Abstract
Objective
We aimed to evaluate longitudinal changes in set‐shifting and central coherence in a predominantly adolescent cohort with anorexia nervosa (AN) and to explore whether these factors predict long‐term eating disorder outcomes.
Method
Ninety‐two female patients with AN (mean age: 16.2, range: 13–21 years) completed neurocognitive tests (Rey Complex Figure Test, Adapted Version of the Wisconsin Card Sorting Test) before and after 12 months of psychotherapeutic treatment (n = 45 Maudsley AN Treatment, MANTRa; n = 47 standard psychotherapy; groups not randomised). Eating disorder severity was assessed at baseline, after 6, 12 and 18 months.
Results
Central coherence (indicated by an increase in the Rey Figure Style Index) and set‐shifting (indicated by a reduction in the percentage of perseverative errors) significantly improved over the course of treatment, with similar outcomes across groups. Lower central coherence was associated with higher eating disorder severity. Individuals with lower baseline set‐shifting ability tended to have worse eating disorder outcomes in the long‐term. However, this trend did not reach statistical significance in a multilevel linear mixed model.
Conclusions
Neurocognitive difficulties in adolescents and young adults with AN can improve after treatment. Interventions specifically addressing flexibility in thinking and behaviour may contribute to treatment success.
Keywords: adolescents, anorexia nervosa, central coherence, maudsley model, set‐shifting
Highlights
Central coherence and set‐shifting significantly improved over the course of treatment, with similar outcomes across groups (MANTRa treatment vs. standard psychotherapy).
Lower central coherence was associated with higher eating disorder severity.
There was a trend towards lower set‐shifting being associated with worse eating disorder outcomes in the long‐term, indicating that interventions addressing cognitive flexibility may contribute to treatment success.
1. INTRODUCTION
Anorexia nervosa (AN) is a severe mental illness marked by serious underweight, poor body perception, and fear of gaining weight (American Psychiatric Association, 2013). Despite treatment often being intensive, the duration of this illness can last for years, with approximately 20% of AN patients experiencing a chronic course (Steinhausen, 2002). Thus, there is an urgent need for research examining predictors of unfavourable treatment outcomes to individualise treatment in the early stages of illness. In this regard, a relatively new yet promising area of interest is neurocognitive functioning.
The most extensively investigated neurocognitive domains in AN research are weak central coherence (a thinking style characterised by excessive attention to detail at the expense of the bigger picture) and set‐shifting (the ability to move flexibly from one task or strategy to another) (Booth, 2006). These neurocognitive processing styles are important in AN research as they reflect the clinical presentation of the illness, including extremely rule‐focused behaviour (e.g. counting calories, exercising), fixation with routines not solely related to food intake but also, for example, to homework, cleaning, and housekeeping, and differences with seeing alternative coping strategies (Tchanturia et al., 2012, 2017) as well as the focus on details, for example, regarding food intake, weight loss or weight gain (Treasure & Schmidt, 2013). Moreover, prominent theoretical frameworks of AN, such as the Cognitive Interpersonal Maintenance Model of AN (Schmidt & Treasure, 2006; Treasure & Schmidt, 2013), postulate that obsessive‐compulsive traits (and consequently inefficiencies in set‐shifting and central coherence) not only elevate the vulnerability to develop AN but also maintain the illness as starvation‐induced alterations in brain function exacerbate rigid and detail‐orientated thinking and behaviour. Consequently, inefficiencies in central coherence and set‐shifting have been discussed on the one hand as endophenotype and on the other hand as important treatment targets.
Numerous studies have investigated central coherence and set‐shifting in the adult population, demonstrating a more detail‐oriented processing style and diminished set‐shifting abilities in adults diagnosed with an eating disorder compared to recovered patients and healthy controls across all eating disorder subtypes (Fuglset, 2019; Keegan et al., 2021; Miles et al., 2020; Stedal et al., 2021; Tchanturia et al., 2012; Wu et al., 2014). These effects are, however, less clear in adolescent populations. For example, two systematic reviews (Lang et al., 2014; Miles et al., 2020) found no significant differences in cognitive flexibility between acutely ill or recovered adolescents and healthy controls. Furthermore, a recent meta‐analysis (Saure et al., 2020) demonstrated that problems in set‐shifting and central coherence are evident among individuals with prolonged AN but not among those with shorter illness duration. Overall, most studies conducted in the adolescent population failed to replicate findings on neurocognitive inefficiencies observed in adults diagnosed with AN (Tchanturia, 2015). However, the absence of neurocognitive inefficiencies among adolescents with AN found in many studies does not exclude the possibility of a subgroup of adolescents experiencing such inefficiencies. Noteworthy, the comparability of the existing studies in adolescents is limited due to for example, inconsistent methodology, inclusion of mixed age groups or utilisation of different neuropsychological tasks (e.g. Wisconsin Card Sorting Test and Trail Making Test), making it challenging to identify patients that may indeed experience inefficiencies with regard to neurocognitive domains. Additionally, neurocognitive functioning undergoes developmental changes during adolescence and thus even in the absence of inefficiencies at beginning of the disorder, the age‐appropriate development of neurocognitive abilities may be disrupted by AN, potentially contributing to the development of a chronic form of AN (Tchanturia, 2015). Early interventions implemented during adolescence may have the potential to prevent cases from progressing to chronicity. In this regard, one core limitation of many previous studies having investigated neurocognitive abilities in patients with AN is the use of a cross‐sectional design which did not allow investigation of whether neurocognitive functioning improves during treatment and whether inefficiencies at treatment start can predict the treatment outcome. Thus, there is a need for longitudinal studies to address these aspects, especially in the adolescent population. Indeed, the present study using a longitudinal design intends to fill this gap in evidence.
Whether neurocognitive difficulties regarding central coherence and set‐shifting in the acute stage of the illness can predict long‐term treatment outcome is still unclear. It is hypothesised that such difficulties could hinder treatment success, as an inflexible thinking style and a detailed rigid focus on food and weight could interfere with adopting a bigger picture perspective about life and the need for behavioural change (Keegan et al., 2022). However, research about the predictive effects of neurocognitive variables on the treatment outcome is limited. A study by Oldershaw et al. (2018) reported that the combination of impaired set‐shifting and central coherence were predictive of poor treatment outcome in adults, whereas other studies did not find an impact of neurocognitive variables on the outcome in both outpatient (Keegan et al., 2022) or day‐hospital treatment (Tenconi et al., 2021). However, these studies focused mostly on adult patients with AN. To the best of our knowledge, there are no studies on neurocognitive predictors in an exclusively or predominantly adolescent population at the present.
Moreover, whether neurocognitive inefficiencies can change with weight‐restoration or throughout the course of treatments is also a matter of debate. Longitudinal studies in the adolescent population demonstrated that weak central coherence and set‐shifting abilities both improved over the course of treatment (Giombini et al., 2022; Rhind et al., 2022; Tchanturia et al., 2017), indicating that inefficiencies in these domains might be reversible or improvable, at least in the younger age. However, it is important to note that the majority of the available literature in adolescent populations primarily evaluated the efficacy of Cognitive Remediation Therapy (CRT), a specialised, supplementary treatment method designed to specifically address set‐shifting and central coherence. Therefore, more longitudinal studies are needed to examine whether neurocognitive improvements are solely attributable to specialised CRT treatment, whether they also occur in the course of other psychotherapeutic approaches or whether they reflect general improvements associated with weight restoration. For example, the Maudsley AN treatment for adolescents (MANTRa; Wittek et al., 2021), although less specific than CRT, incorporated content addressing cognitive flexibility and the balance between detail focus and bigger picture thinking and thus may also promote longitudinal effects in central coherence and set‐shifting. Among adult patients with AN, larger improvements in central coherence were observed in patients having received an adult version of MANTRa compared to cognitive behavioural therapy; however, for set‐shifting no differences between treatment approaches have been found (Keegan et al., 2022).
