Abstract
Dupilumab is a monoclonal antibody that has recently been introduced for the treatment of asthma. It has the potential to significantly alter the progression and severity of T2 -dependent diseases, including severe asthma. However, dupilumab can cause transient hypereosinophilia. In rare cases, hypereosinophilia can reach high levels and manifest with clinical symptoms. We present the case of a female patient who presented with hypereosinophilia of almost 5000 cells/μL accompanied by erythema nodosum during dupilumab treatment. After stopping and then restarting dupilumab, the patient developed eosinophilic pneumonia. This case highlights the need to monitor blood eosinophil count along with clinical and radiological symptoms in patients receiving dupilumab.
Keywords: Asthma, Biologics, Eosinophilia, Erythema nodosum, Dupilumab
Highlights
-
•
Treatment with novel biologicals require precautions.
-
•
Eosinophil level should be monitored during therapy with dupilumab.
-
•
During treatment with dupilumab pulmonary and skin adverse events may occur.
1. Introduction
Dupilumab is a novel human monoclonal antibody that blocks the α-unit of the interleukin IL-4 receptor. It has been shown to be effective in different T2-dependent diseases, including T2 asthma. In addition, it has been shown to be well tolerated by patients with asthma. However, patients receiving dupilumab may experience a transient increase in the eosinophil count, which is usually not clinically significant. We present the case of a patient who developed symptoms of erythema nodosum with blood hypereosinophilia after 6 weeks of dupilumab treatment. After restarting dupilumab, eosinophilic pneumonia occurred. To our knowledge, this is the first such case reported in the literature.
2. Case report
A 52-year-old woman was referred to the Department of Pulmonology and Allergy at the University Hospital in Krakow, Poland, to determine the cause of symptoms that developed during dupilumab treatment administered at another hospital.
Retrospective data and medical records of the patient were reviewed. The patient had a 20-year history of eosinophilic asthma, which in the previous year manifested with frequent exacerbations requiring courses of systemic corticosteroids. Blood eosinophil count before treatment was 750/μL. Treatment with dupilumab at an initial dose of 400 mg was started in June 2023, followed by a maintenance dose of 200 mg every 2 weeks. After the fourth dose, the patient reported a persistent dry cough, and after the sixth dose at the end of August 2023, the patient developed inflammation of the wrists and painful peripheral edema of the legs, especially around the ankle joints, with red-purple nodular lesions on the calves.
On admission to the previous hospital, laboratory tests revealed an eosinophil count of 4880/μL (54.3 % of white blood cell count). The remaining laboratory parameters as well as chest radiograph were normal. Additional tests performed to exclude other causes of high eosinophilia showed no abnormalities. This included testing for antineutrophil cytoplasmic antibodies (ANCAs), antinuclear antibodies (ANAs), rheumatoid factor (RF), anti-cyclic citrullinated peptide antibodies (aCCPs), and parasite infestation. Dupilumab treatment was discontinued, and a short course of systemic corticosteroids (2 weeks with a tapering dose of methylprednisolone) was started, resulting in a normal eosinophil blood count and improved symptoms. One month later, the patient was admitted to the same ward for follow-up. Peripheral edema of the legs and hands, along with reddish painful nodules on the legs, persisted, but their severity was reduced compared with the previous examination. Hypereosinophilia was still present, with an eosinophil count of 1500/μL. A decision was made to consult the patient at the Department of Pulmonology and Allergology at the University Hospital in Krakow.
The patient was admitted to our department 3 months after the onset of symptoms. On admission, she reported a gradual significant worsening of asthma since the discontinuation of dupilumab. A course of systemic corticosteroids was required, and the patient reported discontinuing corticosteroid treatment 2 weeks prior to admission. On physical examination, wheezing throughout the lungs was observed. The eosinophil count was 660/μL. Spirometry revealed obstruction, with a forced expiratory volume in 1 s (FEV1) to vital capacity (FEV1/VC) ratio of 59 % and an FEV1 of 55 % of the predicted value. There were no symptoms in the legs and hands except for some bluish areas on the calves about 10 cm above the ankles. Blood samples for ANCA, ANA, RF, and aCCP testing were obtained. The results were negative. High-resolution chest computed tomography (HRCT) showed bronchial wall thickening and sinus computed tomography revealed subtle mucosal thickening in some ethmoid cells. Bronchoscopy showed normal airways, and bronchial washings taken for various microbiological tests were negative. Bronchial biopsy showed signs of remodeling, including basal membrane thickening, muscle hypertrophy, mucosal swelling, and eosinophilic infiltrates. Standard treatment for severe asthma exacerbation was initiated with systemic corticosteroids and inhaled bronchodilators. One week later, clinical improvement was observed and spirometry showed resolution of obstruction (FEV1/VC, 73.4 %; FEV1, 99 % predicted). The patient was discharged home with a tapering regimen of oral corticosteroids, along with inhaled corticosteroids, a long-acting beta2-agonist, and a long-acting antimuscarinic receptor antagonist.
