Abstract
Saikosaponin A (SSA), the primary active monomer derived from the Radix bupleuri, demonstrates a diverse array of pharmacological activities, including anti-inflammatory, antitumor, analgesic, anti-fibrotic, antidepressant, and immune-modulating properties. Despite its potential therapeutic impact on various human diseases, comprehensive studies exploring SSA's efficacy in these contexts remain limited. This review synthesizes the current research landscape regarding SSA's therapeutic applications across different diseases, highlighting critical insights to overcome existing limitations and clinical challenges. The findings underscore the importance of further investigations into SSA's mechanisms of action, facilitating the development of targeted therapeutic strategies and their translation into clinical practice.
Keywords: Radix bupleuri, Saikosaponin a, Pharmacological mechanism, Anti-inflammatory, Antitumor, Antidepressant, Neuroprotective
Abbreviations
- SSA
Saikosaponin A
- NF-κB
nuclear factor kappa-B
- MAPK
mitogen-activated protein kinases
- ROS
reactive oxygen species
- CKD
chronic kidney disease
- P-gp
P-glycoprotein
- CRH
corticotropin-releasing hormone
- CUMS
chronic unpredictable mild stress
- PPAR
peroxisome proliferator-activated receptor
- LPS
lipopolysaccharide
- CREB
cyclic response element binding protein
- BDNF
brain-derived neurotrophic factor
- ALI
acute lung injury
1. Introduction
The radix bupleurum is obtained from the root of Bupleurum Chinese DC, which belongs to the Umbelliferae family, it typically grows mainly in the subtropical regions of the Northern Hemisphere [1],Chaihu contains abundant natural metabolites, such as phenolics, lignans, flavonoids, terpenoids (triterpenoids and sterols), mono- and sesquiterpenes (essential oils), and polyacetylenes, Pharmacological studies have confirmed that radix bupleurum exhibits various biological functions, including hepatoprotective, antitumor, anticonvulsant, antidepressant, antipyretic, analgesic, antibacterial, antiviral, anti-inflammatory, antioxidant, and immunomodulatory effects [2,3]. It is primarily utilized for the treatment of colds, fevers, cold, imbalances in cold and heat, and stagnation of liver qi. In clinical practice, a range of patented traditional Chinese medicines or herbal formulations containing Bupleurum are frequently utilized in China. These include Xiao-Yao-Wan tablets, Xiao-Chai-Hu-Tang, Da-Chai-Hu-Tang, Chai-Hu-Shu-Gan tablets, and Chai-Hu-Shu-Gan-San [4]. Saikosaponins (SSs) are one of the major active components of radix bupleurum and a triterpenoid saponin extracted only from Bupleurum plants, known for their diverse pharmacological characteristics [5]. Saikosaponins can be further categorized as Saikosaponin A, B1, B2, B3, B4, C, D, E, F, G, H, and I [6]. It demonstrates various effects, including anti-endotoxin and corticosterone hormone properties. It has the ability to inhibit the activity of sodium-potassium ATPase and is extensively used as a widely recognized medicinal herb [7,8]. In recent decades, these herbal medicines have attracted considerable scholarly attention owing to their potential therapeutic properties,including anti-inflammatory, antiviral, anticancer, and hepatoprotective effects [4,9,10]. Among these, SSA and SSD have exhibited biological activity [11]. SSD has been observed to demonstrate antitumor and anti-inflammatory effects through various mechanisms [4,[12], [13], [14]]. The molecular formula of SSA is C42H68O13. It is a triterpenoid saponin compound with a molecular mass of 781. SSA has a melting point that ranges between 224 and 230 °C and is soluble in both water and alcohol [15]. Pharmacological investigations of SSA have predominantly concentrated on its anti-inflammatory properties, particularly through the modulation of nuclear factor activation within signaling pathways, including nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinases (MAPK). In a study involving mice with chronic kidney disease, SSA treatment markedly improved renal histomorphology, evidenced by reduced inflammatory cell infiltration, decreased vacuolization of renal tubular epithelial cells, and repair of interstitial dilatation and renal fibrosis, alongside the modulation of extracellular regulated protein kinases (ERK) in B cells [[16], [17], [18]]. Additionally,SSA has demonstrated significant efficacy in antitumor, neuroprotective, and organ damage prevention [4,19,20]. The development of SSA-based therapeutics could have profound implications for the treating of various human diseases, including tumors, inflammation, depression, and organ damage. This article aims to provide a comprehensive review of SSA research, emphasizing its application in these context. It will also address the limitations and challenges encountered in SSA research and its potential future clinical applications.
2. Search strategy
I conducted searches in online academic databases, namely PubMed, Web of Science, Google Scholar, and CNKI, using the search terms "Saikosaponin A″ OR "Radix Bupleuri" OR "Bu-pleurum" AND "Cancer" OR "Tumor" OR "Inflammatory" OR "Depression" OR "Neuroprotective" OR "Organ Protective". We have included relevant literature for the research topic.
3. Anti-inflammatory activity of SSA
The anti-inflammatory activity of SSA has garnered extensive interest due to its significant therapeutic potential. Evidence suggests that SSA inhibits inflammatory response in chronic kidney disease (CKD) models by reducing levels of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α, while increasing IL-10. Treatment with SSA has demonstrated improvements in renal histomorphology, notably decreasing inflammatory cell infiltration, tubular epithelial cell vacuolization, and alleviating interstitial dilatation and fibrosis [21]. These beneficial effects can be attributed to SSA's potent antioxidant properties, which counteract oxidative stress that exacerbates inflammation, thereby reducing the risk of oxidative damage in CKD models [22]. These findings suggest that SSA effectively reduces oxidative damage in mice with CKD. Prolonged oxidative stress in the kidneys results in chronic inflammation and renal insufficiency, both of which can be significantly alleviated by SSA treatment [23]. Similarly, SSA decreases the inflammatory response in acute pancreatitis, a condition often exacerbated by heightened oxidative stress. This oxidative stress worsens pancreatic injury by elevating glutathione levels. SSA mitigates inflammation in acute pancreatitis by modulating gut microbiota and enhancing antioxidant signaling. Furthermore, SSA enhances antioxidant and anti-inflammatory properties, leading to improved lesions associated with acute pancreatitis in rat models [24]. It has been proposed that SSA inhibits the secretion of inflammatory cytokines through the enhancement of lipid metabolism and modulation of NF-κB signaling, thus reducing the severity of hyperlipidemic pancreatitis in animal models. These alterations in gut microbiota induced by SSA may offer protective effects against acute pancreatitis and contribute to the prevention of inflammatory diseases overall [25].
In vitro studies demonstrate that SSA exhibits anti-inflammatory effects in autoimmune conditions such as ulcerative colitis, characterized by an exaggerated mucosal immune response [26]. The pathogenesis of ulcerative colitis involves a complex interplay of inflammatory responses [27], with elevated levels of cytokines like TNF-α and IL-1β stimulating mucosal immune activation [28]. SSA enhances antioxidant enzyme activity, yielding neuroprotective effects in a mouse model of 5-fluorouracil-induced intestinal mucositis [29]. It also provides protection against dextran sodium sulfate-induced colitis by inhibiting TNF-α and IL-1β levles [30]. Moreover, SSA effectively suppresses chemically induced hepatic inflammation and fibrosis in rats by downregulating pro-inflammatory factors and modulating the NF-κB signaling pathway [10]. The inhibition of pro-inflammatory cytokine expression and the modulation of inflammation via signaling pathways, particularly NF-κB, are recognized mechanisms underlying SSA's anti-inflammatory activity [31]. Experimental evidence supports SSA's regulation of inflammation-related cytokines and signaling pathways, including NF-κB and MAPK [32,33]. Notably, Lu et al. first identified SSA as an NF-κB activation inhibitor, reducing NF-κB target gene expression in macrophages. They also reported that SSA directly inhibits COX2 protein expression, potentially linked to mRNA stability rather than transcriptional effects [31]. Additionally, SSA regulates lipid metabolism and promotes cholesterol efflux in early atherosclerosis stages. It may act as a peroxisome proliferator-activated receptor (PPAR)-γ agonist, enhancing PPAR-γ expression [34]. PPAR-γ interacts closely with NF-κB, inhibiting its signaling and the release of pro-inflammatory factors [35]. Animal studies suggest that SSA stimulates PPAR-γ expression and suppresses NF-κB signaling in pancreatic tissues, ultimately mitigating hyperlipidemic pancreatitis in rats through improved lipid metabolism and reduced pro-inflammatory cytokine release [36]. These findings suggest SSA's potential as a therapeutic agent for pancreatitis, highlighting its multifaceted role in regulating inflammatory responses and metabolic processes. The alterations in gut microbiota induced by SSA may also provide protective effects against acute pancreatitis and contribute to the prevention of various inflammatory diseases.