Therefore, the aim of this study is to assess alterations in central coherence and set‐shifting ability after 1 year of psychotherapeutic treatment, comparing two cohorts of adolescent and emerging adults with AN. The patients either received the specialised AN treatment programme MANTRa or standard psychotherapy (subsequently called optimised treatment as usual, TAU‐O). Specifically, our hypotheses are: (1) Central coherence and set‐shifting improve in the course of psychotherapeutic treatment independently of the intervention type. (2) MANTRa is superior to TAU‐O regarding enhancement of central coherence and set‐shifting. (3) Central coherence and set‐shifting prior to treatment start predict treatment success with regard to eating disorder psychopathology in the long‐term. Moreover, we aim to explore baseline associations between central coherence and set‐shifting with eating disorder psychopathology including Body Mass Index (BMI), eating disorder pathology, duration of illness, and psychiatric comorbidities.
2. METHODS
2.1. Study design
The present study used data from the MANTRa trial, a multi‐centre cohort study examining the efficacy and acceptability of MANTRa (n = 45) compared to TAU‐O (n = 47) in adolescent and emerging adult outpatients with AN. In this trial, we observed significant improvements in eating disorder psychopathology in both groups, with higher eating disorder remission rates in the MANTRa group in the long‐term (Wittek et al., 2023). Details of the study protocol were published in Wittek et al. (2021).
2.2. Eligibility criteria
Potential participants were considered as eligible for participation if they were female, aged between 13 and 21 years, had a full diagnosis of AN (according to DSM‐5), an atypical AN or weight restored AN after inpatient treatment and were willing to attend psychotherapy. Potential participants were excluded if their physical condition required immediate inpatient treatment, had insufficient cognitive abilities or German language skills, were diagnosed with a severe other mental or physical illness that needed treatment in its own right (e.g. psychosis, acute suicidality, borderline personality disorder) or were pregnant. If eligibility was confirmed, written informed consent was obtained from the patient and one of their legal guardians (if age <18).
2.3. Interventions
In both treatments, the participants received dietary consultation as well as medical check‐ups to monitor physical health and weight gain. Moreover, most patients' parents participated in the carers‐only skills training group ‘SUCCEAT’ (Franta et al., 2018; Philipp et al., 2020; Truttmann et al., 2020).
Maudsley Model of Anorexia Nervosa Treatment for Adolescents and Young Adults (MANTRa): MANTRa is the adapted version of the original MANTRA programme for adults (Schmidt et al., 2015) and is based on the Cognitive Interpersonal Maintenance Model of Eating disorders (Schmidt & Treasure, 2006; Treasure & Schmidt, 2013). It is an individual outpatient treatment approach for AN consisting of 20 to 30 weekly treatment sessions followed by 4 monthly booster sessions. The number of sessions is predefined and depends on the severity of the illness. The centrepiece of MANTRa is the formulation of individual aetiological and maintaining factors of the illness like the avoidance of emotions, rigid and inflexible thinking styles, identity development, and unhelpful reactions of close others. Based on the formulation, further interventions from the workbook are chosen. The workbook encompasses one chapter specifically targeting perfectionistic, rigid and detail‐orientated thinking styles via various therapeutic exercises (e.g. self‐observation exercises, checklists, cognitive techniques, exercises in everyday life). For more details about the MANTRa treatment see Wittek et al. (2021).
Optimised treatment as usual (TAU‐O): The TAU‐O group consisted of participants who received outpatient, individual psychotherapeutic treatment as it is standard in Austria. Therapeutic approaches used by therapists of the TAU‐O group can be classified in four broad categories (according to the Federal Ministry of Social Affairs, Health, Care and Consumer Protection, 2020): Systemic Family Therapy (n = 11), Cognitive Behavioural Therapy (n = 12), Psychodynamic Therapy (n = 11) or Humanistic Therapy (n = 12), Unknown (n = 1). Treatment frequency was either weekly or biweekly.
2.4. Measures
Participants completed the neuropsychological tasks prior to the start of treatment and after 12‐month. Eating disorder pathology was assessed at four time points: at baseline, after 6‐month, after 12 months (end of treatment), and after 18 months. Comorbid psychopathology was also assessed at several time points; however, for the purpose of the present study, only baseline values were used. Self‐report questionnaires were administered online via the ‘LimeSurvey’ software.
2.4.1. Neuropsychology:
The Rey Complex Figure Test and Recognition Trial (RCFT; Meyers & Meyers, 1995) is a neuropsychological drawing task measuring visuospatial abilities, non‐verbal memory, and central coherence. In this task, participants are instructed to copy a complex figure consisting of 18 numbered elements as accurately as possible. Without further instructions, they are asked to draw the figure from memory again after 30 min. The drawings were rated by a trained psychologist. According to the traditional scoring method by Osterrieth (1944), the accuracy of each element and the correct placement within the figure were rated in the copy as well as the recall condition to reflect the matching degree of the figure drawn with the original design. The accuracy score ranges from 0 to 36, with higher scores indicating greater accuracy. Additionally, the central coherence index is calculated in the copy condition according to the scoring system by Booth (2006). The central coherence index (which was defined as the main outcome parameter of the Rey Complex Figure Test in the present study) combines the ‘Order of Construction Index’ (whether the first six elements drawn have been global or detailed elements) and the ‘Style Index’ (whether the six global elements of the figure have been drawn in a continuous or fragmented way). The Order of Construction Index can range from 0 to 3.3, the style index and the central coherence index can range from 0 to 2, with higher scores indicating better abilities. The inter‐rater reliability ranges from 0.74 to 0.97 (Booth, 2006).
The Computergestütztes Kartensortierverfahren (CKV; Drühe‐Wienholt & Wienholt, 2013) is the computerised German version of the modified Wisconsin Card Sorting Task (WCST) (Nelson, 1976) measuring cognitive flexibility and set‐shifting abilities. Participants are asked to match 96 stimulus cards to one of four key cards. Cards can be matched by the colour, form, or number of symbols on the cards. Participants do not receive any instruction on the sorting principle; instead, they must figure this out based on feedback they receive after each trial (wrong or right). After 10 correct matches in a row, the sorting rule changes. Participants are not told that the sorting rule has changed and must determine this from the feedback. The scoring procedure of the CKV is comparable to Heaton (1981), while not all outcome parameters as in the face‐to‐face version of the WCST are provided (see below). The measure of set‐shifting is the number of ‘perseverative errors’ (defined by Nelson (1976) as incorrect classifications in which the participant uses the same criterion for a stimulus card as with the previous card, even though they had already received the feedback that this was incorrect), respectively the percentage of perseverative errors (number of perseverative errors divided by the number of trials administered), which is the main outcome recommended by the authors of the CKV and which was also defined as the main outcome parameter in the present study. Of note, the number and percentage of perseverative errors were highly correlated (r = 0.85). A higher number or percentage of perseverative errors indicates poorer set‐shifting ability (Miles et al., 2021). The CKV does not provide the number of perseverative responses (which is considered as the primary outcome in other versions of the WCST) as ambiguous cards are not used in the CKV (for more information see Nelson, 1976). Other variables reported are ‘Number of trials administered’, ‘Total correct responses’, ‘Total response errors’, ‘Non‐perseverative errors’, ‘Trials to complete first category’ (number of assignments needed to complete the first set of 10 consecutive correct assignments), ‘Failure to maintain set’ (number of errors to sort cards by the sorting rule after it can be presumed that the participant has acquired the rule), and ‘Concept perseverations’ (if 3 or more consecutive assignments are made to one of the two incorrect concepts. This corresponds to the ‘perseverated‐to principle’ from Heaton (1981)). The split‐half reliability of the outcome parameters of the CKV was between 0.90 and 0.95 (Drühe‐Wienholt & Wienholt, 2013). For practical reasons, 24 patients completed a paper‐pencil version of the Wisconsin Card Sorting Test at the baseline assessment. However, as the computerised and paper‐pencil versions were not directly comparable, we excluded these 24 patients from the subsequent statistical analysis on set‐shifting.
2.4.2. Eating disorder psychopathology
The BMI is a measurement of a person's weight in relation to the height (kg/m2). The BMI standard deviation score (BMI‐SDS) represents a sex and age specific standardised BMI based on Austrian normative data (Kromeyer‐Hauschild et al., 2001). Additionally, weight suppression defined as the difference between the pre‐morbid maximum weight and the minimum weight (in kg) was calculated.
