Abstract
Millions of individuals worldwide are afflicted by the fatal infectious disease tuberculosis, which accounts for thousands of avoidable deaths. The literature has provided a good description of the clinical manifestation and radiologic features of pulmonary tuberculosis. However, the parenchymal complication of pulmonary tuberculosis presenting as cystic lung disease, has not been widely documented in the literature and is one of the incredibly uncommon causes of diffuse cystic lung disease. It is very uncommon to have a patient with possible pulmonary lymphangioleomyomatosis to be superinfected with bacteriologically confirmed tuberculosis.
This report describes a young female patient who was admitted to the hospital, had repeated chest tube insertions and drainage of recurrent spontaneous pneumothoraxes secondary to likely diffuse cystic lung disease related to pulmonary tuberculosis. First, it was thought that the most likely diagnosis was pulmonary lymphangioleomyomatosis. The patient ultimately diagnosed with diffuse cystic lung disease associated with pulmonary tuberculosis as the most likely cause of her clinical presentation considering the high index of suspicion and her sputum gene xpert results. For drug-susceptible tuberculosis, the patient was finally started on anti-tuberculosis medication. She had both clinical and radiological improvement after completion of her anti tuberculosis treatment.
Thus, it is reasonable to conclude that tuberculosis may contribute to diffuse cystic lung disease (DCLD) in tuberculosis endemic settings such as Ethiopia, and that appropriate diagnostic efforts should be undertaken to make the diagnosis. A high index of clinical suspicion is crucial to prevent delays in the diagnosis of diffuse cystic lung disease associated with pulmonary tuberculosis.
Keywords: Ethiopia, Cyst, Lung lymphangioleomyomatosis, Tuberculosis
1. Introduction
Diffuse cystic lung diseases (DCLD) are identified on high-resolution computed tomography (HRCT) of the chest by the presence of air-filled lucencies in the pulmonary parenchyma, which are discrete, thin-walled lesions (usually less than 2 mm) on multiple lung lobes bilaterally. In patients with DCLD, there should be a minimum of two cysts, but typically there are 10 or more [1], [2]. The main primary causes of DCLDs on high resolution computed tomography (HRCT) of the chest include Pneumocystis jirovecii pneumonia (PJP), Desquamative interstitial pneumonitis (DIP), Pulmonary Langerhans cell histiocytosis (PLCH), Birt-Hogg-Dubé (BHD) syndrome, lymphocytic interstitial pneumonia (LIP), and Lymphangioleomyomatosis (LAM) [1], [2], [3] (Table 1).
Table 1.
Radiologic features of major DCLD.
| Disease | Lung cyst distribution and characteristics | Other radiological features |
|---|---|---|
| Lymphangioleomyomatosis (LAM) |
|
|
| Pulmonary Langerhans cell histiocytosis (PLCH) |
|
|
| Lymphocytic interstitial pneumonia (LIP) |
|
|
| Follicular bronchiolitis |
|
|
| Birt-Hogg-Dubé syndrome (BHD) |
|
|
| Desquamative interstitial pneumonia (DIP) |
|
|
Cystic lung disease as a parenchymal complication of pulmonary tuberculosis has not been frequently reported in the literature and is among the extremely rare causes of DCLD. Cysts emerging as a consequence of pulmonary tuberculosis were determined to be the rarest appearance of this prevalent disease based on the available case reports and case series [3], [4], [5]. Cystic formation in TB can occur due to various mechanisms, including scarring of larger bronchi, secondary bacterial infections, caseating necrosis, granulomatous involvement of bronchioles, ciliated epithelia, and isoniazid, which can lead to inflammatory destruction of the bronchial wall, re-lined healed tubercular cavities, and cystic change [3]. In pulmonary tuberculosis cysts may evolve with varied outcome; they may regress, persist or even progress following disease treatment [6], [7], [8].