In a mouse model of pneumonia, SSA exhibited protective effects against lung inflammation induced by cigarette smoke, attributed to its capacity to suppress both inflammation and oxidative stress. The mechanisms for SSA's suppression of cigarette smoke-induced lung inflammation include the activation of Nrf2 and inhibition of the NF-κB signaling pathway [37]. Conversely, SSA induced apoptosis and suppressed the proliferation of HEKa cells through reactive oxygen species (ROS) production [16]. Previous research indicates that ROS can modulate NF-κB activity bidirectionally [38]. While NF-κB influences ROS production by regulating antioxidant protein expression [39]. Thus, the interaction between SSA-induced ROS production and NF-κB inhibition may be significant. In vitro studies have demonstrated that SSA inhibits the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 while simultaneously increasing IL-1 levels via the NF-κB pathway in macrophages and adipocytes treated with lipopolysaccharide (LPS) [17,40]. In vivo, SSA has been shown to prevent sepsis by inhibiting nucleotide-binding oligomerization domain 2 (NOD2)-mediated NF-κB activation [41].
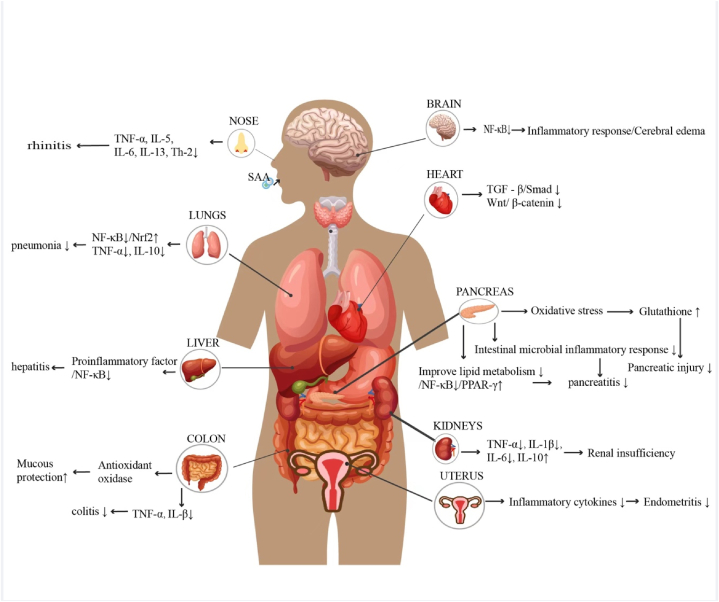
In allergic rhinitis, the levels of TNF-α, IL-5, IL-6, and IL-13 are upregulated. However, SSA administration significantly downregulates these cytokines, indicating its potential to reduce inflammation by inhibiting Th2 cytokines, which are instrumental in eosinophil recruitment [42,43]. Studies have shown a decrease in mast cells, eosinophils, and cup cells in SSA-treated mice, emphasizing SSA's role in preventing inflammatory cell infiltration into nasal tissues [44]. These findings indicate that SSA plays a role in reducing inflammation in allergic rhinitis by regulating the levels of pro-inflammatory cytokines. Moreover, pro-inflammatory cytokines play a crucial role in the progression of endometritis [45], characterized by inflammatory cell infiltration during LPS-induced endometritis [46]. The suppression of these cytokines lessens LPS-induced endometritis, with SSA demonstrating a protective effect likely due to its inhibition of inflammatory responses. This mechanism may involve the activation of the Nrf2 signaling pathway and the inhibition of the NF-κB signaling [47]. Liu et al. highlighted the beneficial effects of SSA on cardiac fibrosis through its inhibition of TGFβ/Smad signaling at higher doses and Wnt/β-cyclin signaling at lower doses [48]. Increasing evidence suggests that SSA exerts strong antioxidant and anti-inflammatory effects on cardiac remodeling [49,50]. In a study of LPS-induced acute lung injury (ALI) in mice, SSA notably reduced TNF-α and IL-1β expression while inhibiting the NF-κB and NLRP3 signaling pathway [51]. Additionally, SSA diminished the inflammatory response and reduced brain edema in rats with craniocerebral trauma, alleviating neuropathic pain via NF-κB inhibition [52,53]. Finally, in human umbilical vein endothelial cells, SSA suppresses oxidative stress and inflammation by reducing TLR4 translocation to lipid rafts [54]. Overall, SSA's ability to reduce inflammatory pathways across various models—including chronic kidney disease, acute pancreatitis, ulcerative colitis, and endometritis—highlights its potential as a therapeutic agent in treating inflammatory diseases. Its dual actions of promoting apoptosis and exerting anti-inflammatory effects necessitate further exploration to fully elucidate their interconnection and therapeutic implications.(Fig. 1, Table 1).
Fig. 1.
SSA exhibits protective effects across multiple organs, including the alleviation of inflammatory responses, preservation of organ function, reduction of oxidative stress, and improvement of metabolic processes. It mitigates rhinitis, brain edema, and inflammation by inhibiting the NF-κB signaling pathway. In cardiac protection, SSA acts through the suppression of TGF-β/Smad and Wnt/β-catenin pathways. For pulmonary inflammation, it reduces symptoms by inhibiting the NF-κB/Nrf2 signaling pathway. In liver inflammation, SSA similarly alleviates symptoms via NF-κB inhibition. It protects the pancreas by reducing oxidative stress and improving lipid metabolism, thereby minimizing pancreatic damage. In cases of renal dysfunction, SSA decreases inflammatory factors such as TNF-α, IL-1β, IL-6, and IL-10. It also alleviates endometrial inflammation by suppressing inflammatory cytokines and reduces colitis symptoms through its antioxidant properties and inhibition of TNF-α and IL-1β.
Table 1.
Anti-inflammatory activity of SSA.