The global score of the Eating Disorder Examination (EDE) Interview (EDE; Hilbert & Tuschen‐Caffier, 2016), was used to assess severity of eating disorder symptoms. The global score was calculated by adding up the 22 items across all subscales (shape concern, weight concern, eating concern, dietary restraint) and dividing by 4. It ranges from 0 to 6, with higher scores indicating greater eating disorder psychopathology. The Cronbach's alpha of the global is excellent (α = 0.93) and convergent validity with other measures of eating disorder pathology was between 0.56 and 0.81 (Hilbert & Tuschen‐Caffier, 2016). The EDE was administered by trained researchers and supervised by a clinical psychologist (G.W.).
2.4.3. Comorbid psychopathology
As not only eating disorder patients but also individuals with depression, obsessive‐compulsive, or anxiety disorders show difficulties in neurocognitive functioning including set‐shifting and central coherence (Grant & Chamberlain, 2023), we included the following instruments assessing comorbid symptoms:
The Beck Depression Inventory (BDI‐2; Hautzinger et al., 2006) is a 21‐question self‐report inventory measuring the severity of depression (e.g., hopelessness, irritability, cognitions such as guilt, or physical symptoms such as fatigue or concentration difficulties). It ranges from 0 to 63, with higher scores indicating more severe depression. The internal consistency of the BDI‐2 score was excellent (Cronbach's α = 0.92).
We used the subscale “state” of the German version of the Stait Trait Anxiety Inventory (STAI; Laux et al., 1981), which measures the current amount of anxiety from a patient's perspective (e.g., tension, concern, nervousness, inner restlessness, fear of future events). It contains 20 items and scores range from 20 to 80, with higher scores indicating more anxiety. The Cronbach's α of the STAI state score was 0.89.
The Obsessive Compulsive Inventory Revised (OCI‐R; Gönner et al., 2007) is a widely used self‐report measure for assessing symptoms of an obsessive‐compulsive disorder (e.g., controlling, washing, hoarding, mental neutralisation, or obsessive thoughts). It contains 18 items with scores ranging from 0 to 72. A higher score indicates greater obsessive‐compulsive symptoms. The Cronbach's α of the OCI‐R total score was 0.86.
Moreover, the presence of any current or lifetime comorbid psychiatric disorders based on DSM‐5 diagnostic criteria was obtained via a semi‐structured clinical interview (Diagnostic Interview for Mental Disorders in Children and Adolescents; Schneider et al., 2009) which was administered by a trained psychologist.
2.4.4. Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics 29.0 and R (version 4.4.0). A global significance level of α = 0.05 was used for all subsequently described analyses. We used t‐tests and Chi 2‐tests to compare differences between the MANTRa and TAU‐O group with regards to baseline characteristics. Analysis of variance models with time as within‐subject factor (baseline, 12‐month‐follow up) and group as between‐subject factor (MANTRa vs. TAU‐O) were performed to analyse changes in neurocognitive outcomes over time and changes by group (time × group interaction). In these models, the presence of a current psychiatric comorbidity was included as covariate as this was the only variable that statistically significantly differed between the MANTRA and TAU‐O group at baseline (see Table 1). Additionally, effect sizes of changes per group were calculated in terms of Cohen's d (including 95% confidence intervals), while effect sizes of d = 0.2, 0.5, and 0.8 are interpreted as small, medium, and large effects.
TABLE 1.
Sample baseline characteristics.
| Total sample (N = 92) | MANTRa (N = 45) | TAU‐O (N = 47) | Group difference (MANTRa vs. TAU‐O) | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Test statistic, p‐Value | |
| Age (years) | 16.25 (1.99) | 16.56 (2.17) | 15.95 (1.78) | t (90) = 1.503, p = 0.136 |
| Age at eating disorder onset (years) | 14.46 (1.76) | 14.64 (1.81) | 14.26 (1.69) | t (83) = 1.001, p = 0.320 |
| Eating disorder duration (in months) | 17.18 (15.63) | 17.93 (15.71) | 16.32 (15.70) | t (82) = 0.469, p = 0.640 |
| Body‐Mass‐index (BMI, kg/m2) | 16.80 (1.47) | 16.64 (1.46) | 16.96 (1.48) | t (89) = −1.014, p = 0.313 |
| BMI standard deviation score | −1.83 (1.01) | −1.97 (0.99) | −1.69 (1.04) | t (89) = −1.314, p = 0.192 |
| Weight suppression (kg) | 14.52 (6.70) | 13.62 (6.56) | 15.40 (6.78) | t (87) = −1.260, p = 0.211 |
| EDE interview global score | 3.40 (1.06) | 3.50 (0.93) | 3.30 (1.18) | t (90) = 0.943, p = 0.348 |
| % | % | % | Test statistic, p‐Value | |
|---|---|---|---|---|
| Inpatient treatment prior to study inclusion (yes) | 51.1 | 51.1 | 51.1 | χ 2 (1) = < 0.001, p = 0.996 |
| AN diagnosis | χ 2 (1) = 0.080, p = 0.778 | |||
| AN | 90.2 | 91.1 | 89.4 | |
| Atypical AN | 9.8 | 8.9 | 10.6 | |
| Anorexia nervosa subtype | χ 2 (1) = 0.131, p = 0.717 | |||
| Restrictive | 87.6 | 88.9 | 86.4 | |
| Binge/purging | 12.4 | 11.1 | 13.6 | |
| Any current psychiatric comorbidity (yes) | 47.3 | 35.6 | 58.7 | χ 2 (1) = 4.887, p = 0.027 |
| Any lifetime psychiatric comorbidity (yes) | 58.2 | 48.9 | 67.4 | χ 2 (1) = 3.202, p = 0.074 |
Abbreviations: BMI, Body‐mass‐index; EDE, Eating Disorder Examination; MANTRa, Maudsley model of anorexia nervosa treatment for adolescents and young adults; TAU‐O, Optimised treatment as usual.
Associations of demographic (age), illness‐specific variables (eating disorder duration, BMI‐SDS, EDE global score, AN subtype, weight suppression) and comorbid psychopathology (level of depression, anxiety, and obsessive‐compulsiveness) with main neurocognitive outcomes (central coherence index of the Rey Complex Figure Task, percentage of perseverative errors of the CKV) were explored using Pearson correlations.
To address the research question of whether baseline neurocognitive functioning can predict the eating disorder long‐term outcome, we performed multilevel linear mixed models using the lme4 package (version 1.1–35.3) in R (Bates et al., 2015). The EDE global score measured at four time points (baseline, 6‐month, 12‐month, 18‐month) was used as outcome variable, the central coherence index of the Rey Complex Figure Task measured at baseline, and the interaction between assessment time point and the central coherence index were included as fixed effects, while the subject was entered as random effect. Additionally, a random slope for assessment time point was included in the model, allowing the subjects to change differently over time. This model is subsequently named as ‘unadjusted’ model. Moreover, an adjusted model was calculated adding additional fixed effects including the patient's age, the presence of a current comorbidity (yes vs. no), weight suppression as well as the group (MANTRa vs. TAU‐O), which allows to evaluate the effect of the central coherence index on the change in eating disorder pathology when controlled for potential covariates. The final adjusted model was as follows: EDE Global Score = Central Coherence Index + Central Coherence Index*Time + Age + Comorbidity + Weight Suppression + Group + (Time | Subject). This procedure was repeated using the CKV percentage of perseverative errors as fixed effect.
3. RESULTS
3.1. Sample characteristics
In total, 92 patients with AN and atypical AN were included in the study. 45 of those patients received MANTRa therapy and 47 patients TAU‐O. Baseline characteristics are shown in Table 1. All patients were female with a mean age of 16.25 years (76% adolescent between 13 and 17 years, 24% emerging adults between 18 and 21 years), their BMI was about 1.8 standard deviations below their expected BMI according to sex and age, and about half of the sample had received inpatient treatment prior to study inclusion. The great majority of patients (87.6%) were diagnosed with AN restrictive type. Patients from the MANTRa and TAU‐O groups were comparable regarding most baseline characteristics; however, the percentage of patients with a current psychiatric comorbidity was higher in the TAU‐O group. More details about the sample are published in Wittek et al. (2023).
