Abstract
Objective
To evaluate changes in hemoglobin A1c (HbA1c) levels and z-height over 3 years based on continuous glucose monitoring (CGM) usage among children with new-onset type 1 diabetes (T1DM) from various Latin American centers.
Study design
Data on z-height, CGM access, and HbA1c (%) were collected for Latin American children aged 6 months to 18 years with T1DM onset from 19 centers in a retrospective analysis of medical records, from 2020 to 2023. A 2-way ANOVA method with repeated measures and multiple regression analyses were performed.
Results
We included 433 children (46.0% female) aged 8.7 ± 3.7 years; 199 (45.9%) used CGM. The mean HbA1c was significantly lower in years 1, 2, and 3 than at baseline in children with CGM, but not those without CGM. The z-height decreased significantly with the years in both groups. However, the CGM users showed a significantly greater height in years 2 and 3 than the nonusers. Multiple linear regression analysis showed that CGM users exhibited a significantly lower incremental area under the curve (AUC) for HbA1c during follow-up than nonusers. Furthermore, a lower incremental AUC for HbA1c was associated with a smaller decremental AUC for z-height (R2 = 0.19). Multiple logistic regression analysis revealed that children with CGM were 80% more likely (OR, 0.22; 95% CI, 0.1-0.6) to achieve an HbA1c of <7% in the third year of follow-up.
Conclusions
This study reveals a significant association between CGM use and lower HbA1c from the onset of T1DM over a 3-year follow-up in Latin American children. Further prospective studies should be performed to confirm this finding.
Keywords: Latin American children, type 1 diabetes, height, CGM
Several studies have demonstrated that continuous glucose monitoring (CGM) is an effective tool for improving glycemic outcomes and enhancing the quality of life for children with type 1 diabetes (T1DM).1, 2, 3 Most children and adolescents with T1DM find it challenging to reach the recommended glycemic targets.1 Additionally, CGMs provide noninvasive glucose values and trends almost immediately, along with rate-of-change arrows indicating the direction and speed of glucose fluctuations.2,3 This information helps children with T1DM and their caregivers to adjust daily insulin doses.2,3 However, glucose levels can fluctuate significantly throughout the day owing to various activities, creating challenges for effective management.4
Several studies have highlighted significant socioeconomic disparities in CGM prescriptions.2,5 Individuals with T1DM from lower socioeconomic backgrounds, historically marginalized groups, those with limited internet access, and those facing transportation issues are lagging behind in crucial diabetes outcomes, including the adoption of CGM technology.2 Additionally, research has shown a connection between metabolic control and factors such as race/ethnicity, socioeconomic background, CGM use, and hemoglobin A1c (HbA1c) levels.6,7 Although earlier studies suggest that integrating CGM can potentially reduce HbA1c levels in children, there is limited research on the impact of CGM on HbA1c and z-height in Latin American children newly diagnosed with T1DM. This retrospective study aims to evaluate the changes in HbA1c and z-height over 3 years based on CGM usage among children with new-onset T1DM from several Latin American centers.
Methods
A retrospective analysis of medical records examined the trends in HbA1c, z-height, and insulin doses in children with new-onset T1DM who used CGM across 19 centers from 2020 to 2023 in 5 Latin American countries: Argentina, Chile, Paraguay, Peru, and Uruguay. Nearly all presumed cases of new-onset T1DM in each area were referred to these centers, which were the sole tertiary pediatric care facilities for diabetes in their respective regions. The data collected included age at diabetes onset, sex, date of birth, anthropometric measurements, parents' educational background, type of school attended (public or private), healthcare insurance coverage, HbA1c values (%), daily insulin dose (IU/kg/day), and use of CGM. Parental educational status was categorized based on years of schooling into 2 groups: an elementary school diploma or less and more than an elementary school education.
Exclusion criteria included children <6 months of age and those >18 years old at diagnosis, those with psychiatric disorders, those undergoing corticosteroid therapy, or with genetic syndromes (eg, Prader-Willi syndrome, Down syndrome), pregnant adolescents, and an absence of information regarding age and sex. Moreover, another exclusion criterion was information regarding HbA1c, insulin dose, and height on ≥2 occasions during follow-up. In addition, those children with biologically implausible height values at the time of diagnosis (height z-score of <−4 or >4), as determined by the Centers for Disease Control and Prevention growth charts8 were also excluded.
Children received either regular home blood glucose monitoring or CGM. In the Latin American countries involved in this study, all children had access to free care for routine diabetes management, including necessary medications, regardless of their insurance status. However, when providing CGM, families without health insurance found more prolonged and complex procedures than those with insurance coverage.
To be considered in the CGM group, users should have continuous access for ≥2 follow-up years. Children who used the sensor less than this period were excluded from the CGM group. The CGM used by most children was an intermittently scanned device, specifically the first generation of the Freestyle Libre. However, the 8 children using automated systems used Medtronic devices and the corresponding Guardian 4 CGM.
Each center designated 1 physician to submit the data to avoid duplication. The information was anonymous and reviewed for transcription errors and missing data. The protocol was submitted to each center's ethics review board for approval. Each center obtained approval from its ethics committee with a waiver of consent. The anonymous data were then sent to the coordinating center.