| Disease Type | Model | SSA dosage | Findings | Suggested mechanism | Ref. |
|---|---|---|---|---|---|
| Chronic kidney disease | 5/6 nephrectomized mice in vivo and in vitro with Dexamethasone (Dex)-managed C2C12 myotubes. | 5 mg/kg; 1uM | decreasing oxidative stress, reduced CKD-related muscle atrophy. |
activated the PI3K/AKT/Nrf2 signaling pathway | [21] |
| Chronic inflammatory pain | rats were initially treated intraplantarly with complete Freund's adjuvant for induction of hyperalgesia | 2 mg/kg | Analgesic, anti-inflammatory | / | [33] |
| Atopic dermatitis | HaCaT cells; BALB/c mice challenged with 2,4-dinitrochlorobenzene | 1uM; 0.5 % | Inhibits production of multiple inflammatory cytokines and chemokines | Inhibition of MAPK signaling-mediated transcriptional activation of EGR1 | [55] |
| Obesity | 3T3-L1 cells | 1.875–15uM | Inhibition of lipogenesis | Activated AMPK-ERK-JNK-p38 signaling pathway | [56] |
| Severe acute pancreatitis | Sprague-Dawley (SD) rats through the injection of sodium taurocholate into the biliopancreatic duct | 10–40 mg/kg | Inhibition of oxidative stress, Anti-inflammatory, Improved gut microbiota composition | Activated Keap1-Nrf2-ARE signaling pathway | [24] |
| Acute pancreatitis | a rat model of hyperlipidemic pancreatitis | 10,20 mg/kg | Suppressing the inflammatory response, regulating the release of proinflammatory cytokines |
Inhibition of NF-κB signaling pathway | [36] |
| Allergic rhinitis | Mice were sensitized by intraperitoneal injection of OVA with aluminum hydroxide adjuvant | 2,10 mg/kg | improved epithelial disruption and interstitial edema, reduce inflammatory factors. | inhibition of IL-6/STAT3/ROR-γt and NF-κB pathway | [44] |
| Chronic pancreatitis and pancreatic fibrosis | Pancreatic stellate cells | 5-10μg/ml | reduced viability, proliferation, migration, and autophagy, promoted apoptosis, promotes collagen degradation and inhibits matrix deposition | regulated the AMPK/mTOR pathway | [57] |
| Psoriasis | HEKa cells and imiquimod (IMQ)-induced mice | 5-20μM; 37.5,75 mg/kg | Promotes apoptosis and promotes ROS production | inhibiting activation of NF-κB signaling |
[16] |
| Ulcerative colitis | dextran sulfate sodium-induced colitis in C57BL/6 mice | 5,10,20 mg/kg | Inhibition of inflammatory response | Activation of LXRα | [30] |
| Intestinal mucositis | 5-FU-induced intestinal mucositis in mice | 1,5,10 mg/kg | Protection against intestinal injury, inhibits oxidative stress, inhibits inflammatory factors | inhibiting activation of NF-κB signaling | [29] |
| Chronic obstructive pulmonary disease | mice were exposed to cigarette smoke | 5,10,20 mg/kg | Inhibits inflammatory mediators, inhibits oxidative stress | Activating Nrf2 and inhibiting NF-κB signaling pathway | [37] |
| Endometritis | lipopolysaccharide (LPS)-induced endometritis in mice | 5,10,20 mg/kg | Inhibits production of inflammatory cytokines | Activating Nrf2 and inhibiting NF-κB signaling pathway | [47] |
| Obesity | 3T3-L1 adipocytes | 100 nM | Inhibition of inflammatory factors | Inhibition of ERK and NF-κB signaling | [40] |
| Sepsis | cecal ligation and puncture | 1,2.5,5 mg/kg | Decreased secretion of pro-inflammatory mediators | inhibition of the NOD2-mediated NF-κB signaling pathway | [41] |
| Inflammation | LPS-stimulated mouse RAW 264.7 | 3.125–25uM | Suppression of pro-inflammatory cytokines | Inhibition of NF-κB and MAPK signaling pathway | [17] |
4. Antitumor activity of SSA
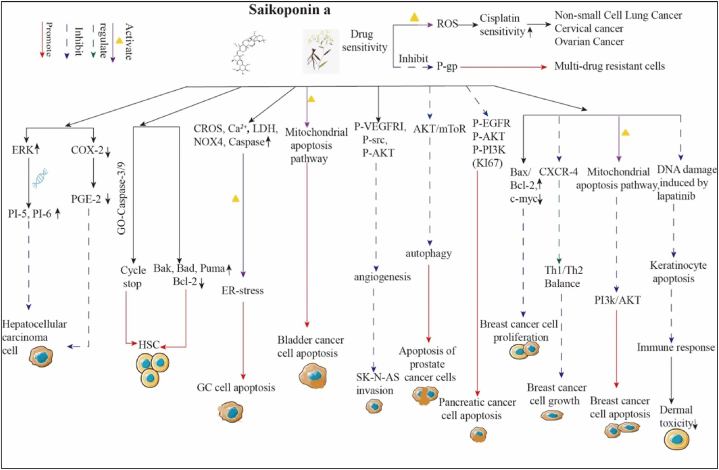
Natural medicines derived from plants, foods, and fruits are often regarded as low in toxicity and considered safe, making them attractive options for enhancing anticancer activity [58,59]. There is growing interest to exploring natural compounds for cancer treatment. A study found that the aqueous extract of Radix bupleurum alleviated DNA damage induced by 5-fluorouracil in HepG2 hepatocellular carcinoma cells, promoting cancer cell death while sparing normal human lymphocytes. Co-treatment with 5-fluorouracil and Radix bupleurum extract increased the expression of the apoptotic protein BAX and decreased the mitochondrial membrane potential in HepG2 cells, indicating potential for targeted hepatocellular carcinoma therapy [60]. Active components in Radix bupleurum, particularly SSA and SSD, have been extensively studied for their potential anticancer properties. SSA inhibits the growth of HepG2 cells by activating ERK and its downstream cell cycle regulators, p15 (INK4b) and p16 (INK4a), inducing growth arrest [61]. Wang et al.'s research suggests that SSA may inhibit the proliferation of hepatocellular carcinoma cells through a dose-time effect. This is achieved by decreasing the expression of COX-2 and down-regulating the levels of prostaglandin E2 (PGE2). This mechanism could potentially contribute to the anticancer effects of SSA in hepatocellular carcinoma [61,62]. Additionally, SSA induces cell death in hematopoietic stem cells via caspase-dependent and independent pathways, as well as through a mitochondria-dependent intrinsic pathway [63]. SSA, a novel inhibitor of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, induces ER stress and apoptotic cell death in gastric cancer cells through the generation of ROS, elevation of intracellular calcium levels, increase in LDH activity, upregulation of Nox4 expression, and activation of cysteine asparaginase activity. Both in vivo and in vitro studies have shown that SSA suppresses the proliferation of bladder cancer cells by triggering the mitochondrial apoptotic pathway and promoting apoptosis [64]. The combination of SSA and radiotherapy (2Gy) was discovered to effectively overcome radioresistance by suppressing the EMT phenotype. However, in PERK knockdown radioresistant AGSR cells, the use of SSA/2 Gy hindered apoptotic cell death by suppressing the PERK-ATF4-CHOP axis [65]. In breast cancer research, SSA inhibits the proliferation of MDA-MB-231 and MCF-7 cells by enhancing the Bax/Bcl-2 protein ratio and upregulating c-myc expression [66]. It has been shown to regulate the balance between Th1 and Th2 immune responses by reducing the expression of CXCR4 [67,68]. Furthermore, SSA inhibits the PI3K/AKT signaling pathway, leading to apoptosis through mitochondrial and ER stress pathways [69]. Additionally, SSA holds promise in counteracting the reduction in HMGB1 transcription induced by lapatinib, thereby mitigating lapatinib-induced DNA damage, preventing apoptosis in keratinocytes, and alleviating dermatological toxicity observed in murine models of breast cancer [70]. This potential suggests that the combination of SSA with lapatinib could offer a more effective therapeutic strategy for breast cancer by reducing toxicity while enhancing efficacy [67]. Furthermore, SSA exhibits significant inhibitory effects on the proliferation and DNA synthesis of hepatocellular carcinoma cell lines, specifically PLC/PRF/5 and Hep-G2 cells, as well as pancreatic cancer BxPC-3 cells [71]. SSA treatment resulted in a marked decrease in levels of phosphorylated EGFR, Akt, and PI3K across these pancreatic cancer models. Xenograft studies corroborated these findings, demonstrating SSA's capability to reduce pancreatic tumor weight and downregulate the expression of Ki67, a marker associated with cell proliferation [72]. Similarly, SSA has been shown to significantly inhibit the Akt/mTOR pathway, which plays a crucial role in negatively regulating autophagy. The cytotoxic effects of SSA on quiescent prostate cancer cells were nearly abolished with the overexpression of Akt,underscoring the necessity of the Akt/mTOR pathway in mediating these effects. Although the AKT inhibitor MK-2206 exhibited cytotoxic properties against quiescent prostate cancer cells, it was less effective compared to SSA [73]. Moreover, SSA has been reported to inhibit the proliferation of rat C6 glioma cells [74], promote their differentiation into astrocytes, and diminish macrophage immune function by targeting the PI3K/AKT pathway [34]. It notably inhibits tumor angiogenesis, displaying enhanced sensitivity in primary human umbilical vascular endothelial cells compared to tumor cells (4T1 and HCT-15). Additionally, SSA inhibits primary human umbilical vascular endothelial cell migration and tube formation in a dose-dependent manner. This antiangiogenic property of SSA was further validated in vivo experiments involving chick embryos [75]. The expression levels of p-VEGFR2, p-Src, and p-Akt were found to increase during angiogenesis, with SSA regulating the VEGFR2/Src/Akt pathway, thereby mitigating the invasion and migration of neuroblastoma (SK-N-AS) cells [76]. In addition, SSA demonstrates potent efficacy in overcoming tumor resistance mechanisms. It triggers an accumulation of ROS, which leads to apoptosis. Notably, SSA enhances the efficacy of the chemotherapeutic agent cisplatin across various cancer lines, including non-small cell lung cancer A549, cervical cancer (HeLa and Siha), and ovarian cancer (SKOV3) cell lines [77]. The association between P-glycoprotein (P-gp) expression and multidrug resistance in tumor cells is well-documented. Importantly, SSA has been observed to induce apoptosis in P-gp-overexpressing MCF-7 and HepG2 multidrug-resistant cells while decreasing P-gp expression, thereby augmenting the sensitivity of these chemotherapy-resistant cells to conventional treatments [78]. Thus, SSA holds promise as a sensitizer for drug-resistant cancer cells and exhibits noteworthy anticancer properties, suggesting its potential utility as an adjuvant therapy in combination with existing anticancer drugs to enhance treatment efficacy (Fig. 2, Table 2).