3.2. Changes in central coherence and cognitive flexibility
Changes from baseline to the 12‐month follow‐up assessment for the Rey Complex Figure and CKV measures are presented in Table 2. Regarding the Rey Complex Figure Task, statistically significant time effects controlled for the presence of a psychiatric comorbidity were observed for the Style Index, indicating an increase in global thinking style. Visuo‐spatial memory (indicated by the Accuracy Recall measure) also improved over time. There were no statistically significant differences between the groups except for the Accuracy Copy outcome which stayed stable in the MANTRa group but decreased in the TAU‐O group.
TABLE 2.
Changes in central coherence and cognitive flexibility over the course of treatment.
| MANTRa | TAU‐O | ANOVA model a | ||||
|---|---|---|---|---|---|---|
| Baseline | 12‐month follow‐up | Baseline | 12‐month follow‐up | Effect of time | Group × time interaction | |
| RCFT (central coherence) | ||||||
| Central coherence index | 1.31 (0.39) | 1.44 (0.36) | 1.33 (0.33) | 1.38 (0.43) | F (1,59) = 3.068, p = 0.085 | F (1,59) = 1.164, p = 0.285 |
| Order construction index | 2.13 (0.70) | 2.27 (0.62) | 2.11 (0.53) | 2.18 (0.75) | F (1,59) = 1.195, p = 0.279 | F (1,59) = 0.320, p = 0.574 |
| Style index | 1.35 (0.44) | 1.52 (0.39) | 1.39 (0.39) | 1.44 (0.47) | F(1,59) = 4.009, p = 0.050 | F (1,59) = 1.817, p = 0.183 |
| Accuracy copy | 30.61 (3.48) | 31.04 (3.10) | 31.69 (2.93) | 29.51 (3.78) | F(1,59) = 4.197, p = 0.045 | F(1,59) = 10.290, p = 0.002 |
| Accuracy recall | 20.14 (5.60) | 22.73 (5.84) | 20.31 (4.34) | 21.31 (4.73) | F(1,59) = 11.195, p = 0.001 | F (1,59) = 3.865, p = 0.054 |
| CKV (set‐shifting) | ||||||
| Number of trials administered (max. 96) | 79.63 (7.90) | 78.69 (17.55) | 86.56 (9.95) | 89.52 (21.46) | F (1,37) = 0.139, p = 0.712 | F (1,37) = 0.025, p = 0.876 |
| Total correct responses | 64.81 (4.61) | 64.31 (5.19) | 61.04 (10.17) | 68.12 (11.52) | F(1,37) = 4.450, p = 0.022 | F (1,37) = 3.357, p = 0.075 |
| Total response errors (%) | 18.25 (5.52) | 16.59 (8.50) | 28.21 (15.37) | 22.12 (12.19) | F(1,37) = 4.727, p = 0.036 | F (1,37) = 1.719, p = 0.198 |
| Number of perseverative errors | 3.38 (3.18) | 2.44 (4.73) | 9.08 (9.11) | 7.53 (12.79) | F (1,37) = 0.731, p = 0.398 | F (1,37) = 0.192, p = 0.664 |
| Perseverative errors (%) | 4.08 (3.64) | 2.42 (3.60) | 9.64 (9.19) | 6.66 (9.84) | F(1,37) = 4.402, p = 0.043 | F (1,37) = 0.435, p = 0.514 |
| Concept perseverations | 0.69 (0.95) | 0.38 (0.62) | 2.00 (2.47) | 1.20 (2.31) | F (1,37) = 4.054, p = 0.051 | F (1,37) = 0.251, p = 0.619 |
| Non‐perseveration errors (%) | 14.16 (2.92) | 12.87 (3.49) | 18.15 (6.44) | 16.28 (5.28) | F (1,37) = 3.214, p = 0.081 | F (1,37) = 0.129, p = 0.721 |
| Trials to complete first category | 12.56 (3.35) | 13.38 (3.63) | 13.64 (5.54) | 14.12 (5.07) | F (1,37) = 0.523, p = 0.474 | F (1,37) = 0.100, p = 0.753 |
| Failure to maintain set | 0.69 (1.25) | 0.50 (0.73) | 1.44 (1.85) | 1.68 (1.35) | F (1,37) = 0.023, p = 0.881 | F (1,37) = 0.236, p = 0.630 |
Abbreviations: ANOVA, analysis of variance; RCFT, rey complex figure test and recognition trial.
Effects were controlled for the presence of a current psychiatric comorbidity.
For the CKV, the number of correct responses significantly increased, and the percentage of total response errors as well as the percentage of perseverative errors significantly decreased at the 12‐month follow‐up, with the latter indicating improvements in cognitive flexibility over the treatment course. We did not observe any differences in change between the groups, indicating that the observed time effects were the same between the MANTRa and the TAU‐O group. Effect sizes of changes in neurocognitive outcomes are provided in Figure 1. The great majority of effect sizes were in the small or small‐to‐medium range.
FIGURE 1.
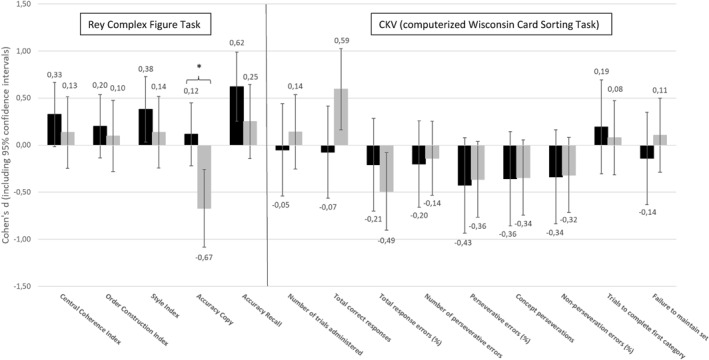
Effect sizes of changes in neurocognitive outcomes for the MANTRa (black bars) and TAU‐O (grey bars) groups. Note: The bars present standardised effect sizes of baseline‐to‐12‐month follow changes (in terms of Cohen's d including 95% confidence intervals) in the Rey Complex Figure Test and the computerised version of the Wisconsin Card Sorting Task (WCST) (CKV) separately for the MANTRa and TAU‐O groups. Statistically significant differences in effect sizes between the groups (as indicated by non‐overlapping confidence intervals) are marked with an asterisk.
3.3. Associations between neurocognitive outcomes and illness‐specific variables at baseline
As shown in Table 3, the EDE global score was statistically significantly associated with the central coherence index of the Rey Complex Figure Task, indicating that a more‐detailed focused thinking style was accompanied by higher eating disorder severity (r = −0.22). No associations were observed between the patient's age, eating disorder duration, BMI‐SDS, weight suppression, AN subtype, level of depression, anxiety, and obsessive‐compulsiveness with the central coherence index and the percentage of perseverative errors.
TABLE 3.
Associations of demographic and illness specific variables with neurocognitive outcomes at baseline (pearson correlation coefficients).
| Age | AN duration | BMI‐SDS | Weight suppression | EDE global score | AN subtype a | BDI‐2 total score | STAI state score | OCI‐R total score | |
|---|---|---|---|---|---|---|---|---|---|
| RCFT central coherence index | −0.16 | −0.11 | 0.11 | −0.14 | −0.22* | −0.16 | −0.20 | −0.08 | −0.05 |
| CKV percentage of perseverations errors | −0.09 | −0.12 | −0.15 | −0.09 | −0.07 | 0.07 | 0.01 | −0.01 | −0.17 |
Abbreviations: BDI‐2, beck depression inventory; BMI‐SDS, BMI standard deviation score; RCFT, rey complex figure test and recognition trial.
Coded as follows: 0 = restrictive type, 1 = binge/purging type.
*p < 0.05.
3.4. Neuropsychology as a predictor of the eating disorder longer‐term outcome
Results of the multilevel linear mixed models evaluating whether the central coherence index and the percentage of perseverative errors are predictive of the EDE global score are shown in Table 4. In all models, the fixed effect of time was statistically significant (p < 0.001), indicating that the EDE global score decreased over time. In the unadjusted model, the central coherence index was a significant predictor of the EDE global score (p = 0.017), indicating that a more detailed thinking style was associated with higher eating disorder pathology across all time points. However, the interaction between time and central coherence index was statistically not significant (p = 0.108), indicating that central coherence at baseline did not influence the course in eating disorder pathology over time. In the adjusted model, the only significant fixed effect except for assessment time was the presence of a current psychiatric comorbidity.