The diagnosis of T1DM was based on the American Diabetes Association criteria.9 Diabetic ketoacidosis (DKA) and severe hypoglycemia were defined according to the International Society for Pediatric and Adolescent Diabetes.10,11 Information on celiac disease was included, and it was determined by measuring tissue transglutaminase antibodies IgA and IgG and anti-endomysial antibodies in the serum. Thyroid function data were collected, including thyrotropin, free T4, thyroglobulin antibodies, and thyroid peroxidase antibodies. Information about acute and chronic complications was also included.
Using the Centers for Disease Control and Prevention z-height enabled comparison with a significant portion of existing published literature. HbA1c (glycated hemoglobin) was expressed as a percentage (National Glycohemoglobin Standardization Program) and measured using the Bio-Rad D-10 Dual Program through ion exchange high-performance liquid chromatography. HbA1c values were mathematically standardized to a reference range of 4.05%-6.05% as per the Diabetes Control and Complications Trial using the multiple of the mean method to account for laboratory method variations. Guidelines recommend that all children aim to achieve and maintain an HbA1c level of ≤7.0%.12,13 Consequently, z-height, HbA1c, and insulin dose were measured for all children at after 3 months of admission (T0) and each year closest to the initial measurement date: +1 year, +2 years, and +3 years.
Data Analysis
Descriptive statistics for the variables were presented as mean ± SD. Proportions were compared using χ2 tests, and Fisher's exact test was used when more than 20% of cells had expected frequencies of <5. Health insurance coverage, use of an insulin pump or CGM, presence of celiac disease, thyroid pathology, severe DKA or hypoglycemia, and microalbuminuria were analyzed as categorical variables (yes/no). The normal distribution of continuous variables was assessed using the Shapiro-Wilk test. A Student t test was conducted to compare 2 groups with normally distributed data. The evolution of HbA1c and z-height in children with new-onset T1DM was evaluated by tracking changes in HbA1c and z-height over time. Profile plots illustrating HbA1c and z-height changes were created using 2-way ANOVA with repeated measures in one of the factors. The area under the curve (AUC) for HbA1c values, insulin doses, and z-height over the follow-up years were calculated using the trapezoid rule. The AUC's arithmetic means and their SD was obtained. Multiple linear regression analysis was performed using the AUC for HbA1c as the dependent variable and the AUC for insulin dose, the AUC for z-height, CGM, health insurance, age, and sex as independent covariates. A multiple logistic regression analysis was performed using HbA1c of <7% in the third year as the dependent variable. Bilateral P-values of less than 0.05 were considered significant. Statistical analyses were performed using SPSS software (IBM SPSS Statistics version 24, IBM Corp., Armonk, NY).
Results
Clinical and Metabolic Characteristics at Baseline
Of the 472 children, 10 were excluded because they were either <6 months or >18 years of age at diagnosis, 2 were excluded owing to pregnancy, 10 were excluded for missing sex data, and 17 were excluded because they lacked height and HbA1c data on ≥2 follow-up occasions. Therefore, 433 children (46.0% female) aged 8.7 ± 3.7 years baseline were included in the study. Every child adhered to intensive insulin therapy through a basal-bolus regimen or continuous subcutaneous insulin infusion. However, only 22 children (5.1%) were treated with continuous subcutaneous insulin infusion. A total of 199 children (45.9%) used CGM for ≥2 years, and 202 (46.6%) had health insurance coverage. Twenty-eight children (6.5%) had celiac disease, and 48 (11.1%) had thyroid pathology, both compensated and treated. There were no significant differences in the prevalence of celiac disease or thyroid pathology among children with and without CGM.
Baseline characteristics according to the use of CGM are displayed in Table I. There was no significant difference in sex between children with and without CGM. However, children with CGM were significantly younger than those without CGM. There was a significantly higher attendance at private schools among children with CGM than those without CGM. The percentage of children with health insurance was significantly higher among those with CGM than those without. There was no significant difference regarding parental educational level; 67.7% of parents (239) had only an elementary school education or less.
Table I.
Baseline characteristics by CGM use
| Characteristics | Without CGM (n = 234 [54%]) | With CGM (n =199 [46%]) | Total (n = 433 [100%]) |
|---|---|---|---|
| Age, years∗ | 9.41 ± 3.68 | 7.95 ± 3.51 | 8.72 ± 3.67 |
| Female sex | 109 (46.6) | 101 (50.8) | 210 (48.5) |
| Health insurance∗ | 95 (40.6) | 101 (50.8) | 196 (45.3) |
| Private schools∗ | 29 (12.4) | 58 (29.1) | 87 (20.1) |
| Parental education (elementary school or less) | 163 (69.7) | 130 (65.3) | 293 (67.7) |
| z-BMI | 0.03 ± 1.19 | 0.05 ± 1.47 | 0.04 ± 1.33 |
| z-Height | 0.18 ± 1.11 | 0.15 ± 1.19 | 0.17 ± 1.15 |
| z-Weight | 0.19 ± 0.07 | −0.18 ± 0.07 | 0.00 ± 1.00 |
| Insulin dose, IU/kg | 0.60 ± 0.30 | 0.57 ± 0.26 | 0.59 ± 0.28 |
| HbA1c, %∗ | 9.50 ± 2.92 | 10.18 ± 2.69 | 9.85 ± 2.82 |
BMI, body mass index.