Fig. 2.
SSA significantly impacts cancer by inhibiting cell proliferation, inducing apoptosis, suppressing invasion, reducing cell growth, and alleviating skin toxicity. It inhibits hepatocellular carcinoma proliferation via ERK pathway suppression and COX-2 expression. In gastric cancer, SSA activates CROS, Ca2+, LDH, NOX4, and Caspase pathways to induce apoptosis. It triggers mitochondrial apoptosis in bladder cancer and inhibits neuroblastoma invasion by targeting P-VEGFR1, P-src, and P-AKT. In prostate cancer, SSA induces apoptosis through AKT/mTOR pathway inhibition, and in pancreatic cancer by suppressing P-EGFR, P-AKT, and P-PI3K signaling. In breast cancer, it inhibits proliferation via Bax/Bcl-2, c-myc, and CXCR-4 modulation, while reducing growth by affecting Th1/Th2 balance and the PI3K/AKT pathway. SSA also induces breast cancer cell apoptosis through mitochondrial pathways and DNA damage and promotes keratinocyte apoptosis by inhibiting the PI3K/AKT pathway, alleviating skin toxicity by suppressing immune responses.
Table 2.
Antitumor activity of SSA in vitro vivo.
| Disease Type | Model | SSA dosage | Findings | Suggested mechanism | Ref. |
|---|---|---|---|---|---|
| Pancreatic cancer | BxPC-3 and MIA PaCa-2; xenograft mouse model | 5,10,20uM | Pro-apoptotic | Inhibition of the EGFR/PI3K/Akt signaling pathway | [72] |
| Gastric Cancer | Gastric Cancer Cells; GC xenograft mouse model | 1-20μM; 5,10 mg/kg | Pro-apoptotic, enhancing radiation sensitivity | Activates ER stress and Ca2+ release | [65] |
| Prostate cancer | prostate cancer cells; xenograft mouse model | 2-30μM; 7.5 mg/kg | Induction of apoptosis, activation of autophagy, enhances chemosensitivity of Docetaxel | Inhibition of Akt/mTOR activity | [73] |
| Colorectal cancer | HCT 116 cells | 2-16uM | Promoting apoptosis | NR | [79] |
| Bladder cancer | T24 and 5637, orthotopic xenograft mice model | 3.75–15uM; 2,5,10 mg/kg | Inhibits proliferation, promotes apoptosis | NR | [64] |
| Cutaneous adverse reactions | HaCaT and NHEK cells; lapatinib-induced cutaneous toxicity | 15μM; 20 mg/kg | Inhibition of skin toxicity of lapatinib | mitigates lapatinib-induced cutaneous toxicity by restoring HMGB1 expression |
[70] |
| Antiangiogenesis | umbilical vein endothelial cells; chick embryo chorioallantoic membrane (CAM) and Matrigel plug model | 1-100uM | Inhibition of migration and angiogenesis | Suppressing VEGFR2 Signaling Pathway and Its Downstream Proteins |
[75] |
| Neuroblastoma | SK-N-AS cells | 2.5–20uM | Inhibition of migration and invasion, promoting apoptosis | Inhibition of VEGFR/Src/Akt pathway | [76] |
| Cervical cancer | HeLa cells; cervical cancer xenograft model | 5-20μM; 15 mg/kg | Inhibits growth and promotes apoptosis | Inhibition of PI3K/AKT signaling | [69] |
| Breast cancer | SUM149 and MDA-MB-231 cells; MDA-MB-231-Luc injected orthotopically into left mammary fat pads | 0.625–30μM; 12 mg/kg | Inhibition of migration and invasion, inhibition of lung metastasis | Inhibition of PI4K/Akt/mTOR and MMP signaling pathways | [67] |
| Breast cancer | 7,12-dimethyl-benz[a]anthracene induces breast cancer by gavage | 35 mg/kg | Inhibits growth and regulates immune infiltration | Activated the IL-12/STAT4 Pathway | [68] |
| Breast cancer | MCF, MCF-7/ADR | 2.5,5uM | Enhancing chemosensitivity and promoting apoptosis | Inhibition of P-glycoprotein-mediated multidrug resistance | [78] |
| Colon carcinoma | human colon carcinoma cell lines | 5-20uM | Promotes apoptosis and increases release of LDH | proteolytic caspase-2 activation and the caspase-8/Bid pathway |
[71] |
| Cervical cancer, ovarian cancer, non-small cell lung cancer | human cancer cells | 2,6,10uM | Enhances cisplatin sensitivity, promotes apoptosis, and increases ROS accumulation | NR | [77] |
| Breast cancer | MDA-MB-231 and MCF-7 cell lines | 2-10μg/ml | Inhibits proliferation and promotes apoptosis | Activation of p53/p21 signaling | [66] |
| Liver cancer | HepG2 | 12.5ug/ml | Inhibited growth | Activation of ERK signaling pathway by induction of p15INK4b/p16INK4a expression | [61] |
5. Antidepressant effects of SSA
Depression is a mental disorder characterized by persistent low mood and is often accompanied by various physical symptoms, including reduced appetite, bodily discomfort, and various physiological dysfunction. It is associated with high rates of prevalence, relapse, and disability [80]. Currently, four primary hypotheses have been proposed regarding the etiology of depression: the monoamine neurotransmitter hypotheses, the hypothalamus-pituitary-adrenal (HPA) axis hypothesis, the cytokine hypothesis, and the excitatory amino acid and endocrine hormone hypothesis [[81], [82], [83], [84]]. The prevailing consensus in research indicates that depression is primarily attributed to insufficient or dysregulated activity of neurotransmitters, particularly 5-hydroxytryptamine (5-HT) and noradrenaline (NE) in the central nervous system. Consequently, investigations targeting potential antidepressant drug mechanisms frequently concentrate on NE or 5-HT [85]. Recently, SSA has emerged as an compound exhibiting antidepressant properties [86]. Chronic elevation of HPA axis mediators, such as corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and glucocorticoids, has been linked to depressive-like behaviors, including impairments in memory, cognition, and neurogenesis [87]. However, the administration of SSA over four weeks resulted solely in the reversal of corticosterone elevation without significant changes in CRH levels in conditions of chronic unpredictable mild stress (CUMS) or serum following SSA treatment. Analysis of CRH mRNA and protein levels in the hypothalamus demonstrated increased CRH expression in the CUMS group and a decrease in the SSA group, aligning with clinical findings that suggest that perimenopausal depression does not correlate with altered basal hormone concentrations of the HPA axis [88]. This observation implies that SSA primarily exerts its influence on the HPA axis through central effects rather than peripheral modulation. Within the HPA circuit, the activity of the axis is primarily regulated by feedback mechanisms activated by glucocorticoid receptors in the hippocampus [89]. Although reduced glucocorticoid receptors have been associated with responses to persistently high glucocorticoid levels in depression [90], related studies indicate that CUMS can increase glucocorticoid receptor mRNA levels in the hippocampus —a response that is reversed by SSA [91]. A recent study supports this notion, showing that both mRNA and protein expression of glucocorticoid receptors were upregulated by CUMS [92]. In CUMS mice, however, glucocorticoid sensitivity is diminished due to the previously noted association where glucocorticoid receptor down-regulation reflects an increase in function rather than a decrease [93]. Consequently, down-regulation of glucocorticoid receptors by SSA suggests heightened sensitivity to glucocorticoids, illustrating the modulatory effect of SSA on HPA axis dysfunction. In conclusion, SSA seems to exert antidepressant-like effects, partially by enhancing neuroendocrine responses. Evidence suggesting that intracerebroventricular administration of SSA does not directly modify CRH and ACTH levels supports the inference that SSA does not directly impact the HPA axis [94]. Instead, SSA treatment appears to alleviate depressive symptoms by modulating the neuroendocrine, neuroinflammatory, and neurotrophic systems within the hippocampus of perimenopausal rats [94]. Therefore, it is hypothesized that the restoration of the HPA axis may arise indirectly through the suppression of neuroinflammation or enhancement of neurotrophic factors. Furthermore, SSA is proposed to exert antidepressant effects by elevating levels of proline-rich transmembrane protein 2 (PRRT2) and dopamine (DA) in the hippocampus of rats suffering from CUMS-induced depression [95]. Studies have demonstrated that SSA can effectively mitigates depressive symptoms in CUMS rats by augmenting PRRT2 expression and elevating DA levels in the hippocampus while simultaneously reducing the neurotoxic