TABLE 4.
Results of multilevel linear mixed models predicting the outcome in eating disorder pathology by baseline set‐shifting and central coherence.
| Model 1 (central coherence index as a predictor for the EDE global score) | ||||
|---|---|---|---|---|
| Fixed effects | Unadjusted | Adjusted | ||
| Estimate [95% CI] | p‐Value | Estimate (95% CI) | p‐Value | |
| Intercept | 4.84 [3.91; 5.78] | <0.001 | 4.16 [2.05; 6.28] | <0.001 |
| Time | −0.90 [−1.28; −0.51] | <0.001 | −0.91 [−1.29; −0.53] | <0.001 |
| Central coherence index | −0.83 [−1.52; −0.15] | 0.017 | −0.59 [−1.28; 0.11] | 0.099 |
| Time*Central coherence index | 0.23 [−0.05; 0.50] | 0.108 | 0.23 [−0.05; 0.50] | 0.104 |
| Age | 0.02 [−0.01; 0.05] | 0.192 | ||
| Psychiatric comorbidity a | 0.53 [0.11; 0.94] | 0.013 | ||
| Weight suppression | 0.02 [−0.01; 0.05] | 0.192 | ||
| Group b | −0.32 [−0.74; 0.10] | 0.131 | ||
| Model 2 (CKV percentage of perseverative errors as a predictor for the EDE global score) | ||||
|---|---|---|---|---|
| Fixed effects | Unadjusted | Adjusted | ||
| Estimate (95% CI) | p‐Value | Estimate (95% CI) | p‐Value | |
| Intercept | 3.94 [3.51; 4.37] | <0.001 | 4.29 [1.81; 6.76] | 0.001 |
| Time | −0.67 [−0.82; −0.52] | <0.001 | −0.67 [−0.82; −0.52] | <0.001 |
| Percentage of perseverative errors | −0.02 [−0.05; 0.02] | 0.272 | −0.02 [−0.05; 0.02] | 0.346 |
| Time*Percentage of perseverative errors | 0.01 [−0.00; −0.02] | 0.069 | 0.01 [−0.00; 0.02] | 0.078 |
| Age | −0.03 [−0.17; 0.11] | 0.673 | ||
| Psychiatric comorbidity a | 0.96 [0.43; 1.49] | <0.001 | ||
| Weight suppression | 0.04 [−0.00; 0.08] | 0.069 | ||
| Group b | −0.60 [−1.17; −0.02] | 0.044 | ||
Coded as follows: 0 = no psychiatric comorbidity, 1 = at least one psychiatric comorbidity.
Coded as follows: 1 = MANTRa, 2 = TAU‐O.
Bold p‐values indicate statistically significant fixed effects (p < 0.05), p‐values printed in italic are between 0.05 and 0.10 The multilevel linear mixed models include a random factor for subject and random slopes for assessment time point.
In model 2 (unadjusted), the percentage of perseverative errors did not significantly predict the EDE global score (p = 0.272). Neither, the interaction between assessment time and the percentage of perseverative errors was statistically significant; however, the p‐value was close to 0.05 (p = 0.069). In the adjusted model, this interaction also just failed statistical significance (p = 0.078). To further explore this tendency, we descriptively compared extreme groups of patients with a low percentage of perseverative errors (≤33. percentile) and a high percentage of perseverative errors (≥66. percentile) with regard to their change in eating disorder pathology over time. As shown in Figure S1, patients with a high percentage of perseverative errors tended to show smaller improvements in the EDE global score compared to patients with a low percentage of perseverative errors.
4. DISCUSSION
The main objectives of this study were to evaluate long‐term changes in set‐shifting and central coherence in a sample of adolescents and emerging adults with AN treated with 2 different interventions (MANTRa vs. TAU‐O), and to analyse whether set‐shifting and central coherence were predictors of the long‐term eating disorder outcome. After 1 year of psychotherapeutic treatment, set‐shifting and, to some extent, also central coherence significantly improved with small to medium effect sizes. These changes were independent from the type of psychotherapeutic approach. At baseline, only marginal associations of central coherence and eating disorder pathology were observed. Pre‐treatment ability in set‐shifting and central coherence did not statistically significantly predict the long‐term course in eating disorder severity. However, there was a trend towards lower set‐shifting ability being associated with a lower reduction in eating disorder pathology until the 18‐month follow‐up.
The findings from the present study support the hypothesis that neurocognitive inefficiencies in adolescents with AN can—to some extent—improve over the course of psychotherapeutic treatment. Set‐shifting abilities, as indicated by a reduction in the percentage of perseverative errors, as well as central coherence as indicated by an increase in the Rey Complex Figure Style Index, improved over a 1‐year period. This in line with previous studies in adolescent and adult patients that reported significant improvements in set‐shifting and central coherence following CRT (Giombini et al., 2022; Rhind et al., 2022) and other types of treatment, including an adult version of MANTRa (Keegan et al., 2022). The effect size for improvements in central coherence was similar to those reported in a meta‐analysis of CRT studies (Tchanturia et al., 2017). For set‐shifting, however, the meta‐analysis published by Tchanturia et al. (2017) found no effect. This may be explained by the relatively short treatment period of CRT (few weeks) and short‐term follow‐up assessments, while in the present study the treatment duration was approximately 1 year. Longer evaluation periods may be needed to detect alterations in set‐shifting abilities. Furthermore, some studies highlighted that adolescent patients with a relative short illness duration do not show neurocognitive inefficiencies in comparison to healthy individuals (Lang et al., 2014; Miles et al., 2020), which may also explain the absence of treatment effects reported in some studies (Herbrich et al., 2017; Tchanturia et al., 2017). The predominantly adolescent sample in the present study, however, showed baseline central coherence being comparable to adult patients with AN (Lang et al., 2016). For the computerised version of the WCST (CKV) used in the present study, no normative data based on AN or healthy samples are currently available to which the degree of impairment of the present sample can be compared. However, a study having used the CKV in a sample of healthy adolescents reported only a slightly lower number of perseverative errors as in our sample, indicating that set‐shifting inefficiencies in our AN patients at baseline were only marginal (Sarrar et al., 2013).
Nevertheless, our study contributes to the ongoing discussion whether neurocognitive inefficiencies in patients with AN are state or trait‐related. It was argued that set‐shifting and central coherence difficulties are rather predisposing factors, as the majority of studies (primarily conducted in adult patients) demonstrated that they persist after recovery (Fuglset, 2019). In our predominantly adolescent sample, we showed that set‐shifting and central coherence were to a certain degree sensitive to change over the course of treatment. This is in line with the Cognitive Interpersonal Maintenance Model of AN that assumes that reduced set‐shifting and weak central coherence are trait‐related, but also aggravated by starvation in the acute stage of the illness and may also partly remit in weight‐restored patients (Treasure & Schmidt, 2013), which indicates that difficulties in set‐shifting and central coherence also reflect the patient's state to a certain degree. Noteworthy, although longitudinal changes in set‐shifting and central coherence were statistically significant in the present study, effect sizes of these changes were rather low, which raises the question whether the observed improvements also represent a clinically significant change.
Interestingly, we did not find differences in treatment effects between MANTRa (which incorporates content targeting central coherence and set‐shifting) and TAU‐O (which does not explicitly target central coherence and set‐shifting). However, effect sizes of improvements in central coherence tended to be slightly higher in the MANTRa group, which may indicate that conducting specific exercises to strengthen bigger picture thinking may be beneficial. However, more adequately powered studies are needed to confirm this hypothesis. Of note, the MANTRa chapter targeting thinking styles was addressed in only 2.6 out of 34 therapeutic session on average, which indicates that the full potential of MANTRa in working on inflexible thinking and detail‐focus has possibly not yet been reached. A randomised controlled trial comparing immediate and delayed provision of CRT reported overall positive effects of CRT, which were similar for the immediate and delayed condition (Giombini et al., 2022) and thus further supports the value of cognitive interventions to improve cognitive flexibility and big‐picture thinking.