Data are number (%) or mean ± SD. The z-score is a quantitative measure of the deviation of a specific variable taken from the mean of that population. Centers for Disease Control and Prevention z-height and BMI takes into account age and sex. Children were divided into 2 groups according to the use of CGM.
P < .05.
Follow-up
During the follow-up period, regarding acute complications, 94 (21.7%) had severe DKA and 36 (8.3%) experienced severe hypoglycemia events. There were no significant differences in the prevalence of acute complications between groups. Only 16 (3.7%) developed microalbuminuria or other chronic complications during follow-up.
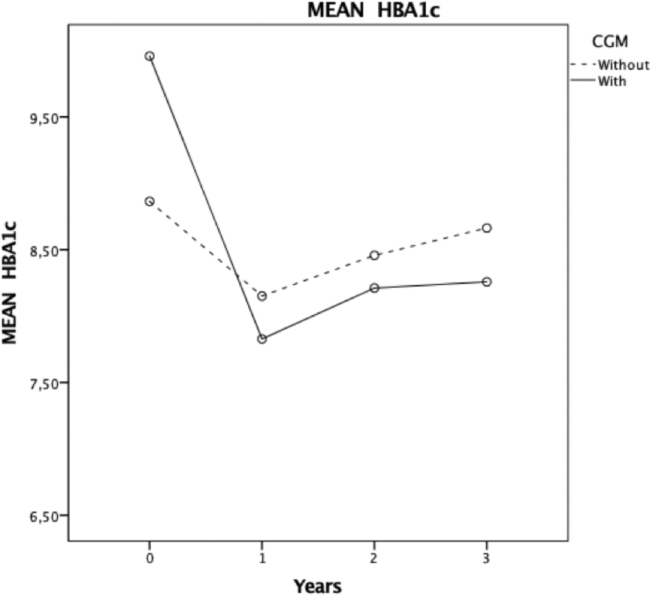
Table II provides HbA1c, insulin dose, and z-height levels at baseline and follow-up in children with and without CGM. The mean HbA1c values were significantly lower in years 1, 2, and 3 compared with baseline in children with CGM (Table II and Figure 1). In contrast, no significant differences in HbA1c values were observed in the group without CGM (Table II and Figure 1). Although the basal HbA1c levels were significantly higher in children using CGM, after 3 years of follow-up, the HbA1c levels were significantly lower in those using CGM than those not using CGM (Table II and Figure 1).
Table II.
HbA1c, insulin dose, and z-height at baseline and follow-up
| Year | With CGM | Without CGM | P value vs baseline with GCM | P value vs baseline without GCM | P value with CGM vs without CGM |
|---|---|---|---|---|---|
| HbA1c∗ | |||||
| Baseline (0) | 10.18 ± 2.69 | 9.50 ± 2.92 | .004 | ||
| 1 | 7.92 ± 1.48 | 8.42 ± 2.04 | <.001 | .134 | .131 |
| 2 | 8.25 ± 1.50 | 8.82 ± 1.80 | <.001 | .959 | .223 |
| 3 | 8.38 ± 1.48 | 8.98 ± 1.85 | <.001 | .999 | .041 |
| Insulin dose | |||||
| Baseline (0) | 0.57 ± 0.26 | 0.60 ± 0.30 | .995 | ||
| 1 | 0.64 ± 0.28 | 0.61 ± 0.32 | .912 | .783 | .989 |
| 2 | 0.71 ± 0.26 | 0.70 ± 0.31 | <.001 | .01 | .191 |
| 3 | 0.72 ± 0.25 | 0.77 ± 0.32 | <.001 | <.001 | .924 |
| z-Height∗ | |||||
| Baseline (0) | 0.15 ± 1.19 | 0.18 ± 1.11 | .653 | ||
| 1 | 0.05 ± 0.98 | −0.08 ± 1.11 | .941 | <.001 | .073 |
| 2 | −0.03 ± 1.01 | −0.23 ± 1.15 | .049 | <.001 | .020 |
| 3 | −0.14 ± 1.01 | −0.44 ± 1.11 | <.001 | <.001 | .032 |
Data are presented as mean ± SD. The z-score is a quantitative measure of the deviation of a specific variable taken from that population's mean. Centers for Disease Control and Prevention z-height takes age and sex into account. Significance was indicated for values with P < .05.
Significant interaction with time (2-way ANOVA with repeated measures at one of the factors; Bonferroni post hoc test).
Figure 1.
HbA1c trajectory by CGM use. Repeated measures model for the follow-up of HbA1c for 3 years. x axis, years; y axis, HbA1c means in children with and without CGM.
In years 2 and 3, the insulin dose increased significantly in both groups, but there were no significant differences between them (Table II).