Tuberculosis is a deadly infectious disease, causing 3,500 preventable deaths daily. Untreated individuals can spread the disease, potentially infecting 10–15 more people annually. Efforts to eradicate TB have saved 75 million lives since 2000, but in 2022, over 10 million contract the disease [9]. In 2021, the estimated TB incidence in Ethiopia was 143,000, and an estimated 21,000 people died from Tuberculosis [10]. Despite being one of the most uncommon complications of tuberculosis, DCLD can have a life-threatening secondary spontaneous pneumothorax that necessitates repeated chest tube insertions, hospital stays, and unclear long-term care plans. As a result, tuberculosis should be considered when making differential diagnoses for cystic lung disease, as it is among the rare causes of DCLD, particularly in tuberculosis-endemic areas. In this report, a young woman with diffuse cystic lung disease coupled with recurrent pneumothorax and drug-susceptible pulmonary tuberculosis is described. The therapy for tuberculosis was first delayed because the treating physician mistook it for lymphangioleomyomatosis alone.
2. Case summary
On October 13, 2023, a 30-year-old female patient arrived at the Wolaita Sodo comprehensive specialized hospital emergency room with complaints of shortness of breath and left-side pleuritic chest pain of 5 days duration. Over the last 48 h, she experienced worsening of shortness of breath and her chest pain. For the previous three months, she has experienced a low-grade fever, nocturnal sweats, coughing up yellowish sputum, and easy fatigability. Two-months ago, she had the history of hospitalization at Sodo Christian hospital with similar complaints and diagnosed with bilateral spontaneous pneumothorax. She was treated with bilateral chest tube drainage for 2 weeks and discharged with improvement (Supplementary video 1). During the recent admission at nearby general hospital, she was screened for pulmonary tuberculosis with sputum gene xpert but the result was negative.
She had a history of smear positive drug susceptible pulmonary tuberculosis treatment three years ago. She took first-line anti-tuberculosis drugs (2RHZE, 4RH) for the rifampicin-susceptible pulmonary tuberculosis diagnosed from sputum gene xpert. During the intensive phase of anti-tuberculosis treatment, she developed a right-side pneumothorax and was treated with chest tube drainage. She completed anti-tuberculosis drug for six-months and declared cured with sputum examination for acid fast bacilli. She was relatively healthy over the past three years. She denies history of smoking cigarette, and drinking alcohol. No history of diabetes mellitus, hypertension or HIV/AIDS. No history of similar illness in the family. No history of change in mentation, history of seizure disorder or psychiatric illness. She denies contact history with tuberculosis patient.
Up on physical examination, she was acute sick looking and in respiratory distress. Her oxygen saturation was 71 % in ambient air and 92 % in intra-nasal oxygen at a rate of 5 L/min. Her blood pressure was 125/70 mmHg, her pulse rate was 104 beats per minute, and her respiratory rate was 28 breaths per minute. She had a pink conjunctiva. No lymph-node enlargement was detected. She had intercostal and sub-costal retractions, hyper-resonant percussion notes from the bilateral posterior chest and course crepitations on both lung fields. Upon assessment of the cardio-vascular system, S1 and S2 were clearly audible; neither a murmur nor a gallop rhythm was found. The abdomen was flat moves with the respirations; there was no mass, no organomegaly, or sign of fluid collection. Examinations of the genito-urinary system, neurological system, musculoskeletal system, and integumentary system revealed nothing noteworthy.