In the present study, we observed only weak correlations between central coherence and eating disorder severity markers. At baseline, a higher eating disorder psychopathology was associated with central coherence. No associations at all were found for the main outcome of set‐shifting (percentage of perseveration errors). Most likely, this can be explained by the young age of our sample with a relatively short duration of illness (<1.5 years on average). It has been repeatedly discussed that neurocognitive inefficiencies may consolidate when the illness progresses and that mild neurocognitive inefficiencies in adolescent patients may worsen when the illness persists into adulthood (Harper et al., 2017; Herbrich et al., 2017). Moreover, we did not include individuals currently in inpatient treatment and thus no patients under extreme starvation. We hypothesise that in these patients, associations between neurocognitive performance and eating disorder severity might have been stronger.
One of the most interesting and potentially clinically relevant finding from our study was that lower set‐shifting ability (percentage of perseverative errors) at baseline tended to be associated with a lower reduction in eating disorder pathology (EDE global score) until the 18‐month follow‐up, although this finding just failed to reach statistical significance, which may have been the case due to the low sample size. However, this trend still expands the existing limited literature on neurocognitive performance as a prognostic marker for the outcome reported in adult AN patients. While in Keegan et al. (2022) and Tenconi et al. (2021) set‐shifting and central coherence were not predictive for the treatment outcome, early change, or remission, Oldershaw et al. (2018) reported that poorer set‐shifting and central coherence were significantly associated with post‐treatment body weight. However, the explained variance in these prediction models was minimal (4% and 3%, respectively). Our findings indicate that low cognitive flexibility may be a maintaining factor of AN in the long‐term and subsequently may hamper recovery. Neuropsychological assessments, including set‐shifting, are not typically part of routine diagnostic procedures even though it could be useful to detect inefficiencies in cognitive flexibility in adolescent patients with AN at an early stage. Therapeutic approaches specifically promoting flexibility in thinking could then be administered to those with low set‐shifting ability, which may improve overall treatment outcome. CRT (Giombini et al., 2022; Rhind et al., 2022) but also the tools targeting inflexible thinking styles integrated in MANTRa (Keegan et al., 2022; Wittek et al., 2021) can be regarded as helpful in this context. However, future studies should also evaluate whether improvements in cognitive flexibility assessed via neuropsychological tests can be transferred to real‐life situations (e.g., less rigidity with regard to diet and exercise) as previous studies using self‐reported measures of flexibility yielded inconsistent results (Herbrich et al., 2017; McAnarney et al., 2011).
This study has several strengths and limitations. Strengths refer to the longitudinal design of this study. In contrast to most previous longitudinal studies investigating the change in set‐shifting and central coherence, we used a relatively long follow‐up period of 12 months for neurocognitive outcomes and of 18 months to assess the predictive value of set‐shifting and central coherence on eating disorder pathology. Moreover, we included a relatively homogenous sample of AN patients who had just started their outpatient psychotherapeutic treatment and excluded inpatients. Thus, we can be near certain that general problems with attention and concentration resulting from extreme starvation or organic brain disease affected performance in set‐shifting and central coherence. The following limitations need to be mentioned: We did not use a randomised‐controlled trial design to evaluate differences in neurocognitive effects between the MANTRa and TAU‐O group. We also did not have data from a healthy control group to which the neurocognitive outcomes of AN patients could have been directly compared. Moreover, we do not have information about the number of completed psychotherapeutic sessions in the TAU‐O group and thus were not able to use this variable as a covariate in the statistical models. Although we assume that the number of psychotherapeutic sessions was similar between the groups, we cannot prove this assumption. Moreover, set‐shifting and central coherence outcomes were not controlled for patient intelligence quotient (IQ) as no intelligence test was conducted in our sample. However, it has been shown that full scale IQ in adolescent patients with AN is comparable to that of healthy controls (Kjaersdam Telléus et al., 2015) and thus it is unlikely that the intelligence level had a substantial impact on the results. Another limitation of our study is the absence of measurements examining autism spectrum disorder, respectively autistics traits. As difficulties in cognitive flexibility are also evident among individuals with autism spectrum disorder (Westwood et al., 2016) and autistic traits are also prevalent in AN, future studies investigating neurocognitive features in AN patients should also incorporate autistic traits as a potential influencing variable. General limitations of neurocognitive tests discussed in the literature (Herbrich et al., 2017; Tenconi et al., 2021), which are not specific to the present study but are also relevant here, include potential practice effects when neuropsychological tests are conducted longitudinally (however, we assume that practice effects were low due to the 1‐year testing interval in our study), differences in scoring procedures of neurocognitive tests across studies (making it difficult to directly compare results), as well as the fact that neuropsychological tests, including those used in the present study, were originally developed for adult populations and normative data for children and adolescents were not available. As we did not recruit a healthy control group of adolescents, we were not able to directly assess how severe neurocognitive difficulties in our sample actually have been.
5. CONCLUSION
The findings from this study, together with the knowledge from the existing literature, indicate that inefficiencies in set‐shifting and central coherence may be less severe in adolescent patients with AN and sensitive to change over the course of treatment. As lower levels of cognitive flexibility prior to the start of psychotherapy tended to be associated with worse eating disorder outcomes in the long term, interventions specifically addressing flexibility in thinking and behaviour may contribute to the treatment success. The adolescent age group may be particularly sensitive to intervention approaches targeting neurocognitive inefficiencies. More studies, including randomised‐controlled trials, are needed to evaluate the longitudinal effects of integrating tools to promote set‐shifting and central coherence in the treatment of adolescent AN.
AUTHOR CONTRIBUTIONS
Conceptualisation: Gudrun Wagner, Andreas Karwautz, Stefanie Truttmann; Methodology: Michael Zeiler, Tanja Wittek; Investigation and Recruitment: Tanja Wittek, Stefanie Truttmann, Leonie Kahlenberg, Andrea Schneider, Ellen Auer‐Welsbach, Michaela Mitterer and Clarissa Laczkovics; Resources: Tanja Wittek, Julia Philipp, Konstantin Kopp, Stefanie Truttmann, Gudrun Wagner, Sonja Werneck‐Rohrer, Petra Sackl‐Pammer; Writing – Original Draft preparation: Tanja Wittek, Michael Zeiler; Writing – Review & Editing: Tanja Wittek, Michael Zeiler, Gudrun Wagner, Andreas Karwautz, Ulrike Schmidt; Visualisation: Tanja Wittek, Michael Zeiler; Supervision: Ulrike Schmidt, Suanne Ohmann; Project Administration: Tanja Wittek; Funding Acquisition: Gudrun Wagner, Andreas Karwautz. The authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
This study was funded by ‘Gemeinsame Gesundheitsziele – Pharma Master Agreement’ (a cooperation between the Austrian pharmaceutical industry and the Austrian social insurance) – project code: 99901006800. US is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at South London and Maudsley National Health Service Foundation Trust (SLaM) and King's College London (KCL) and the Medical Research Council (grant MR/W002418/1: ‘Eating Disorders: Delineating illness and recovery trajectories to inform personalised prevention and early intervention in young people (EDIFY)).
Wittek, T. , Zeiler, M. , Truttmann, S. , Philipp, J. , Kopp, K. , Krauss, H. , Auer‐Welsbach, E. , Ohmann, S. , Sackl‐Pammer, P. , Werneck‐Rohrer, S. , Laczkovics, C. , Mitterer, M. , Schneider, A. , Kahlenberg, L. , Schmidt, U. , Karwautz, A. , & Wagner, G. (2025). Exploring neurocognitive features in adolescents and young adults with anorexia nervosa: Evidence from a longitudinal study. European Eating Disorders Review, 33(1), 20–34. 10.1002/erv.3127
Handling Editor: Daniel Le Grange
Tanja Wittek and Michael Zeiler are contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data related to this manuscript are available from the corresponding author upon reasonable request.