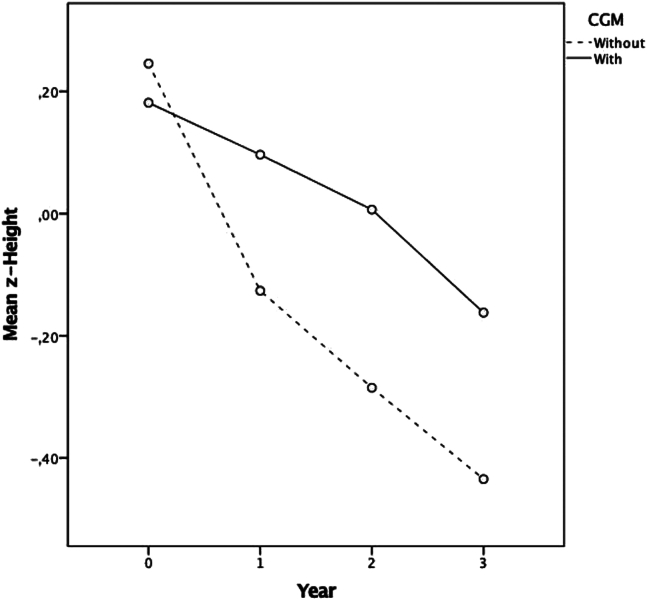
The z-height decreased significantly in years 2 and 3 compared with the baseline in the CGM group and in years 1, 2, and 3 in the group without CGM (Table II and Figure 2). However, the CGM group showed a significantly greater height in years 2 and 3 than in the non-CGM group. Only 48 children (11.1%) achieved the HbA1c target of <7% in the third year of follow-up. A significantly higher proportion of children in the CGM group met the HbA1c targets (<7.0%) compared with children without CGM in the third year of follow-up (39 [16.1%] vs 14 [6.0%]; P < .01).
Figure 2.
z-Height trajectories by CGM. Repeated measures model for the follow-up of z-height for 3 years. x axis, years; y axis, z-height means in children with and without CGM.
Univariate and Multivariate Associations
Nonparametric correlations indicated that the incremental AUC for HbA1c over 3 years was inversely associated with the CGM group (r = −0.29; P < .01). Additionally, the decremental AUC for z-height was inversely associated with the CGM group (r = −0.18; P = .01).
Multiple linear regression analysis showed that CGM users exhibited a significantly lower incremental AUC for HbA1c during follow-up than nonusers. Furthermore, a lower incremental AUC for HbA1c was also associated with a smaller decremental AUC for z-height (R2 = 0.19) (Table III); adjusted for age, sex, and health insurance. These findings suggest that lower HbA1c levels were associated with CGM use and better z-height growth over the 3-year follow-up.
Table III.
Multiple regression linear analysis
| Variables | Unstandardized coefficients |
Standardized coefficients |
t | P value | |
|---|---|---|---|---|---|
| B | Standard error | Beta | |||
| Age | −0.19 | 0.18 | −0.10 | −1.07 | .29 |
| Sex | −1.72 | 1.23 | −0.13 | −1.40 | .17 |
| CGM (yes/no)∗ | −3.86 | 1.28 | −0.29 | −3.02 | <.001 |
| Insurance (yes/no) | 0.80 | 1.28 | 0.06 | 0.62 | .54 |
| Insulin dose (AUC)∗ | 2.46 | 1.08 | 0.22 | 2.28 | .03 |
| z-Height (AUC)∗ | −0.91 | 0.45 | −0.19 | −2.02 | .05 |
Dependent variable: incremental HbA1c AUC.
P < .05.
Multiple logistic regression analysis revealed that children utilizing CGM were 80% more likely (OR, 0.22; 95%, CI 0.1-0.6) to achieve an HbA1c level of <7% in the last year of follow-up (Table IV), adjusted for age, sex, body mass index, insulin dose, z-height, and health insurance.
Table IV.
Multiple logistic regression analysis
| Variables | B | SE | Wald | Degree of freedom | P value | OR | 95% CI for OR |
|
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Sex | 0.37 | 0.44 | 0.70 | 1.00 | .40 | 1.44 | 0.61 | 3.41 |
| Age | −0.08 | 0.08 | 1.03 | 1.00 | .31 | 0.92 | 0.79 | 1.08 |
| Insulin dose | 1.42 | 0.85 | 2.79 | 1.00 | .10 | 4.14 | 0.78 | 21.94 |
| BMI | −0.02 | 0.07 | 0.08 | 1.00 | .78 | 0.98 | 0.87 | 1.12 |
| z-Height | −0.35 | 0.22 | 2.59 | 1.00 | .11 | 0.71 | 0.46 | 1.08 |
| GCM∗ | −1.53 | 0.54 | 8.02 | 1.00 | .01 | 0.22 | 0.08 | 0.62 |
| Insurance | −0.63 | 0.44 | 2.10 | 1.00 | .15 | 0.53 | 0.23 | 1.25 |
BMI, body mass index.
Dependent variable: HbA1c<7%.
P < .05.
Discussion
The findings from this study reveal a significant association between CGM use and lower HbA1c from the onset of T1DM over a 3-year follow-up in Latin American children. Additionally, children using CGM showed better z-height growth over the 3 years, suggesting the effectiveness of CGM in enhancing metabolic control and its practicality in real-world clinical settings.
