceriello A., Buysschaert M., Chapple I., Demmer R.T., Graziani F., et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45(2):138–149. doi: 10.1111/jcpe.12808. [DOI] [PubMed] [Google Scholar]
- 46.Chapple I.L., Bouchard P., Cagetti M.G., Campus G., Carra M.C., Cocco F., et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl 18):S39–s51. doi: 10.1111/jcpe.12685. [DOI] [PubMed] [Google Scholar]
- 47.Pamuk F., Kantarci A. Inflammation as a link between periodontal disease and obesity. Periodontol 2000. 2022;90(1):186–196. doi: 10.1111/prd.12457. [DOI] [PubMed] [Google Scholar]
- 48.The Prevention of Diabetes Mellitus JAMA. 2021;325(2):190. doi: 10.1001/jama.2020.17738. [DOI] [PubMed] [Google Scholar]
- 49.Boyko E.J., Zelnick L.R., Braffett B.H., Pop-Busui R., Cowie C.C., Lorenzi G.M., et al. Risk of foot ulcer and lower-extremity amputation among participants in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2022;45(2):357–364. doi: 10.2337/dc21-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.10 Cardiovascular disease and risk management: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179–s218. doi: 10.2337/dc24-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subramanian S., Khan F., Hirsch I.B. New advances in type 1 diabetes. BMJ. 2024;384 doi: 10.1136/bmj-2023-075681. [DOI] [PubMed] [Google Scholar]
- 52.Perrier Q., Moro C., Lablanche S. Diabetes in spotlight: current knowledge and perspectives of photobiomodulation utilization. Front Endocrinol. 2024;15 doi: 10.3389/fendo.2024.1303638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carter W., Tiwari T., Elangovan S., Johnson L., Parsegian K., Chandrasekaran S. Patient awareness of the association between periodontal and systemic diseases in an academic setting. J Periodontol. 2024 doi: 10.1002/JPER.23-0635. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Lalla E., Papapanou P.N. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7(12):738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 55.Dicembrini I., Serni L., Monami M., Caliri M., Barbato L., Cairo F., et al. Type 1 diabetes and periodontitis: prevalence and periodontal destruction-a systematic review. Acta Diabetol. 2020;57(12):1405–1412. doi: 10.1007/s00592-020-01531-7. [DOI] [PubMed] [Google Scholar]
- 56.Sun K.T., Chen S.C., Lin C.L., Hsu J.T., Chen I.A., Wu I.T., et al. The association between Type 1 diabetes mellitus and periodontal diseases. J Formosan Med Assoc= Taiwan yi zhi. 2019;118(6):1047–1054. doi: 10.1016/j.jfma.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Li M., Huang S., Zhang Y., Song Z., Fu H., Lin Z., et al. Regulation of the unfolded protein response transducer IRE1α by SERPINH1 aggravates periodontitis with diabetes mellitus via prolonged ER stress. Cell Signal. 2022;91 doi: 10.1016/j.cellsig.2022.110241. [DOI] [PubMed] [Google Scholar]
- 58.de Oliveira P., Bonfante E.A., Bergamo E.T.P., de Souza S.L.S., Riella L., Torroni A., et al. Obesity/metabolic syndrome and diabetes mellitus on peri-implantitis. Trends Endocrinol Metabol: TEM (Trends Endocrinol Metab) 2020;31(8):596–610. doi: 10.1016/j.tem.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Javed F., Romanos G.E. Chronic hyperglycemia as a risk factor in implant therapy. Periodontol 2000. 2019;81(1):57–63. doi: 10.1111/prd.12283. [DOI] [PubMed] [Google Scholar]
- 60.Huo S., Wang Q., Shi W., Peng L., Jiang Y., Zhu M., et al. ATF3/SPI1/SLC31A1 signaling promotes cuproptosis induced by advanced glycosylation end products in diabetic myocardial injury. Int J Mol Sci. 2023;24(2) doi: 10.3390/ijms24021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iacopino A.M., Cutler C.W. Pathophysiological relationships between periodontitis and systemic disease: recent concepts involving serum lipids. J Periodontol. 2000;71(8):1375–1384. doi: 10.1902/jop.2000.71.8.1375. [DOI] [PubMed] [Google Scholar]
- 62.Nakahara Y., Ozaki K., Matsuura T. Long-term hyperglycemia naturally induces dental caries but not periodontal disease in type 1 and type 2 diabetic rodents. Diabetes. 2017;66(11):2868–2874. doi: 10.2337/db17-0291. [DOI] [PubMed] [Google Scholar]
- 63.Salvi G.E., Carollo-Bittel B., Lang N.P. Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. J Clin Periodontol. 2008;35(8 Suppl):398–409. doi: 10.1111/j.1600-051X.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 64.Jansen P., Conrads G., Wenzler J.S., Krause F., Braun A. Bacteremia prevention during periodontal treatment-an in vivo feasibility study. Antibiotics. 2023;12(10) doi: 10.3390/antibiotics12101555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Itoh N., Akazawa N., Ishibana Y., Hamada S., Hagiwara S., Murakami H. Femoral osteomyelitis caused by oral anaerobic bacteria with mixed bacteremia of Campylobacter rectus and Parvimonas micra in a chronic periodontitis patient: a case report. BMC Infect Dis. 2022;22(1):613. doi: 10.1186/s12879-022-07573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shao A., He Q., Jiao X., Liu J. Hemoptysis caused by Parvimonas micra: case report and literature review. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1307902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe T., Hara Y., Yoshimi Y., Fujita Y., Yokoe M., Noguchi Y. Clinical characteristics of bloodstream infection by Parvimonas micra: retrospective case series and literature review. BMC Infect Dis. 2020;20(1):578. doi: 10.1186/s12879-020-05305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duan Y., Feng W., Shen Y., Li Y., Li N., Chen X., et al. Severe pneumonia with empyema caused by Parvimonas micra and Streptococcus constellatus co-infection: a case report. J Int Med Res. 2023;51(11) doi: 10.1177/03000605231210657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pishan B., Andrukhov O., Rausch-Fan X. Dental implant failure in post-menopausal women on oral bisphosphonates: a systematic review and meta-analysis. J Oral Implantol. 2024;50(3):288–295. doi: 10.1563/aaid-joi-D-23-00069. [DOI] [PubMed] [Google Scholar]
- 70.Baker J.L., Mark Welch J.L., Kauffman K.M., McLean J.S., He X. The oral microbiome: diversity, biogeography and human health. Nat Rev Microbiol. 2024;22(2):89–104. doi: 10.1038/s41579-023-00963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]