REFERENCES
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders DSM‐5 (5th ed.). [DOI] [PubMed]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Booth, R. D. L. (2006). Local‐global processing and cognitive style in autism spectrum disorders and typical development. [Ph.D]. University of London. Retrieved from https://kclpure.kcl.ac.uk/portal/en/theses/localglobal‐processing‐and‐cognitive‐style‐in‐autism‐spectrum‐disorders‐and‐typical‐development(99cff8ab‐25d8‐4530‐8e66‐dc089e8db9fd).html [Google Scholar]
- Drühe‐Wienholt, C.‐M. , & Wienholt, W. (2013). Computergestütztes Kartensortierverfahren (CKV). Modifizierte Version des Wisconsin Kartensortiertests. Pearson. [Google Scholar]
- Federal Ministry of Social Affairs, Health, Care and Consumer Protection . (2020). Patientinnen/Patienten‐Information über die in Österreich anerkannten psychotherapeutischen Verfahren.
- Franta, C. , Philipp, J. , Waldherr, K. , Truttmann, S. , Merl, E. , Schöfbeck, G. , Koubek, D. , Laczkovics, C. , Imgart, H. , Zanko, A. , Zeiler, M. , Treasure, J. , Karwautz, A. , & Wagner, G. (2018). Supporting carers of children and adolescents with eating disorders in Austria (SUCCEAT): Study protocol for a randomised controlled trial. European Eating Disorders Review, 26(5), 447–461. 10.1002/erv.2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglset, T. S. (2019). Set‐shifting, central coherence and decision‐making in individuals recovered from anorexia nervosa: A systematic review. Journal of Eating Disorders, 7(1), 22. 10.1186/s40337-019-0251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giombini, L. , Nesbitt, S. , Kusosa, R. , Fabian, C. , Sharia, T. , Easter, A. , & Tchanturia, K. (2022). Neuropsychological and clinical findings of Cognitive Remediation Therapy feasibility randomised controlled trial in young people with anorexia nervosa. European Eating Disorders Review, 30(1), 50–60. 10.1002/erv.2874 [DOI] [PubMed] [Google Scholar]
- Gönner, S. , Leonhart, R. , & Ecker, W. (2007). Das Zwangsinventar OCI‐R ‐ die deutsche Version des Obsessive‐Compulsive Inventory‐Revised. PPmP ‐ Psychotherapie · Psychosomatik · Medizinische Psychologie, 57(9/10), 395–404. 10.1055/s-2007-970894 [DOI] [PubMed] [Google Scholar]
- Grant, J. E. , & Chamberlain, S. R. (2023). Impaired cognitive flexibility across psychiatric disorders. CNS Spectrums, 28(6), 688–692. 10.1017/S1092852923002237 [DOI] [PubMed] [Google Scholar]
- Harper, J. A. , Brodrick, B. , Van Enkevort, E. , & McAdams, C. J. (2017). Neuropsychological and cognitive correlates of recovery in anorexia nervosa. European Eating Disorders Review, 25(6), 491–500. 10.1002/erv.2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger, M. , Keller, F. , & Kühner, C. (2006). Beck depressions inventar, 2. Auflage (BDI‐II). Pearson. [Google Scholar]
- Heaton, R. K. (1981). Wisconsin card sorting test manual. Psychological Assessment Resources. [Google Scholar]
- Herbrich, L. , van Noort, B. , Pfeiffer, E. , Lehmkuhl, U. , Winter, S. , & Kappel, V. (2017). Follow‐up assessment of cognitive remediation therapy in adolescent anorexia nervosa: A pilot study. European Eating Disorders Review, 25(2), 104–113. 10.1002/erv.2501 [DOI] [PubMed] [Google Scholar]
- Hilbert, A. , & Tuschen‐Caffier, B. (2016). Eating disorder examination—questionnaire (2 edition). PAG Institut für Psychologie AG. Retrieved from http://www.vfp‐muenster.de/publikationen/online/EDE_VfP_1.pdf [Google Scholar]
- Keegan, E. , Byrne, S. , Hay, P. , Touyz, S. , Treasure, J. , Schmidt, U. , McIntosh, V. V. W. , & Wade, T. D. (2022). An exploratory examination of executive functioning as an outcome, moderator, and predictor in outpatient treatment for adults with anorexia nervosa. Journal of Eating Disorders, 10(1), 83. 10.1186/s40337-022-00602-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan, E. , Tchanturia, K. , & Wade, T. D. (2021). Central coherence and set‐shifting between nonunderweight eating disorders and anorexia nervosa: A systematic review and meta‐analysis. International Journal of Eating Disorders, 54(3), 229–243. 10.1002/eat.23430 [DOI] [PubMed] [Google Scholar]
- Kjaersdam Telléus, G. , Jepsen, J. R. , Bentz, M. , Christiansen, E. , Jensen, S. O. W. , Fagerlund, B. , & Thomsen, P. H. (2015). Cognitive profile of children and adolescents with anorexia nervosa. European Eating Disorders Review, 23(1), 34–42. 10.1002/erv.2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromeyer‐Hauschild, K. , Wabitsch, M. , Kunze, D. , Geller, F. , Geiß, H. C. , Hesse, V. , von Hippel, A. , Jaeger, U. , Johnsen, D. , Korte, W. , Menner, K. , Müller, G. , Müller, J. M. , Niemann‐Pilatus, A. , Remer, T. , Schaefer, F. , Wittchen, H. U. , Zabransky, S. , Zellner, K. , …, Hebebrand, J. (2001). Perzentile für den Body‐mass‐Index für das Kindes‐und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschrift Kinderheilkunde, 149(8), 807–818. 10.1007/s001120170107 [DOI] [Google Scholar]
- Lang, K. , Roberts, M. , Harrison, A. , Lopez, C. , Goddard, E. , Khondoker, M. , Treasure, J. , & Tchanturia, K. (2016). Central coherence in eating disorders: A synthesis of studies using the Rey Osterrieth complex figure test. PLoS One, 11(11), e0165467. 10.1371/journal.pone.0165467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, K. , Stahl, D. , Espie, J. , Treasure, J. , & Tchanturia, K. (2014). Set shifting in children and adolescents with anorexia nervosa: An exploratory systematic review and meta‐analysis. International Journal of Eating Disorders, 47(4), 394–399. 10.1002/eat.22235 [DOI] [PubMed] [Google Scholar]
- Laux, L. , Glanzmann, P. , Schaffner, P. , & Spielsberger, C. D. (1981). Stai ‐ das state‐trait‐angstinventar. Hogrefe. [Google Scholar]
- McAnarney, E. R. , Zarcone, J. , Singh, P. , Michels, J. , Welsh, S. , Litteer, T. , Wang, H. , & Klein, J. D. (2011). Restrictive anorexia nervosa and set‐shifting in adolescents: A biobehavioral interface. Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine, 49(1), 99–101. 10.1016/j.jadohealth.2010.11.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers, J. E. , & Meyers, K. R. (1995). Rey complex figure test and recognition trial (RCFT). Par. [Google Scholar]
- Miles, S. , Gnatt, I. , Phillipou, A. , & Nedeljkovic, M. (2020). Cognitive flexibility in acute anorexia nervosa and after recovery: A systematic review. Clinical Psychology Review, 81, 101905. 10.1016/j.cpr.2020.101905 [DOI] [PubMed] [Google Scholar]
- Miles, S. , Howlett, C. A. , Berryman, C. , Nedeljkovic, M. , Moseley, G. L. , & Phillipou, A. (2021). Considerations for using the Wisconsin Card Sorting Test to assess cognitive flexibility. Behavior Research Methods, 53(5), 2083–2091. 10.3758/s13428-021-01551-3 [DOI] [PubMed] [Google Scholar]
- Nelson, H. E. (1976). A modified card sorting test sensitive to frontal lobe defects. Cortex, 12(4), 313–324. 10.1016/S0010-9452(76)80035-4 [DOI] [PubMed] [Google Scholar]
- Oldershaw, A. , Lavender, T. , & Schmidt, U. (2018). Are socio‐emotional and neurocognitive functioning predictors of therapeutic outcomes for adults with anorexia nervosa? European Eating Disorders Review, 26(4), 346–359. 10.1002/erv.2602 [DOI] [PubMed] [Google Scholar]