To our knowledge, there is limited research on the long-term impact of CGM on HbA1c and z-height, extending beyond 1 year, in Latin American children newly diagnosed with T1DM. Despite the significant improvement in HbA1c levels, the mean HbA1c levels among these children remained above the recommended values throughout the 3 years. Moreover, only 11% of the children achieved the HbA1c target of <7% in the last year of follow-up. There was also a decrease in height growth in both groups, likely associated with fair metabolic control. Future studies are needed to confirm these findings.
HbA1c
Research indicates that children with T1DM using CGM devices experience a greater reduction in HbA1c and improved quality of life.2,3 CGM devices have revolutionized diabetes management by continuously monitoring blood glucose levels through subcutaneously inserted sensors, providing extensive and frequent data that allows for personalized insulin regimen adjustments based on each patient's specific glucose patterns and lifestyle.14 This outcome is consistent with most randomized controlled trials, which have demonstrated HbA1c improvements of 0.3%-0.6%.15,16 Consistently, the present study showed a significant improvement in HbA1c AUC in children using CGM in the multiple regression analysis, adjusted for confounding variables. Additionally, children using CGM were 80% more likely to achieve an HbA1c level of <7% in the last year of follow-up than nonusers of CGM. Despite the advancements in T1DM management with CGM, achieving the recommended HbA1c target in children remains challenging.13,14 The present study found that only 11% of children with T1DM reached the HbA1c target of <7%. Similarly, the T1DM Exchange Clinic Registry reported that only 14% of children with T1DM achieved an HbA1c level of <7%.2 Consequently, many children worldwide still struggle to meet HbA1c targets.
A variety of additional factors, including multiple social determinants of health, can contribute to poorer outcomes that this study found among Latin American children with T1DM beyond CGM use. These factors may include disparities in healthcare access and differences in the educational status of children and their parents.17,18 Nevertheless, we did not find a significant association between healthcare coverage and HbA1c in the multiple regression analysis, suggesting that healthcare coverage was not associated significantly with metabolic control when adjusting for confounding variables. It is essential to clarify that, in Latin American countries, all children have access to health care regardless of their medical insurance status. They receive free care for routine diabetes management, including necessary medications. However, when delivering CGM, families without health insurance face longer and more complicated procedures than those with insurance coverage.19 Anyhow, access to and use of CGM and insurance coverage are only 2 of many forms of health inequality that may affect Latin American children with T1DM. Individual, social, and environmental factors that may impact the efficacy of CGM include adequate income, safe neighborhoods for physical activity, access to healthy food, positive health behaviors, and a healthy psychological state.20 Consistently, we found a lower use of CGM among children attending public schools, suggesting that children from lower socioeconomic backgrounds had less access to CGM. Other reasons might be associated with these outcomes, such as subcutaneously administered insulin with a prolonged pharmacodynamic profile that makes it less effective than endogenously secreted insulin.21 Moreover, the known lag time of CGM in monitoring glucose levels can pose a challenge to the response of an algorithmic correction.22 Therefore, metabolic control in children with T1DM is much more complex than merely having access to CGM.23
Linear Growth
Poor metabolic control in individuals with T1DM may adversely impact linear growth.24 This study successfully tested and validated this hypothesis by analyzing repeated height measurements in children with new-onset T1DM throughout 3 years. This study found that CGM users had better z-height growth in years 2 and 3 than non-CGM users. However, height curves decreased significantly over the 3 years in children with and without CGM. Although modern therapies involving multiple daily injections, new devices such as CGM, continuous subcutaneous insulin infusion, or new insulin analogs offer more physiological insulin supplementation than previous treatments, anomalies in the GH/IGF-1 axis may persist owing to ongoing hepatic hypo-insulinization.25,26 Subcutaneous insulin therapy cannot fully mimic the pancreatic insulin secretion into the portal circulation, resulting in decreased levels of insulin-like growth factor 1 owing to low intraportal insulin concentrations.25 These abnormalities are particularly evident in children and adolescents with T1DM who have poor metabolic control.26,27 This study found a decrease in height growth, likely linked to poor metabolic control.
Elevated HbA1c levels and inadequate glycemic control are associated with diminished height growth in children with T1DM.25,28 Contemporary intensive insulin regimens, which result in enhanced metabolic control, have the potential to achieve a normal adult height consistent with patients' target parameters.24,29 However, this progress is not mirrored in many middle-income or low-income countries, such as Latin American countries. Although all children in Latin America have access to free insulin regardless of insurance status, barriers related to low socioeconomic status can lead to poor metabolic control and low z-height values. Moreover, understanding instructions, such as carbohydrate counting and interpreting information on current glucose values and trends to make appropriate corrections according to CGM, may have been compromised in this study; 68% of the parents had only an elementary school education or less. Furthermore, essential components of diabetes care can often be unavailable or inaccessible.30 Even though we found a decrease in height growth during the follow-up, children with access to CGM had a lower decrease in height than those without CGM, suggesting that those with access to more advanced technology had better metabolic control.