effects of excessive corticosterone on neural cells. Thus, PRRT2 is positioned as a promising target protein through which SSA mediates its antidepressant effects. Long-term administration of SSA at a dose of 50 mg/kg may upregulate PRRT2 expression levels, potentially increasing the likehood of PRRT2 and subsequent DA release [95]. The hippocampus is known to plays a significant role in the pathophysiology of depression [96], with reduced hippocampal volume recognized a reliable structural marker for the disorder [97]. CUMS has been found to induce hippocampal volume reduction by activating neuronal apoptotic pathways and diminishing neurogenesis [98]. The combination of postischemic separation and CUMS stimulation has been found to significantly increase the expression of neuronal apoptotic and pro-apoptotic proteins in the hippocampus, while SSA has been shown to reverse this effect [91]. Additionally, neurotrophic transcription factor phosphorylated cyclic response element binding protein (CREB) and brain-derived neurotrophic factor (BDNF) are crucial in the prevention and treatment of post-stroke depression [99,100]. Notably, nearly all treatments for depression, including antidepressants, exercise, and electroconvulsive therapy, have been shown to increase BDNF levels in both clinical and experimental contexts [[101], [102], [103]]. Therefore, the neurotrophic hypothesis emphasizes the role of BDNF and its function in neuronal survival and synaptic plasticity [104]. Parallel to this hypothesis, it has been demonstrated that CUMS reduces BDNF expression, which is subsequently reversed four weeks of SSA administration. Additionally, CUMS was found to diminish phosphorylated tropomycin receptor kinase B (TrkB) receptor levels, with SSA reversing this decline. These findings suggest that SSA induces antidepressant-like effects by upregulating the BDNF signaling pathway in the perimenopausal hippocampus [94]. Phosphorylated CREB, representing the active form of CREB, stimulates BDNF expression, which, conversely, promotes CREB activation through TrkB [105]. Repeated administration of antidepressants has been observed to enhance CREB activity and BDNF expression, leading to the protection of damaged nerves in depressed mice [106]. Additionally, rapid BDNF release has been associated with the reversal of stress-induced depressive behavior in mice [107]. Such findings emphasize the potential role of CREB and BDNF in the mechanisms underpinning antidepressant treatments and stress-related depressive behaviors. The involvement of p-CREB in neuronal apoptosis, partially mediated through BDNF, has been documented, revealing that various stimuli that alter CREB expression levels intricately influence the progression of neuronal apoptotic pathways [108,109]. Studies indicate that SSA treatment significantly upregulates p-CREB and BDNF expression while concurrently downregulating Bax and Caspase-3 levels. Consequently, the prevention of hippocampal neuronal apoptosis ultimately results in the improvement of depression-like behaviors in rats with post-stroke depression [110].
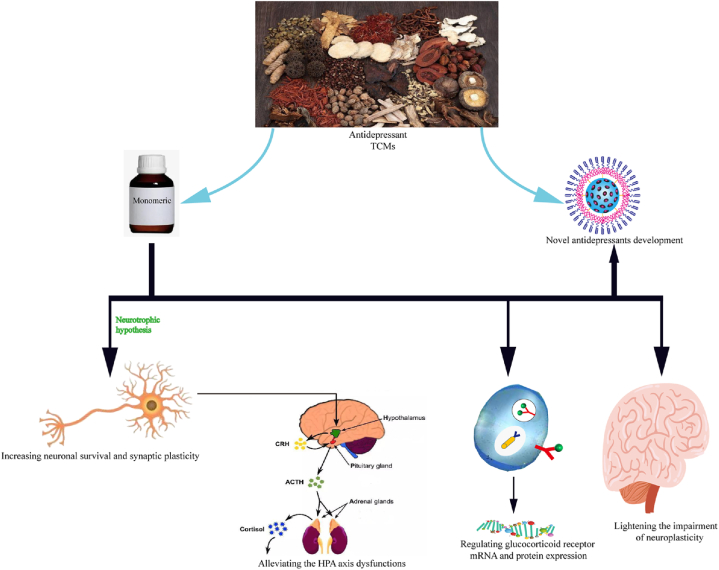
In conclusion, the therapeutic effect of SSA on depression may align more closely with the neurotrophic hypothesis rather than the direct modulation of the HPA axis. It is suggested that SSA may indirectly impact the HPA axis by influencing neurotrophic factors, thereby exerting an antidepressant effect (Table 3, Fig. 3).
Table 3.
Mechanism of action of SSA in antidepressants.
| Disease Type | Model | SSA dosage | Findings | Suggested mechanism | Ref. |
|---|---|---|---|---|---|
| Depression | CUMS combined with solitary confinement establish a depression model | 1-8uM | Increased 5-HT levels, decreased cortisol levels, antidepressant effects | NR | [111] |
| Post-stroke depression | middle cerebral artery occlusion + isolation + CUMS model | 5 mg/kg | Improving neurological function, inhibition of neuronal apoptosis | Activates p-CREB/BDNF signaling | [110] |
| Stress‐induced depression | CUMS depression model | 50 mg/kg | Increased body weight, increased sucrose consumption, increased dopamine | Regulates expression levels of PRRT2 | [95] |
| Perimenopausal depression | depression model induced by CUMS | 25,50,100 mg/kg | Reduce inflammatory factors, corticosterone decreased | Upregulated BDNF-TrkB signaling pathway | [94] |
| Depression | PC12 cells | 0.156-5uM | Regulation of metabolic disorders, inhibition of apoptosis | Inhibits NF-κB signaling pathway | [112] |
| Attention deficit hyperactivity disorder | spontaneously hypertensive rat | 12.5,25,50 mg/kg | DA levels in prefrontal cortex and striatum | Increased protein levels of BDNF | [113] |
Fig. 3.
Mechanism of action of SSA in antidepressants. SSA exerts its antidepressant effects by enhancing neuronal survival and synaptic plasticity, alleviating dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis, regulating the expression of glucocorticoid receptor mRNA and proteins, and mitigating neuroplasticity damage, thereby paving the way for novel antidepressant therapies.
6. Organ protective effects of SSA
The preservation of organs function following injury is crucial for the recovery of the affected tissues. Chinese medicine has garnered considerable interest due to its extensive clinical experience in addressing organ injuries [114]. Inflammation plays a pivotal role in the pathophysiology ALI [115],with research demonstrating that the inhibition of inflammatory cytokines such as TNF-α and IL-1β can attenuate ALI induced by LPS [116]. Additionally, previous research has shown that inhibition of NF-κB activation can also attenuate LPS-induced ALI in mice models [117]. Interestingly, treatment with SSA has been shown to significantly reduce the lung wet-to-dry weight ratio in a dose-dependent manner compared to LPS-treated controls. Furthermore, SSA treatment has alleviated LPS-induced pulmonary edema in mice, suggesting a potential protective effect against ALI-induced lung injury [118]. Histological analyses have demonstrated a visible reduction in lung injuries, including inflammatory cell infiltration, cell proliferation, and bleeding, following treatment with SSA [119]. Additionally, previous studies have proposed that the activation of the Nrf2 signaling pathway can mitigate renal injury. Observations indicate that SSA increases the expression of catalase and heme oxygenase-1(HO-1) in a dose-dependent manner, thereby providing a protective effect against kidney injury [120]. Furthermore, SSA has been demonstrated to protect against renal injury by activating the Nrf2 signaling pathway in response to lead-induced oxidative stress [121]. In the context of traumatic brain injury, SSA treatment has been associated with the attenuation of blood-brain barrier disruption and improved functional recovery, potentially through the suppression of inflammation via inhibition of the MAPK signaling pathway [52]. Moreover, in studies involving cerebral ischemia-reperfusion injury, SSA has been shown to attenuate the extent of cerebral infarction in rats subjected to middle cerebral artery occlusion while minimizing cerebral edema and neurological deficits [122,123]. These findings highlight the potential multi-organ protective effects of SSA in various injury and disease models. LXRα, a member of the nuclear hormone receptor superfamily, has been implicated in the regulation of inflammatory responses [124]. Activated LXRα has been shown to compete with IRF3 for binding and inhibit LPS-induced NF-κB transcriptional activity induced by LPS Kupffer cells. Additionally, SSA has been found to increase LXRα expression in a dose-dependent manner in LPS/D-GalN-induced liver injury [125]. Furthermore, supplementation with SSA has been demonstrated to improve hepatic antioxidant status and inhibit the formation of malondialdehyde, thereby protecting the liver by attenuating hepatic lipids and lipid peroxidation while enhancing antioxidant defense [126]. Thus, SSA may provide protection against liver injury by inhibiting the inflammatory response mediated by the NF-κB signaling pathway (Table 4).