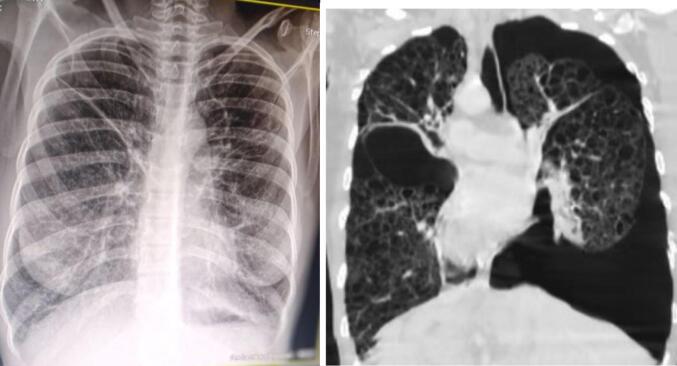
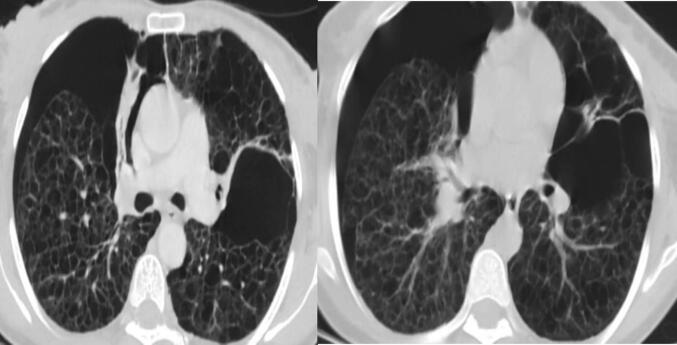
Her complete blood count (CBC) revealed a white blood cell of 12,800 cells (neutrophil 80.2 %, lymphocyte 14.8 %), hematocrit 52 %, hemoglobin 16.5 g/dl (which was explained to be due to secondary polycythemia) and platelet count of 233, 000 cells/mcl. Her Erythrocyte sedimentation rate (ESR) was 74 mm in the first hour. Emergency chest x-ray (Fig. 1) and HRCT of the chest (Fig. 2) were taken. The results of the HRCT of the chest showed that there was bilateral pneumothorax, larger on the right. There are spherical, thin-walled, bilateral diffuse cystic abnormalities in the lungs. On the left side, there are noticeable big bullae. The right lung's spared region has bilateral patchy consolidations. The pericardium, pleura, and pulmonary vasculature all seem normal. The finding was concluded to be diffuse cystic lung disease with bilateral pneumothorax likely representing severe form of LAM. Sever emphysema can be a differential diagnosis. on computed tomography of abdomen and pelvis, internal organs like liver, spleen and pancreas appear normal radiologically. Kidneys are normal in appearance bilaterally with no evidence of hydronephrosis, or angiomyolipoma. Adrenal glands appear normal. There is no evidence of mass, lymphadenopathy in the abdomen or pelvis. There was no evidence for ascites.
Fig. 1.
Initial chest radiograph and high-resolution chest Computed tomography (HRCT): Findings are suggestive of bilateral pneumothorax, larger on the right side and apical on the left side.
Fig. 2.
Initial HRCT: There are spherical, thin-walled, bilateral diffuse cystic abnormalities in the lungs. On the left side, there are noticeable big bullae. The right lung's spared region has bilateral patchy consolidations.
Her liver enzymes were within normal range (ALT 46.5, AST 40) and her serum creatinine was 0.9 mg/dl. Her transthoracic echocardiography was normal. She had emergency thoracostomy inserted bilaterally and was put on five liters of intra-nasal oxygen per minute. Her oxygen requirement increased and she began to experience a high-grade fever on the tenth day following the insertion of chest drainage. For the second time, sputum culture and gene xpert were sent. Her sputum gene Xpert tested positive for the rifampicin-sensitive Mycobacterium tuberculosis, but there was no other bacterial growth from the sputum culture. On 12th day, chest tube drainage was removed and she was started on anti-tuberculosis treatment (2RHZE/4RH).
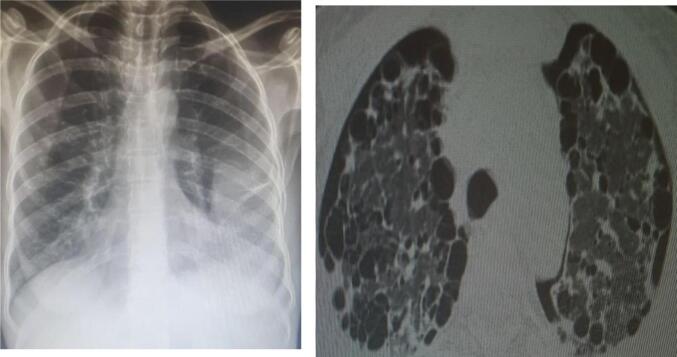
One day before the tube was removed, she had repeat PA-CXR (Fig. 3), which revealed the bilateral peripheral consolidation and loculated effusion on the left upper lobe. She was referred for cardiothoracic surgeon evaluation for possible lung biopsy and determination of vascular endothelial growth factor (VEGF). Due to financial problems, she did not visit cardiothoracic surgeon. VEGF is not available in our country. Therefore, she continued her follow-up in our out-patient department. Pneumothorax was resolved. She has been receiving anti-TB treatment for the last six months. During the follow-up period, there was no history of pneumothorax. She began gain weight and felt better overall. Sputum microscopy was done at the end of intensive phase of anti-TB treatment and at the end of anti-TB treatment. In both case her sputum microscopy for acid fast bacilli (AFB) came back negative.