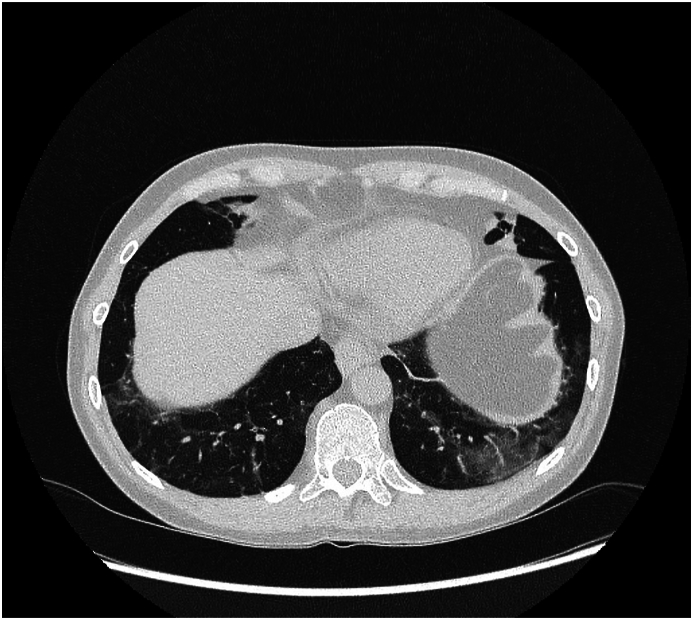
Based on the reported symptoms and previous medical history, erythema nodosum was suspected. To confirm that erythema nodosum and hypereosinophilia were linked to dupilumab treatment, the decision was made to restart dupilumab. On restart, the eosinophil count was 310/μL. The patient was closely monitored. Two weeks after the first dose, improvement in asthma control was noted. As there were no skin lesions, the second dose of dupilumab was administered. Oral methylprednisolone was tapered and then discontinued. After the third dose of dupilumab, the patient reported a persistent dry cough and fatigue, which she attributed to persistent common cold. Spirometry revealed moderate obstruction (FEV1/VC, 61 %; FEV1, 69 % predicted), and complete blood count showed an eosinophil count of 3100/μL (34 % of white blood cell count). Nevertheless, the patient received the fourth dose of dupilumab. Over the next 2 weeks, spirometry results improved (FEV1/VC, 65 %; FEV1, 77 % predicted) and eosinophil count decreased slightly (2700/μL), but dry cough and fatigue persisted. A further reduction in eosinophil count to 2360/μL was noted with 2 subsequent doses of dupilumab. However, after the seventh dose, the eosinophil count increased to 3200/μL. The patient reported a persistent dry cough and interphalangeal and ankle pain without symptoms of erythema nodosum. Detailed examination and chest HRCT revealed ground-glass opacities and some fibrotic opacities over the diaphragm (Fig. 1). Bronchoscopy with bronchoalveolar lavage showed 60 % of eosinophils, and bronchial biopsy showed eosinophilic infiltration of the bronchial mucosa as well as basal membrane thickening of the epithelium and smooth muscle. No signs of vasculitis were observed. ANCA titers, including myeloperoxidase- and proteinase-specific ANCAs, were negative. Parasite infestation and hypereosinophilia syndrome were excluded. Eosinophilic pneumonia was diagnosed and oral corticosteroids were started (0.5 mg/kg/24 h methylprednisolone). The decision was made to discontinue dupilumab.
Fig. 1.
Chest HRCT revealing ground-glass opacities.
3. Discussion
Erythema nodosum is the most common form of panniculitis and is characterized by tender erythematous nodules, mainly in the pretibial area of the legs. The exact cause of erythema nodosum is unknown, although it appears to be a hypersensitivity response to various antigenic stimuli. It was suggested that erythema nodosum may be caused by the formation of immune complexes and their deposition in the venules of the septae in the subcutaneous fat. However, some authors reported absence of circulating immune complexes in uncomplicated erythema nodosum, and a delayed type IV hypersensitivity reaction was proposed. Although the etiology is often idiopathic, it is important to exclude an underlying disease before diagnosing primary erythema nodosum. Erythema nodosum may be the first sign of systemic disease triggered by a variety of processes, including infection, inflammation, neoplasia, and medication. The most common identifiable causes are streptococcal infections, primary tuberculosis, sarcoidosis, Behçet disease, inflammatory bowel disease, drugs, and pregnancy. Among adults, women are about 5-fold more likely to have erythema nodosum than men.