Table 4.
Organ-protective and neuroprotective effects of SSA.
| Disease Type | Model | SSA dosage | Findings | Suggested mechanism | Ref. |
|---|---|---|---|---|---|
| Peripheral nerve injury | sciatic nerve injury model | 10 mg/kg | Inhibits nerve scarring, promoted recovery of lower extremity motor function and neurophysiological function | NR | [127] |
| Acute lung injury | LPS-induced ALI mice | 2.5,10 mg/kg | Inhibition of pulmonary edema, reduce pro-inflammatory cytokine levels, reduce lung tissue injury | Inhibition of TLR4/NF-κB pathway | [119] |
| Acute lung injury | LPS-induced ALI mice | 5,10,20 mg/kg | Decrease MPO activity, decreased lung W/D ratio, inhibition of inflammatory factors | Inhibits NF-κB and NLRP3 signaling pathways | [118] |
| Drug-induced liver injury | L-02 cells; ICR mice | 1-20μM; 75,150,300 mg/kg | Causing autophagy | up-regulate autophagy by triggering ER stress | [128] |
| Kidney injury | Lead-Induced Fish Kidney Injury Model | 5,10,20 mg/kg | Suppression of inflammatory factor levels, decreases SOD and MPO activity | Inhibits NF-κB signaling pathway and activates Nrf2 signaling pathway | [121] |
| Brain damage | Rat middle cerebral artery occlusion (MCAO) model | 0.001 %,0.01 %,0.1 % | Enhancing motor function, decreases brain water content, reduce inflammatory cytokine levels | Inhibits HMGB1 release and attenuates NF-κB activation | [123] |
| Liver injury | LPS/ D-galactosamine -induced mice liver injury model | 5,10,20 mg/kg | Decreases SOD and MPO activity, decreased AST and ALT levels, suppression of inflammatory factor levels | Inhibits NF-κB signaling pathway | [125] |
| Liver injury | CCl(4)-induced liver injury model in rats | 0.004 % | Decreased AST and ALT levels, improving liver fibrosis | NR | [126] |
| Insomnia | C57BL/6J mice | 0.625,1.25, 2.5 mg/kg | Increased sleep duration, improving sleep quality | Decreases neuronal activity in LH | [129] |
7. Neuroprotective effects of SSA
Saikosaponin, particularly its primary component SSA, has been associated with both antidepressant and neuroprotective properties [52,95]. A direct correlation has been established between scar formation, the release of inflammatory cytokines, and Saikosaponin 's activity [130]. Pro-inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6 have been shown to facilitate neural scarring, while anti-inflammatory factors like IL-10 have been observed to inhibit this process [131]. In a study by Ngeow et al. [132], the local administration of IL-10 peptide fragments at the site of sciatic nerve injury in C57/B6 mice significantly reduced nerve scarring and enhanced nerve regeneration. These findings suggest that SSA and Saikosaponin may have a potential role in modulating the inflammatory response and promoting neural regeneration. In further investigations, it has been shown that following sciatic nerve injury in rats, SSA promotes the recovery of motor and nerve conduction functions by increasing the expression of the anti-inflammatory cytokine IL-10 and inhibiting nerve scar formation [127]. Preconditioning with SSA appears to reduce the nuclear accumulation of NF-κB, which may lead to enhanced anti-inflammatory responses and contribute to the neuroprotective effects of SSA in the context of cerebral ischemia-reperfusion injury [123]. Inflammation often triggers the release of glutamate from glial cells into the extrasynaptic gap. This excess glutamate can activate extrasynaptic N-methyl-D-aspartate receptors, resulting in the disruption of synaptic integrity and, ultimately, neuronal loss [133]. Conversely, treatment with SSA can modulate glutamate metabolic disorders by inhibiting glutaminase activity, thereby exerting neuroprotective effects through mechanisms that regulate metabolic disturbances and attenuate neuroinflammation. These findings collectively underscore the potential of SSA and Saikosaponin in therapeutic strategies aimed at neural repair and inflammation modulation [112] (Table 4).
8. Other
Current research predominantly concentrates on the exploration of the anti-inflammatory, antitumor, antidepressant, post-organ injury protection, and neuroprotective properties of SSA, however, recent studies have revealed additional pharmacological effects. Notably, SSA exhibits properties as an analgesic, antiepileptic agent, as well as a modulator of osteoclast differentiation to a certain extent. Saikosaponin has demonstrated efficacy in mitigating neuropathic pain in rats with chronic contraction injury by modulating the p38-MAPK and NF-κB signaling pathways within the spinal cord [53]. The alleviation of neuropathic pain induced by chronic constriction injury may not solely be attributed to the anti-inflammatory effects of SSA but also to its antagonistic properties on TRPA1 channels. This idea is supported by previous research highlighting the role of TRPA1 in the development of neuropathic pain in mice models [134]. The analgesic activity of SSA has been demonstrated in thermal pain and formalin-induced pain tests conducted on mice [135]. In this context, thermal hyperalgesia, which is characterized by acute pain elicited by a thermal stimuli, involves signal transmission to the central nervous system through the spinal cord [136]. While SSA demonstrated an ability to inhibit thermal hyperalgesia, its analgesic effects did not exhibit a dependence on dosage. In the formalin-induced pain test, SSA did not exhibit significant inhibitory effects in phase I, however, it did demonstrated a concentration-dependent inhibition of formalin-induced pain in phase II [135]. Notably, SSA displayed a marked inhibitory effect on inflammatory pain by suppressing nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines, effectively reducing inflammatory pain through the NF-κB pathway in rats [137]. Recent investigations have also indicated that SSA can prevent and treat osteoporosis and cancer-induced bone loss through its inhibitory effects on osteoclast formation [138,139]. The data suggest that SSA not only promotes the differentiation of BMSC into osteoblasts both in vitro and in vivo settings but also helps counteract bone loss caused by estrogen deficiency. Notably, SSA enhances osteogenic differentiation of BMSCs during both early and mature stages, possibly through the WNT/β-linker signaling pathway [50]. Additionally, SSA has been found to inhibit osteoclastogenesis and mediate osteoclast activity in vitro. These effects occur through the inhibition of NF-κB and MAPK activation [138]. Moreover, SSA exhibits anticonvulsant and antiepileptic effects in a model of electroshock-induced epilepsy [140]. These effects may be achieved by inhibiting glial fibrillary acidic protein expression and glutamate-induced activation of rat hippocampal astrocytes. These findings highlight the diverse pharmacological properties of SSA [141]. It has been demonstrated that treatment with SSA significantly reduces the frequency and duration of recurrent seizures and enhances the production of voltage-gated potassium currents over an eight-week period [142,143]. However, further investigation is needed to fully elucidate the underlying mechanisms. In line with this, Yu et al. [144] have shown that SSA alleviates pentylenetetrazole-induced seizures and modulates the expression of inflammatory cytokines by inhibiting the activation of the p-mTOR/p-70S6K pathway. This suggests that SSA plays a role in mediating inflammatory cytokines in the context of analgesia, osteoblast differentiation, and antiepilepsy. The multifaceted effects of SSA highlight its potential as a versatile pharmacological agent.