Association Between CGM and Age, DKA, and Hypoglycemia
Burnside et al,31 in a study involving approximately 1200 children in New Zealand, demonstrated that those who used CGMs were notably younger than those who did not. Our findings align with this result, showing that younger children were more likely to use CGMs than their older counterparts. This trend may be attributed to the increased vulnerability of younger children with T1DM, which could prompt doctors to recommend CGMs earlier for them compared with older children. In addition, health systems generally prioritize the youngest patients and cover CGM expenses more quickly for them.
We did not observe significant differences in the prevalence of severe hypoglycemia episodes between CGM users and nonusers. One possible explanation for this finding is that the CGMs used—an intermittently scanned device, specifically the first generation of the Freestyle Libre—lacked alarm features, which may have contributed to the absence of significant differences in severe hypoglycemia episodes. Additionally, we did not observe significant differences in the number of DKA cases between the 2 groups. This result may be because the children in the study were assessed from the time of their diagnosis, and most DKA cases occurred at diagnosis before CGM treatment had begun. This factor could explain the lack of significant differences in DKA prevalence between users and nonusers.
Strengths and Limitations
The strengths include the study design, the multisite evaluation, and the longitudinal study conducted over an extended period among Latin American children newly diagnosed with T1DM. Furthermore, the information for this study was sourced from the clinics or academic medical centers located in different Latin American countries.
However, it is important to acknowledge the limitations inherent in our research, because transparency is a key aspect of comprehensive research. The data collection was based on retrospective information, which comes with inherent limitations, including recall bias and incomplete documentation. Since the CGM information was based on self-reports, the results may be subject to participant recall bias. Recall information may influence the reliability of device usage and the effectiveness of using the provided glucose data.32 There was the possibility of unmeasured variables confounding the results. HbA1c measurements were not conducted in a centralized laboratory. This study covers the COVID-19 pandemic, incorporating pre- and post-pandemic data, which greatly influenced diabetes care at this time. Many families receive government assistance, discouraging them from taking additional jobs owing to the risk of losing their benefits. Consequently, these families may not disclose if they have stable employment elsewhere. As a result, the information on this issue could be biased and, therefore, could not be included in the analysis.
Conclusions
Our findings revealed a significant association between CGM use and lower HbA1c from the onset of T1DM over a 3-year follow-up in Latin American children, suggesting the potential of CGM in improving HbA1c and demonstrating its practicality in real-world clinical settings. However, HbA1c levels reached by Latin American children were far from the recommended values throughout the 3-year follow-up period. Moreover, there was a decrease in height growth, probably associated with poor metabolic control. This finding underscores the need for future research to increase our understanding of other factors that may contribute to improved metabolic control in Latin American children, including those operating outside medical centers. This understanding may inspire the development of specific strategies to tailor support and resources to enhance treatment satisfaction, improve adherence, and increase technology use in Latin American children with T1DM.
CRediT authorship contribution statement
Valeria Hirschler: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Claudia Molinari: Validation, Software, Methodology. Claudio D. Gonzalez: Supervision, Formal analysis, Data curation.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Footnotes
CODIAPED Investigators: Maria Eugenia Andres (Hospital de Niños Pedro de Elizalde, Nutrition, Buenos Aires, Argentina), Angela Figueroa Sobrero (Hospital San Roque, Parana, Argentina), Victoria Femenia (Hospital H. Notti, Mendoza), Guadalupe Pietropaolo (Hospital Sor Maria Ludovica La Plata, Buenos Aires, Argentina), Maria L. Major (Hospital Materno Infantil de San Isidro, San Isidro, Argentina), Edit Scaiola (Private Clinic former Ushuaia, Ushuaia, Argentina), Sandra Mazzetti (Hospital de Niños Victor J Vilela, Rosario, Argentina), Patricia Pasayo (Hospital Materno Infantil Dr Hector Quintana, San Salvador de Jujuy, Argentina), Amanda J. Benitez (Hospital Pediatrico Juan Pablo Segundo, Corrientes, Argentina), Andrea Escalante Marassi (Hospital Pediatrico Fernando Barreyro, Posadas, Argentina), Laura Pardo (Hospital Pereira, Montevideo, Uruguay), Julie Pelicand (Hospital San Camilo Hospital—Universidad de Valparaiso, San Felipe, Chile), Carlos M. Del Aguila Villar (Universidad Nacional Federico Villarreal, Lima Perú), Alejandra Franchello (Hospital de Niños Pedro de Elizalde, Nutrition, Buenos Aires, Argentina), Ernesto Bogado (Hospital San Roque, Parana, Argentina), Julieta Pomilio (Hospital H. Notti, Mendoza), Rosa Flores (Hospital H. Notti, Mendoza), Elizabeth Garcia Rusca (Hospital Sor Maria Ludovica La Plata, Buenos Aires, Argentina), Andrea Soledad Reinoso (Hospital Sor Maria Ludovica La Plata, Buenos Aires, Argentina), Analia Morin (Hospital Sor Maria Ludovica La Plata, Buenos Aires, Argentina), Eduardo Rossi (Hospital de Niños Victor J Vilela, Rosario, Argentina), Veronica Vacarezza (Argentina Private Clinic former Pilar, Pilar, Argentina), Daniela Rodríguez Fuentes (Hospital de Niños de la Santísima Trinidad de Cordoba, Cordoba, Argentina), Paula Paz Povedano (Hospital de Niños de la Santísima Trinidad de Cordoba, Cordoba, Argentina), Ana L. Garcia (Hospital de Niños Orlando Alassia, SANTA FE, Argentina), Teresa Martinez (Medical Assistance of Maldonado, Uruguay), Fabiola Blanco (Instituto de Previsión Social, Asunción, Paraguay), Oswaldo Núñez Almache (Instituto Nacional de Salud del Niño, Lima, Peru), Ana Parada (Hospital Regional Rio Grande, Rio Grande, Argentina), Patricia Bocco (Hospital Regional Ushuaia, Ushuaia, Argentina), M Laura Arzamendia (Hospital de Niños Pedro de Elizalde, Nutrition, Buenos Aires, Argentina), Carla Mannucci (Hospital de Niños Pedro de Elizalde, Nutrition, Buenos Aires, Argentina), Patricia Taberner (Hospital de Niños Pedro de Elizalde, Nutrition, Buenos Aires, Argentina), Laura Braguinsky (Hospital de Niños Pedro de Elizalde, Nutrition, Buenos Aires, Argentina).