Fig. 3.
Follow-up CXR and HRCT: Lung volume has increased, minimal bilateral pneumothorax, and there are is bilateral middle and lower lung consolidations.
3. Discussion
Diffuse cystic lung diseases are a broad category of rare conditions marked by several, spherical or irregularly shaped, thin-walled, air-filled pulmonary cysts involving multiple lobes of the lung. [11]. The cysts originating from various mechanisms, including the expansion of the distal airspaces due to airway obstruction, necrosis of the airway walls, and parenchymal destruction [1], [2]. The etiologic diagnosis of DCLD is challenging due to myriad differentials and overlapping features of different diseases. Cystic lung disease needs to be differentiated from cavity, bleb, bullae, emphysema, honeycombing, and pneumatocele, all of which may mimic a cyst [12].
A greater understanding of diffuse cystic lung diseases has been made possible by HRCT, which narrows the differential diagnosis and frequently eliminates the need for lung biopsy for diagnostic confirmation. It also improves the identification of cyst characteristics, including their distribution, and associated lesions, including the assessment of the presence of extrapulmonary changes. When combined with clinical and laboratory findings, HRCT is often sufficient for the etiological definition of diffuse lung cysts [13]. The differential diagnoses of diffuse cystic lung diseases are myriad, including neoplastic, inflammatory, and infectious etiologies (Table 1).
The combination of appearance upon imaging and clinical features, together with extrapulmonary manifestations, when present, permits confident and accurate diagnosis of the majority of these diseases without recourse to open-lung biopsy.
(1) Chioncel, O., et al (2017)
According to the LAM registries, pulmonary manifestations, most commonly spontaneous pneumothorax, were the primary events leading to the diagnosis in 86.5 % of cases of LAM and the average age at presentation is typically between the mid-thirties and mid-forties. Recurrent pneumothorax was high and associated with significant morbidities and mortalities [14]. Thus, it was advocated in the past that pathologic findings and immunohistochemical staining of lung biopsy specimens taken via the chest wall or during endoscopic surgery for pneumothorax are typically the basis for a final diagnosis of LAM [15]. Society guidelines however recommend non-invasive tools to diagnose LAM such as characteristic HRCT findings in appropriate clinical settings. This means that, once other diagnoses have been ruled out, multiple (>10) round, well-defined, thin-walled, air-filled cysts with preserved or increased lung volume and no other significant pulmonary involvement are the hallmarks of LAM on HRCT and can suffice its diagnosis [16].
Since LAM has a strong epidemiologic and clinical correlation with women of reproductive age, it was first considered the most likely differential diagnosis for our patient. Furthermore, the characteristic chest HRCT findings such as diffuse cystic lung disease on bilateral lung also support our clinical considerations of LAM as the most likely diagnosis in our patient [17], [18]. Even though LAM does not impair cellular immunity, the structural abnormalities of pulmonary parenchyma due to the natural history of LAM facilitate the growth of any microbes, including Mycobacterium tuberculosis [19]. However, in our patient, the history of pulmonary TB treatment in the past three years and the history of spontaneous pneumothorax either during the course of anti-TB treatment or subsequent life of the patient speak more for PTB-related cystic lung disease than LAM. In addition, the presence of bilateral lung consolidations in addition to cystic lesions and regressions of major cystic lesions following the recent anti-TB treatment also speaks more for PTB than LAM as a primary underlying disease. Additionally, the negative abdominal-pelvic CT scan rules out DCLD linked to LAM. Up to two-thirds of individuals with LAM were reported to have abnormal abdominopelvic imaging findings, including renal angiomyolipoma, enlarged abdominal lymph nodes, and lymphangiomyoma. The diagnosis of LAM can be made with the help of the typical abdominal signs in these patients as well as the traditional thin-section CT finding of pulmonary cysts [20].