9. Conclusions and perspectives
The research on traditional Chinese medicines (TCM) has received considerable attention in recent years, especially regarding their potential effectiveness and lower occurrence of adverse reactions compared to Western medicines for various difficult-to-treat diseases [145]. SSA, a crucial pharmacological component of traditional Chinese medicine, has been extensively investigated for its pharmacological effects and mechanisms of action, particularly in the fields of anti-inflammation, antitumor effects, organ protection, neuroprotection, and antidepressant properties [4]. The dual mechanisms of apoptosis mediated by SSA, which in involve the up-regulation of pro-apoptotic proteins and the concurrent down-regulation of Bcl-2, suggest that SSA may have a multifaceted impact on cellular apoptosis pathways. Such complexity in its mode of action could contribute to its potential effectiveness in targeting various types of cells and diseases [63]. The combination of SSA with conventional chemotherapeutic agents such as adriamycin, vincristine, or paclitaxel has shown promising results in overcoming chemoresistance by downregulating P-gp in multidrug-resistant (MDR) cells. Furthermore, the combination of these drugs has demonstrated significant induction of prostate cancer cell death and reduction of tumor volume compared to using doxorubicin or SSA alone. These findings underscore the potential synergistic effects of combining SSA with conventional chemotherapeutic agents for enhanced anticancer activity [73,78]. The anti-inflammatory properties of SSA have received significant attention for potential clinical application due to its low toxicity [146]. SSA has demonstrated the ability to inhibit the secretion of pro-inflammatory cytokines and promote the production of anti-inflammatory cytokines, thereby mitigating inflammatory responses [36]. Moreover, its modulation of the NF-κB signaling pathway suggests a protective role in inflammation by reducing the expression of pro-inflammatory cytokines. While existing studies have provided insights into its effectuveness against various diseases and clearer understanding of its molecular mechanisms, these findings are primarily based on cell and cell line xenograft models. To validate these observations, further exploration using preclinical organoid models and human tumor xenograft models is necessary.
Traditional Chinese prescriptions that are made from or contain Radix bupleuri, such as Xiao-Yao-Wan tablets, Chai-Hu-Shu-Gan tablets, and Chai-Hu-Shu-Gan-San, have been utilized in clinical settings. However, the clinical application and development of these prescriptions have been restricted due to the toxic side effects they may have on organs such as the liver, kidneys, and lungs when used excessively or for prolonged periods of time [4,147]. However, recent studies have found that the use of SSA in Radix bupleuri not only does not rsult in hepatotoxicity, but also helps to reduce acute liver injury. SSA is considered a crucial compound that exerts hepatoprotective effects. The protective effects are observed in a dose-dependent manner, with concentrations of the drug ranging from 10 to 100μM. Additionally, in in vivo experiments, the oral administration of Radix bupleuri significantly reduced CCL4-induced liver injury [148]. In addition, SSA has been found to attenuate LPS-induced acute lung injury [119]. A growing body of research has demonstrated that SSA has an anti-tumor effect in various types of cancer, including breast cancer, gastric cancer, liver cancer, and leukemia [[149], [150], [151], [152]]. Interestingly, SSA has also been shown to alleviate skin toxicity induced by chemotherapeutic drugs [70,153],and to attenuate a model of heart failure induced by Adriamycin [154]. These findings suggest that SSA may hold promise as a potential treatment option for depression, tumors, inflammation with comorbidities, or in cases where toxic side effects of treatment are present, thereby providing patients with optimized therapeutic choices.
Through a comprehensive review of the research advancements concerning SSA in various domains, including anti-inflammatory, anti-tumor, neuroprotective, and anti-depressant effects, this study delves into the mechanistic actions of SSA via the inhibition of inflammatory signaling pathways such as NF-κB and MAPK. It further explores SSA's functions in apoptosis, antioxidant activity, and immune modulation, highlighting its potential as a therapeutic agent in clinical applications, particularly in diseases such as cancer, inflammation, depression, and organ injury. As a compound derived from traditional Chinese medicine, SSA exhibits extensive biological activity and therapeutic potential for the treatment of a variety of conditions. The research elucidates specific mechanisms by which SSA exerts its anti-inflammatory and anti-tumor effects, providing a theoretical foundation for the development of novel therapeutics based on SSA. Moreover, studies conducted in animal models and cell lines lay the groundwork for future clinical trials, with the promise of yielding more effective treatment modalities. The findings indicate that SSA possesses low toxicity, offering significant safety assurance for its clinical applications as a therapeutic intervention. In addition, the study delineates future research directions, including further pharmacokinetic and toxicological studies, as well as in vivo and clinical application research, to facilitate the clinical translation of SSA. In conclusion, this research not only summarizes the progress of SSA across multiple disease areas but also emphasizes its importance and novelty as a potential therapeutic agent, providing valuable references for subsequent research and clinical applications.
SSA, which is the primary active ingredient found in Radix bupleuri, has the potential to be an effective and relatively safe natural compound for treating various diseases including inflammation, tumors, depression, and organ protection. However, the transition of SSA from basic experiments to clinical applications is a time-consuming process, particularly due to the contradictory experimental evidence regarding the toxicity of Radix bupleuri to the liver and kidney, as well as its hepatorenal protective effect [4,147], SSA demonstrates a broad spectrum of pharmacological activities, encompassing anti-inflammatory, anti-tumor, analgesic, anti-fibrotic, anti-depressant, and immunomodulatory effects, thereby positioning it as a promising candidate for the treatment of various diseases. Nonetheless, the current body of research regarding SSA as a therapeutic modality remains insufficiently systematic and comprehensive. Furthermore, investigations into the pharmacokinetic properties of SSA—such as its metabolism, distribution, and excretion—as well as toxicological assessments are markedly limited. While SSA has exhibited favorable activity in vitro, its application in animal models and clinical trials is still constrained, particularly due to a lack of long-term safety and efficacy data. Additionally, the exploration of SSA's metabolic pathways and its metabolites is not sufficiently thorough, which restricts our holistic understanding of its pharmacodynamic and toxicological profiles.
To address these deficiencies, future work should prioritize enhancing the pharmacokinetic and toxicological research of SSA to ensure its safety and efficacy. Additionally, there is a pressing need for more in vivo and clinical studies to validate the effectiveness and safety of SSA, as well as to determine optimal dosing regimens and routes of administration. Furthermore, further investigation into the metabolic pathways of SSA and its metabolites is essential for a comprehensive understanding of its pharmacodynamics and toxicity. Finally, building upon the existing research findings, the development of novel therapeutics based on SSA should be pursued, alongside the formulation of more precise and personalized treatment strategies, with the aim of achieving broader clinical application.
In summary, despite the extensive pharmacological activities and potential therapeutic value of SSA, several challenges must be addressed to facilitate its translation into practical clinical applications. Future research should focus on bridging these knowledge gaps to optimize the utilization of SSA's potential in the treatment of human diseases.
CRediT authorship contribution statement
Xiao-Hong Sun: Writing – original draft. Yi-Hong Chai: Writing – original draft. Xiao-Teng Bai: Software. Hong-Xing Li: Data curation. Ya-Ming Xi: Writing – review & editing.
Informed consent
For this type of study formal consent is not required.