Contributor Information
Valeria Hirschler, Email: vhirschler@gmail.com.
CODIAPED Study Group:
Maria Eugenia Andres, Angela Figueroa Sobrero, Victoria Femenia, Guadalupe Pietropaolo, Maria L. Major, Edit Scaiola, Sandra Mazzetti, Patricia Pasayo, Amanda J. Benitez, Andrea Escalante Marassi, Laura Pardo, Julie Pelicand, Carlos M. Del Aguila Villar, Alejandra Franchello, Ernesto Bogado, Julieta Pomilio, Rosa Flores, Elizabeth Garcia Rusca, Andrea Soledad Reinoso, Analia Morin, Eduardo Rossi, Veronica Vacarezza, Daniela Rodríguez Fuentes, Paula Paz Povedano, Ana L. Garcia, Teresa Martinez, Fabiola Blanco, Oswaldo Núñez Almache, Ana Parada, Patricia Bocco, M Laura Arzamendia, Carla Mannucci, Patricia Taberner, and Laura Braguinsky
References
- 1.Johnson S.R., Holmes-Walker D.J., Chee M., Earnest A., Jones T.W., Craig M., et al. Universal subsidized continuous glucose monitoring funding for young people with type 1 diabetes: uptake and outcomes over 2 years, a population-based study. Diabetes Care. 2022;45:391–397. doi: 10.2337/dc21-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster N.C., Beck R.W., Miller K.M., Clements M.A., Rickels M.R., DiMeglio L.A., et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66–72. doi: 10.1089/dia.2018.03842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laffel L.M., Kanapka L.G., Beck R.W., Bergamo K., Clements M.A., Criego A., et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323:2388–2396. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajjan R.A. How can We realize the clinical benefits of continuous glucose monitoring? Diabetes Technol Ther. 2017;19(S2):S27–S36. doi: 10.1089/dia.2017.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker A.F., Hood K.K., Gurka M.J., Filipp S.L., Anez-Zabala C., Cuttriss N., et al. Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care. 2021;44:1480–1490. doi: 10.2337/dc20-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addala A., Auzanneau M., Miller K., Maier W., Foster N., Kapellen T., et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44:133–140. doi: 10.2337/dc20-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer-Davis E.J., Bell R.A., Dabelea D., D'Agostino R., Imperatore G., Lawrence J.M., et al. The many faces of diabetes in American youth: type 1 and type 2 diabetes in five race and ethnic populations: the SEARCH for diabetes in youth study. Diabetes Care. 2009;32 Suppl 2:S99–S101. doi: 10.2337/dc09-S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuczmarski R.J., Ogden C.L., Grummer-Strawn L.M., Flegal K.M., Guo S.S., Wei R., et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 9.American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S15–S33. doi: 10.2337/dc21-S002. [published correction appears in Diabetes Care. 2021;44:2182. doi: 10.2337/dc21-ad09] [DOI] [PubMed] [Google Scholar]
- 10.Glaser N., Fritsch M., Priyambada L., Rewers A., Cherubini V., Estrada S., et al. ISPAD clinical practice consensus guidelines 2022: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2022;23:835–856. doi: 10.1111/pedi.13406. [DOI] [PubMed] [Google Scholar]
- 11.Abraham M.B., Karges B., Dovc K., Naranjo D., Arbelaez A.M., Mbogo J., et al. ISPAD clinical practice consensus guidelines 2022: assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2022;23:1322–1340. doi: 10.1111/pedi.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association Professional Practice Committee 14. Children and adolescents: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S208–S231. doi: 10.2337/dc22-S014. [DOI] [PubMed] [Google Scholar]
- 13.DiMeglio L.A., Acerini C.L., Codner E., Craig M.E., Hofer S.E., Pillay K., et al. ISPAD Clinical practice consensus guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;(19 Suppl 27):105–114. doi: 10.1111/pedi.12737. [DOI] [PubMed] [Google Scholar]
- 14.Monitoring diabetes. 2022. https://www.joslin.org/patient-care/diabeteseducation/diabetes-learning-center/monitoring-diabetes
- 15.Beck R.W., Riddlesworth T., Ruedy K., Ahmann A., Bergenstal R., Haller S., et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371–378. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 16.Beck R.W., Bergenstal R.M., Laffel L.M., Pickup J.C. Advances in technology for management of type 1 diabetes. Lancet. 2019;394:1265–1273. doi: 10.1016/S0140-6736(19)31142-0. [DOI] [PubMed] [Google Scholar]