Tuberculosis is a deadly infectious disease, causing 3,500 preventable deaths daily. Untreated individuals can spread the disease, potentially infecting 10–15 more people annually. Efforts to eradicate TB have saved 75 million lives since 2000, but in 2022, over 10 million contract the disease [9]. According to published case reports and case series that are now accessible, pulmonary TB-related DCLD is acknowledged as the rarest manifestation and its diagnosis is difficult, despite the clinco-pathological and epidemiologic components of pulmonary TB is being thoroughly characterized in literature [3], [4], [5], [7]. One case report shows that TB-related DCLD was mistaken for PLCH, leading to the patient receiving needless glucocorticoids, which have not been shown to be helpful in treating TB-related DCLD [5]. Majority of the reports identified TB related DCLD during the treatment of active infection, cystic lung disease was reported to be a rare complication of post-TB infection complicated with spontaneous pneumothorax in Malaysian case series [8]. As compared to most other cystic lung diseases, pulmonary TB related cystic lung disease showed an excellent response to anti-tubercular therapy [4]. Similar result was also seen in our patient.
In this report, we describe a young female patient who was previously treated for pulmonary tuberculosis and was subsequently diagnosed with smear-positive pulmonary tuberculosis. The patient may have had tuberculosis-related DCLD coupled with recurrent spontaneous pneumothorax. It was challenging for us to arrive at the right diagnosis. We have been considering various conditions such as post-TB bronchiectasis, LAM, and bullous emphysema as a cause for her condition. More than a month passed before it was determined that the patient's DCLD was most likely caused by tuberculosis, following the discovery of Mycobacterium tuberculosis by a second sputum gene expert. We hypothesize that pulmonary TB-related DCLD is likely to develop during the active phase of the infection and that it can be treated with anti-tuberculosis medication alone, based on the pattern of clinical presentation. Nevertheless, the pathophysiology of pulmonary TB-related DCLD may advance and it may fall within the post-TB lung disease spectrum. Therefore, additional study with a bigger sample size is desperately needed to validate these findings.
4. Conclusion
Before moving on to other uncommon causes like lymphangioleomyomatosis as a cause to explain the clinical scenarios, it is appropriate to rule out tuberculosis-related sequelae such as diffuse cystic lung disease as a cause of recurrent pneumothorax in settings where tuberculosis infection is endemic, like our case. This is due to the fact that administering anti-TB medication to the patient in a suitable therapeutic context helps prevent death from pulmonary tuberculosis and its associated deadly consequences, such as spontaneous pneumothorax. If clinical evidence supports tuberculosis, it is smart for physicians working in resource-constrained settings where examining alternative reasons is both expensive and inaccessible to begin looking into tuberculosis. It can be concluded that pulmonary tuberculosis-related DCLD can regress, leaving some clinically insignificant sequalae, and the risk of related pneumothorax also decreases with anti-tuberculosis treatment alone. Detailed history, careful follow-up and high index of suspension is required to include TB related cystic lung disease into a final diagnosis. To support the conclusions of this paper, it is also advised that more research need to be done with a larger sample size and a more diverse range of patient profiles.
Ethical statement
This is proving that we are presenting the de-identified clinical data of the patient associated with this report. A written consent was obtained from the patient and ethical clearance was also obtained from institutional ethical review board of Wolaita Sodo University, Ethiopia.
Ethical clearance
A written consent of the patient was taken before publishing this report. Ethical clearance was also received from Wolaita Sodo University college of health science and medicine Ethical review board.
CRediT authorship contribution statement
Haba Churako: Conceptualization, Methodology, Case summary, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision. Melese Tesema: Conceptualization, Methodology, Radiological descriptions, Case summary, Writing – original draft, Writing – review & editing. Lijalem Tema: Case summary, Writing – review & editing. Tsion Ababiya: Conceptualization, Writing – review & editing. Desalegn Wodajo: Radiological descriptions, Writing – original draft, Writing – review & editing. Teshome Hadaro: Writing – original draft, Writing – review & editing. Amanuel Tateso: Case summary, Writing – original draft, Writing – review & editing. Eyosiyas Anjajo: Case summary, Writing – review & editing. Temesgen Sidamo: . Abenezer Bekele: Radiological descriptions, Resources.
Funding
Is not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2024.100494.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
This her follow-up HRCT video. There is bilateral minimal pneumothorax, bilateral upper lung predominant peripheral lung cysts. There is associated honey combing, bilateral lung fibrosis. The size and number of cysts are decreasing as compared to the initial HRCT.