- Osterrieth, P. (1944). Test of copying a complex figure: Contribution to the study of perception and memory. Archives de Psychologie, 30, 206–356. [Google Scholar]
- Philipp, J. , Truttmann, S. , Zeiler, M. , Franta, C. , Wittek, T. , Schöfbeck, G. , Mitterer, M. , Mairhofer, D. , Zanko, A. , Imgart, H. , Auer‐Welsbach, E. , Treasure, J. , Wagner, G. , & Karwautz, A. F. K. (2020). Reduction of high expressed emotion and treatment outcomes in anorexia nervosa—Caregivers’ and adolescents’ perspective. Journal of Clinical Medicine, 9(7), 2021. 10.3390/jcm9072021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind, C. , Mahdi, M. , Simic, M. , Espie, J. , & Tchanturia, K. (2022). Group cognitive remediation therapy for children and adolescents in intensive day‐patient treatment for anorexia nervosa: A feasibility study. Neuropsychiatrie: Klinik, Diagnostik, Therapie Und Rehabilitation: Organ Der Gesellschaft Osterreichischer Nervenarzte Und Psychiater, 36(3), 125–135. 10.1007/s40211-022-00420-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrar, L. , Warschburger, P. , Pfeiffer, E. , Lehmkuhl, U. , & Schneider, N. (2013). Kognitive Flexibilität bei jugendlichen Patienten mit unipolaren Affektiven Störungen unter Berücksichtigung geschlechtsbezogener Unterschiede. Zeitschrift für Kinder‐ und Jugendpsychiatrie und Psychotherapie, 41(4), 261–270. 10.1024/1422-4917//a000240 [DOI] [PubMed] [Google Scholar]
- Saure, E. , Laasonen, M. , Lepistö‐Paisley, T. , Mikkola, K. , Ålgars, M. , & Raevuori, A. (2020). Characteristics of autism spectrum disorders are associated with longer duration of anorexia nervosa: A systematic review and meta‐analysis. International Journal of Eating Disorders, 53(7), 1056–1079. 10.1002/eat.23259 [DOI] [PubMed] [Google Scholar]
- Schmidt, U. , Magill, N. , Renwick, B. , Keyes, A. , Kenyon, M. , Dejong, H. , Lose, A. , Broadbent, H. , Loomes, R. , Yasin, H. , Watson, C. , Ghelani, S. , Bonin, E.‐M. , Serpell, L. , Richards, L. , Johnson‐Sabine, E. , Boughton, N. , Whitehead, L. , Beecham, J. , …, Landau, S. (2015). The Maudsley outpatient study of treatments for anorexia nervosa and related conditions (mosaic): Comparison of the Maudsley model of anorexia nervosa treatment for adults (MANTRA) with specialist supportive clinical management (SSCM) in outpatients with broadly defined anorexia nervosa: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 83(4), 796–807. 10.1037/ccp0000019 [DOI] [PubMed] [Google Scholar]
- Schmidt, U. , & Treasure, J. (2006). Anorexia nervosa: Valued and visible. A cognitive‐interpersonal maintenance model and its implications for research and practice. British Journal of Clinical Psychology, 45(3), 343–366. 10.1348/014466505x53902 [DOI] [PubMed] [Google Scholar]
- Schneider, S. , Unnewehr, S. , & Margraf, J. (2009). Diagnostisches Interview bei psychischen Störungen im Kindes‐und Jugendalter (Kinder‐DIPS) (2. aktualisierte und erweiterte Auflage). Springer. [Google Scholar]
- Stedal, K. , Broomfield, C. , Hay, P. , Touyz, S. , & Scherer, R. (2021). Neuropsychological functioning in adult anorexia nervosa: A meta‐analysis. Neuroscience & Biobehavioral Reviews, 130, 214–226. 10.1016/j.neubiorev.2021.08.021 [DOI] [PubMed] [Google Scholar]
- Steinhausen, H.‐C. (2002). The outcome of anorexia nervosa in the 20th century. American Journal of Psychiatry, 159(8), 1284–1293. 10.1176/appi.ajp.159.8.1284 [DOI] [PubMed] [Google Scholar]
- Tchanturia, K. (Hrsg). (2015). Cognitive remediation therapy (CRT) for eating and weight disorders. Routledge. [DOI] [PubMed] [Google Scholar]
- Tchanturia, K. , Davies, H. , Roberts, M. , Harrison, A. , Nakazato, M. , Schmidt, U. , Treasure, J. , & Morris, R. (2012). Poor cognitive flexibility in eating disorders: Examining the evidence using the Wisconsin card sorting task. PLoS One, 7(1), e28331. 10.1371/journal.pone.0028331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchanturia, K. , Giombini, L. , Leppanen, J. , & Kinnaird, E. (2017). Evidence for cognitive remediation therapy in young people with anorexia nervosa: Systematic review and meta‐analysis of the literature. European Eating Disorders Review: The Journal of the Eating Disorders Association, 25(4), 227–236. 10.1002/erv.2522 [DOI] [PubMed] [Google Scholar]
- Tenconi, E. , Collantoni, E. , Meregalli, V. , Bonello, E. , Zanetti, T. , Veronese, A. , Meneguzzo, P. , & Favaro, A. (2021). Clinical and cognitive functioning changes after partial hospitalization in patients with anorexia nervosa. Frontiers in Psychiatry, 12, 653506. 10.3389/fpsyt.2021.653506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure, J. , & Schmidt, U. (2013). The cognitive‐interpersonal maintenance model of anorexia nervosa revisited: A summary of the evidence for cognitive, socio‐emotional and interpersonal predisposing and perpetuating factors. Journal of Eating Disorders, 1(1), 13. 10.1186/2050-2974-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truttmann, S. , Philipp, J. , Zeiler, M. , Franta, C. , Wittek, T. , Merl, E. , Schöfbeck, G. , Koubek, D. , Laczkovics, C. , Imgart, H. , Zanko, A. , Auer‐Welsbach, E. , Treasure, J. , Karwautz, A. F. K. , & Wagner, G. (2020). Long‐term efficacy of the workshop vs. Online SUCCEAT (supporting carers of children and adolescents with eating disorders) intervention for parents: A quasi‐randomised feasibility trial. Journal of Clinical Medicine, 9(6), 1912. 10.3390/jcm9061912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood, H. , Stahl, D. , Mandy, W. , & Tchanturia, K. (2016). The set‐shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin card sorting test: A systematic review and meta‐analysis. Psychological Medicine, 46(9), 1809–1827. 10.1017/S0033291716000581 [DOI] [PubMed] [Google Scholar]
- Wittek, T. , Truttmann, S. , Zeiler, M. , Philipp, J. , Auer‐Welsbach, E. , Koubek, D. , Ohmann, S. , Werneck‐Rohrer, S. , Sackl‐Pammer, P. , Schöfbeck, G. , Mairhofer, D. , Kahlenberg, L. , Schmidt, U. , Karwautz, A. F. K. , & Wagner, G. (2021). The Maudsley model of anorexia nervosa treatment for adolescents and young adults (MANTRa): A study protocol for a multi‐center cohort study. Journal of Eating Disorders, 9(1), 33. 10.1186/s40337-021-00387-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek, T. , Zeiler, M. , Truttmann, S. , Philipp, J. , Kahlenberg, L. , Schneider, A. , Kopp, K. , Krauss, H. , Auer‐Welsbach, E. , Koubek, D. , Ohmann, S. , Werneck‐Rohrer, S. , Sackl‐Pammer, P. , Laczkovics, C. , Mitterer, M. , Schmidt, U. , Karwautz, A. , & Wagner, G. (2023). The Maudsley model of anorexia nervosa treatment for adolescents and emerging adults: A multi‐centre cohort study. European Eating Disorders Review, 1–16. 10.1002/erv.2996 [DOI] [PubMed] [Google Scholar]
- Wu, M. , Brockmeyer, T. , Hartmann, M. , Skunde, M. , Herzog, W. , & Friederich, H.‐C. (2014). Set‐shifting ability across the spectrum of eating disorders and in overweight and obesity: A systematic review and meta‐analysis. Psychological Medicine, 44(16), 3365–3385. 10.1017/S0033291714000294 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
The data related to this manuscript are available from the corresponding author upon reasonable request.