- 17.Valenzuela J.M., Seid M., Waitzfelder B., Anderson A.M., Beavers D.P., Dabelea D.M., et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369–1375.e1. doi: 10.1016/j.jpeds.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparud-Lundin C., Ohrn I., Danielson E., Forsander G. Glycaemic control and diabetes care utilization in young adults with type 1 diabetes. Diabet Med. 2008;25:968–973. doi: 10.1111/j.1464-5491.2008.02521.x. [DOI] [PubMed] [Google Scholar]
- 19.https://www.argentina.gob.ar/justicia/derechofacil/leysimple/diabetes
- 20.Kahkoska A.R., Pokaprakarn T., Alexander G.R., Crume T.L., Dabelea D., Divers J., et al. The impact of Racial and ethnic health disparities in diabetes management on clinical outcomes: a Reinforcement Learning analysis of health Inequity among youth and young adults in the SEARCH for diabetes in youth study. Diabetes Care. 2022;45:108–118. doi: 10.2337/dc21-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt R.I.G., DeVries J.H., Hess-Fischl A., Hirsch I.B., Kirkman M.S., Klupa T., et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2021;64:2609–2652. doi: 10.1007/s00125-021-05568-3. [published correction appears in Diabetologia. 2022;65:255. doi: 10.1007/s00125-021-05600-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cengiz E., Tamborlane W.V. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;(11 Suppl 1):S11–S16. doi: 10.1089/dia.2009.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill-Briggs F., Adler N.E., Berkowitz S.A., Chin M.H., Gary-Webb T.L., Navas-Acien A., et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44:258–279. doi: 10.2337/dci20-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonfig W., Kapellen T., Dost A., Fritsch M., Rohrer T., Wolf J., et al. Growth in children and adolescents with type 1 diabetes. J Pediatr. 2012;160:900–903.e2. doi: 10.1016/j.jpeds.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Santi E., Tascini G., Toni G., Berioli M.G., Esposito S. Linear growth in children and adolescents with type 1 diabetes mellitus. Int J Environ Res Public Health. 2019;16:3677. doi: 10.3390/ijerph16193677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz M.T., Barrios V., Pozo J., Argente J. Insulin-like growth factor I, its binding proteins 1 and 3, and growth hormone-binding protein in children and adolescents with insulin-dependent diabetes mellitus: clinical implications. Pediatr Res. 1996;39:992–998. doi: 10.1203/00006450-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Dills D.G., Allen C., Palta M., Zaccaro D.J., Klein R., D'Alessio D. Insulin-like growth factor-I is related to glycemic control in children and adolescents with newly diagnosed insulin-dependent diabetes. J Clin Endocrinol Metab. 1995;80:2139–2143. doi: 10.1210/jcem.80.7.7608267. [DOI] [PubMed] [Google Scholar]
- 28.Marcovecchio M.L., Heywood J.J., Dalton R.N., Dunger D.B. The contribution of glycemic control to impaired growth during puberty in young people with type 1 diabetes and microalbuminuria. Pediatr Diabetes. 2014;15:303–308. doi: 10.1111/pedi.12090. [DOI] [PubMed] [Google Scholar]
- 29.Cianfarani S., Bonfanti R., Bitti M.L., Germani D., Boemi S., Chiumello G., et al. Growth and insulin-like growth factors (IGFs) in children with insulin-dependent diabetes mellitus at the onset of disease: evidence for normal growth, age dependency of the IGF system alterations, and presence of a small (approximately 18-kilodalton) IGF-binding protein-3 fragment in serum. J Clin Endocrinol Metab. 2000;85:4162–4167. doi: 10.1210/jcem.85.11.6996. [DOI] [PubMed] [Google Scholar]
- 30.Saiyed M., Hasnani D., Alonso G.T., Richmond E., Besançon S., Cotterill A., et al. Worldwide differences in childhood type 1 diabetes: the SWEET experience. Pediatr Diabetes. 2021;22:207–214. doi: 10.1111/pedi.13137. [DOI] [PubMed] [Google Scholar]
- 31.Burnside M.J., Williman J.A., Davies H.M., Jefferies C.A., Paul R.G., Wheeler B.J., et al. Inequity in access to continuous glucose monitoring and health outcomes in paediatric diabetes, a case for national continuous glucose monitoring funding: a cross-sectional population study of children with type 1 diabetes in New Zealand. Lancet Reg Health West Pac. 2023;31 doi: 10.1016/j.lanwpc.2022.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battelino T., Conget I., Olsen B., Schütz-Fuhrmann I., Hommel E., Hoogma R., et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155–3162. doi: 10.1007/s00125-012-2708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]