References
- 1.Baldi B.G., Carvalho C.R.R., Dias O.M., Marchiori E., Hochhegger B. Diffuse cystic lung diseases: differential diagnosis. J Bras Pneumol. 2017;43(2):140. doi: 10.1590/S1806-37562016000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francisco F.A.F., Souza A.S., Zanetti G., Marchiori E. Multiple cystic lung disease. Eur Respir Rev. 2015;24(138):552–564. doi: 10.1183/16000617.0046-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray A., Suri J.C., Sen M.K., Khanna A. Cystic lung disease in tuberculosis: An unusual presentation. Lung India. 2013;30(4):351–353. doi: 10.4103/0970-2113.120620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinay V., Abdullah Y., Garg A., Verma P., Singh G.K., Sharma A. The master impersonator: Pulmonary tuberculosis mimicking diffuse cystic lung disease - A mini case series of a rare presentation. J Family Med Prim Care. 2022;11(10):6590–6592. doi: 10.4103/jfmpc.jfmpc_331_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L., Liu J., Yang H., Peng L. Diffuse cystic lung disease caused by tuberculosis infection: case series. J Infect Public Health. 2023;16(4):526–530. doi: 10.1016/j.jiph.2023.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Govindasaami V. Diffuse cystic lung diseases-Tuberculosis has to be included in the differentials for Indian context! Lung India. 2023;40(2):190. doi: 10.4103/lungindia.lungindia_509_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johari B., Khor B.H., Musa A.N., Abdul Kadir R.F. Cystic lung disease as a complication of post-tuberculosis infection in young patients: A rare manifestation of a common disease. Respirol Case Rep. 2022;10(3):e0911. doi: 10.1002/rcr2.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.TB T. World TB Day 2024.
- 9.Chakaya J., Petersen E., Nantanda R., Mungai B.N., Migliori G.B., Amanullah F., et al. The WHO Global Tuberculosis 2021 Report – not so good news and turning the tide back to End TB. Int J Infect Dis. 2022;124(Suppl 1):S26–S29. doi: 10.1016/j.ijid.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 11.Araki T., Nishino M., Gao W., Dupuis J., Putman R.K., Washko G.R., et al. Pulmonary cysts identified on chest CT: are they part of aging change or of clinical significance? Thorax. 2015;70(12):1156–1162. doi: 10.1136/thoraxjnl-2015-207653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira Francisco F.A., Soares Souza A., Jr., Zanetti G., Marchiori E. Multiple cystic lung disease. Eur Respir Rev. 2015;24(138):552–564. doi: 10.1183/16000617.0046-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryu J.H., Moss J., Beck G.J., Lee J.C., Brown K.K., Chapman J.T., et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nijmeh J., El-Chemaly S., Henske E.P. Emerging biomarkers of lymphangioleiomyomatosis. Expert Rev Respir Med. 2018;12(2):95–102. doi: 10.1080/17476348.2018.1409622. [DOI] [PubMed] [Google Scholar]
- 15.Johnson S.R., Cordier J.-F., Lazor R., Cottin V., Costabel U., Harari S., et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35(1):14. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 16.Johnson SR. Lymphangioleiomyomatosis. Orphan Lung Diseases: A Clinical Guide to Rare Lung Disease. 2023:335-51.
- 17.McCarthy C., Gupta N., Johnson S.R., Jane J.Y., McCormack F.X. Lymphangioleiomyomatosis: pathogenesis, clinical features, diagnosis, and management. Lancet Respir Med. 2021;9(11):1313–1327. doi: 10.1016/S2213-2600(21)00228-9. [DOI] [PubMed] [Google Scholar]
- 18.Lewandowska K., Oniszh K., Augustynowicz-Kopeć E., Radwan-Röhrenschef P., Kuś J. Bacteriologically confirmed pulmonary tuberculosis in a patient with lymphangioleiomyomatosis accompanying tuberous sclerosis syndrome. Adv Respir Med. 2011;79(4):309–314. [PubMed] [Google Scholar]
- 19.Avila N.A., Kelly J.A., Chu S.C., Dwyer A.J., Moss J. Lymphangioleiomyomatosis: abdominopelvic CT and US findings. Radiology. 2000;216(1):147–153. doi: 10.1148/radiology.216.1.r00jl42147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This her follow-up HRCT video. There is bilateral minimal pneumothorax, bilateral upper lung predominant peripheral lung cysts. There is associated honey combing, bilateral lung fibrosis. The size and number of cysts are decreasing as compared to the initial HRCT.