A transient increase in eosinophil count is often observed during dupilumab treatment. It is usually not significant clinically and is related to the mechanism of action of the drug. However, rare cases of hypereosinophilia associated with clinical conditions such as eosinophilic granulomatosis with polyangiitis and hypereosinophilic syndrome were reported. They are often related to the steroid-sparing effect of the drug or the natural course of the underlying disease rather than a direct cause-and-effect relationship with dupilumab. Rare cases of eosinophilic pneumonia were also described [[1], [2], [3], [4]].
A potential mechanism underlying a transient increase in blood eosinophil count is related to dupilumab inhibiting eosinophil trafficking to tissues. Chemokines and adhesion molecules, such as vascular cell adhesion molecule (VCAM)-1, are present as receptors on the endothelial cells lining the blood vessels, facilitating the adhesion of circulating eosinophils to surrounding tissues. The expression of VCAM-1 is regulated by IL-4. On binding, eosinophils enter the tissue and their migration is guided by chemokines, including thymus- and activation-regulated chemokines, eotaxins, IL-5, and IL-13. By blocking both IL-4 and IL-13 signaling, dupilumab may inhibit VCAM-1 expression and the eosinophil migration process. This is also evidenced by reduced serum levels of eotaxin-3 and thymus- and activation-regulated chemokines reported in clinical trials of dupilumab. Since IL-4 and IL-13 do not mediate the maturation and release of eosinophils into the blood (which is the role of IL-5), this reduced eosinophil migration into the tissue may lead to a transient increase in blood eosinophil count. In our patient, the increase of eosinophil count was significant and reached almost 5000 cells/μL. This raises the question of whether the observed increase in blood eosinophil count is solely due to the above mechanism or if it is also a response to a hypersensitivity reaction to dupilumab.
Our literature search revealed one case report describing the development of erythema nodosum in a woman treated with dupilumab for severe asthma. Similar to our case, she also developed hypereosinophilia [5]. The authors suggested that the association between dupilumab and erythema nodosum cannot be confirmed without re-exposure. They decided to restart dupilumab treatment because discontinuation led to loss of asthma control. However, a week after restarting treatment, skin nodules recurred. Based on this report, we decided to follow a similar approach. However, in our case, erythema nodosum did not recur but the patient developed eosinophilic pneumonia. Another case of erythema nodosum was recently published, describing a patient treated with dupilumab for atopic dermatitis [6].
4. Conclusion
Dupilumab is a relatively new biologic agent that was introduced for the treatment of asthma in 2022. Although adverse effects related to dupilumab-induced hypereosinophilia have been rarely reported, our case emphasizes the need for close monitoring of patients receiving this treatment.
CRediT authorship contribution statement
Agnieszka Gawlewicz-Mroczka: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Marek Przybyszowski: Writing – review & editing, Writing – original draft, Visualization, Software. Grażyna Bochenek: Writing – review & editing, Supervision, Formal analysis, Conceptualization. Maria Mroczka: Writing – original draft, Visualization. Krzysztof Sładek: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: DR AC Amit Chopra
References
- 1.Menzella F., Montanari G., Patricelli G., Cavazza A., Galeone C., Ruggiero P., et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Therapeut. Clin. Risk Manag. 2019;15:869–875. doi: 10.2147/TCRM.S207402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiyama Y., Koya T., Nagano K., Abe S., Kimura Y., Shima K., et al. Two cases of dupilumab-associated eosinophilic pneumonia in asthma with eosinophilic chronic rhinosinusitis: IL-5-driven pathology? Allergol. Int. 2022;71:548–551. doi: 10.1016/j.alit.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Nishida K., Takaaki N., Shiori T., Satsuki I., Yasuhiro T., Gakuya T., et al. A case of eosinophilic pneumonia in a patient receiving dupilumab therapy. J. Japan Soc. Respi. Endos. 2022;4:377–382. [Google Scholar]
- 4.Kurihara M., Masaki K., Matsuyama E., Fujioka M., Hayashi R., Tomiyasu S., et al. How can dupilumab cause eosinophilic pneumonia? Biomolecules. 2022;12:1743. doi: 10.3390/biom12121743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mustin D.E., Cole E.F., Blalock T.W., Kuruvilla M.E., Stoff B.K., Feldman R.J. J Dupilumab-induced erythema nodosum. JAAD Case Rep. 2021 Nov 17;19:41–43. doi: 10.1016/j.jdcr.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanami Y., Yamamoto T. Erythema nodosum of the extremities following dupilumab treatment in a patient with atopic dermatitis. J. Dermatol. 2024 Apr 8;51:291–292. doi: 10.1111/1346-8138.17199. [DOI] [PubMed] [Google Scholar]