Data availability statement
All data reported are included and represented in the manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
No funding was received for this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Qu X., Hu S., Li T., et al. Metabolomics analysis reveals the differences between bupleurum chinense DC. And bupleurum scorzonerifolium willd. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.933849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan H., Zhou L., Wu B., et al. Integrated metabolomics and transcriptomics analysis of roots of Bupleurum chinense and B. scorzonerifolium, two sources of medicinal Chaihu. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-27019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang Y., Gao Y., Li X., et al. Bupleurum chinense exerts a mild antipyretic effect on LPS-induced pyrexia rats involving inhibition of peripheral TNF-alpha production. J. Ethnopharmacol. 2023;310 doi: 10.1016/j.jep.2023.116375. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Li X., Huang N., et al. A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine. 2018;50:73–87. doi: 10.1016/j.phymed.2018.09.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Shi W., He X.Y., et al. Saikosaponin D: review on the antitumour effects, toxicity and pharmacokinetics. Pharm. Biol. 2021;59(1):1480–1489. doi: 10.1080/13880209.2021.1992448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han X., Ma D., Wang J., et al. Spatial mapping of bioactive metabolites in the roots of three bupleurum species by matrix-assisted laser desorption/ionization mass spectrometry imaging. Molecules. 2024;29(16) doi: 10.3390/molecules29163746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L., Liu J., Qi G. Mechanism of the effect of saikosaponin on atherosclerosis in vitro is based on the MAPK signaling pathway. Mol. Med. Rep. 2017;16(6):8868–8874. doi: 10.3892/mmr.2017.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng L., Guo X., Ma Y., et al. A comprehensive review on traditional and modern research of the genus Bupleurum (Bupleurum L., Apiaceae) in recent 10 years. J. Ethnopharmacol. 2023;306 doi: 10.1016/j.jep.2022.116129. [DOI] [PubMed] [Google Scholar]
- 9.Chang G.R., Lin W.L., Lin T.C., et al. The ameliorative effects of saikosaponin in thioacetamide-induced liver injury and non-alcoholic fatty liver disease in mice. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S.J., Tam K.W., Tsai Y.H., et al. Curcumin and saikosaponin a inhibit chemical-induced liver inflammation and fibrosis in rats. Am. J. Chin. Med. 2010;38(1):99–111. doi: 10.1142/S0192415X10007695. [DOI] [PubMed] [Google Scholar]
- 11.Yang F., Dong X., Yin X., et al. Radix bupleuri: a review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/7597596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun K., Du Y., Hou Y., et al. Saikosaponin D exhibits anti-leukemic activity by targeting FTO/m(6)A signaling. Theranostics. 2021;11(12):5831–5846. doi: 10.7150/thno.55574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G., Guan Y., Liu Y., et al. Saikosaponin D inducing apoptosis and autophagy through the activation of endoplasmic reticulum stress in glioblastoma. BioMed Res. Int. 2022;2022 doi: 10.1155/2022/5489553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao T., Zhang L., Fu Y., et al. Saikosaponin-d alleviates renal inflammation and cell apoptosis in a mouse model of sepsis via TCF7/FOSL1/matrix metalloproteinase 9 inhibition. Mol. Cell Biol. 2021;41(10) doi: 10.1128/MCB.00332-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maccioni P., Chin Y.W., Corelli F., et al. Reducing effect of intragastrically administered saikosaponin A on alcohol and sucrose self-administration in rats. Nat. Prod. Res. 2023:1–5. doi: 10.1080/14786419.2023.2177848. [DOI] [PubMed] [Google Scholar]
- 16.Liu M., Zhang G., Naqvi S., et al. Cytotoxicity of Saikosaponin A targets HEKa cell through apoptosis induction by ROS accumulation and inflammation suppression via NF-kappaB pathway. Int Immunopharmacol. 2020;86 doi: 10.1016/j.intimp.2020.106751. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J., Luo C., Wang P., et al. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-kappaB pathways in LPS-stimulated RAW 264.7 cells. Exp. Ther. Med. 2013;5(5):1345–1350. doi: 10.3892/etm.2013.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi X., Liu J., Li X., et al. Saikosaponin a contributed to CCIN treatment by promoting neutrophil bactericidal activity via activation CBL-dependent ERK pathway. Phytomedicine. 2021;82 doi: 10.1016/j.phymed.2020.153444. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z., Wei B., Mu T., et al. Facile synthesis of saikosaponins. Molecules. 2021;26(7) doi: 10.3390/molecules26071941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maccioni P., Colombo G., Lorrai I., et al. Suppressing effect of a saikosaponin-enriched extract of Bupleurum falcatum on alcohol and chocolate self-administration in rats. Nat. Prod. Res. 2022;36(17):4502–4505. doi: 10.1080/14786419.2021.1986816. [DOI] [PubMed] [Google Scholar]
- 21.Huang M., Yan Y., Deng Z., et al. Saikosaponin A and D attenuate skeletal muscle atrophy in chronic kidney disease by reducing oxidative stress through activation of PI3K/AKT/Nrf2 pathway. Phytomedicine. 2023;114 doi: 10.1016/j.phymed.2023.154766. [DOI] [PubMed] [Google Scholar]
- 22.He Y., Peng L., Zhao X., et al. Selenium deficiency induces inflammatory response and decreased antimicrobial peptide expression in chicken jejunum through oxidative stress. Biol. Trace Elem. Res. 2023;201(7):3461–3473. doi: 10.1007/s12011-022-03442-w. [DOI] [PubMed] [Google Scholar]
- 23.Zhou F., Zou X., Zhang J., et al. Jian-pi-yi-shen formula ameliorates oxidative stress, inflammation, and apoptosis by activating the Nrf2 signaling in 5/6 nephrectomized rats. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.630210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Han J., Lv J., et al. Saikosaponin A-induced gut microbiota changes attenuate severe acute pancreatitis through the activation of keap1/nrf2-ARE antioxidant signaling. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/9217219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Morsy E.M., Ahmed M. a E. Carvedilol attenuates l-arginine induced acute pancreatitis in rats through modulation of oxidative stress and inflammatory mediators. Chem. Biol. Interact. 2020;327 doi: 10.1016/j.cbi.2020.109181. [DOI] [PubMed] [Google Scholar]
- 26.Awad A., Hollis E., Goyanes A., et al. 3D printed multi-drug-loaded suppositories for acute severe ulcerative colitis. Int J Pharm X. 2023;5 doi: 10.1016/j.ijpx.2023.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Shao Z., Song C., et al. Clinopodium chinense Kuntze ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by reducing systematic inflammation and regulating metabolism. J. Ethnopharmacol. 2023;309 doi: 10.1016/j.jep.2023.116330. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y., Liu Y., Lu J., et al. Effects of traditional Chinese medicine on serum cytokines for the dampness-heat syndrome of ulcerative colitis: a systematic review and meta-analysis. Altern Ther Health Med. 2023;29(5):386–395. [PubMed] [Google Scholar]
- 29.Ali J., Khan A.U., Shah F.A., et al. Mucoprotective effects of Saikosaponin-A in 5-fluorouracil-induced intestinal mucositis in mice model. Life Sci. 2019;239 doi: 10.1016/j.lfs.2019.116888. [DOI] [PubMed] [Google Scholar]
- 30.Zhou F., Wang N., Yang L., et al. Saikosaponin A protects against dextran sulfate sodium-induced colitis in mice. Int Immunopharmacol. 2019;72:454–458. doi: 10.1016/j.intimp.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Lu C.N., Yuan Z.G., Zhang X.L., et al. Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-kappaB signaling pathway. Int Immunopharmacol. 2012;14(1):121–126. doi: 10.1016/j.intimp.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Yuan B., Yang R., Ma Y., et al. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm. Biol. 2017;55(1):620–635. doi: 10.1080/13880209.2016.1262433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lobina C., Lee J.H., Pel P., et al. Analgesic effects of saikosaponin A in a rat model of chronic inflammatory pain. Nat. Prod. Res. 2023;37(16):2732–2736. doi: 10.1080/14786419.2022.2124985. [DOI] [PubMed] [Google Scholar]
- 34.He D., Wang H., Xu L., et al. Saikosaponin-a attenuates oxidized LDL uptake and prompts cholesterol efflux in THP-1 cells. J. Cardiovasc. Pharmacol. 2016;67(6):510–518. doi: 10.1097/FJC.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 35.Huang D., Zhao Q., Liu H., et al. PPAR-Alpha agonist WY-14643 inhibits LPS-induced inflammation in synovial fibroblasts via NF-kB pathway. J. Mol. Neurosci. 2016;59(4):544–553. doi: 10.1007/s12031-016-0775-y. [DOI] [PubMed] [Google Scholar]
- 36.Feng P., Xu Y., Tong B., et al. Saikosaponin a attenuates hyperlipidemic pancreatitis in rats via the PPAR-gamma/NF-kappaB signaling pathway. Exp. Ther. Med. 2020;19(2):1203–1212. doi: 10.3892/etm.2019.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen R.J., Guo X.Y., Cheng B.H., et al. Saikosaponin a inhibits cigarette smoke-induced oxidant stress and inflammatory responses by activation of Nrf2. Inflammation. 2018;41(4):1297–1303. doi: 10.1007/s10753-018-0778-7. [DOI] [PubMed] [Google Scholar]
- 38.Ma X., Ma J., Leng T., et al. Advances in oxidative stress in pathogenesis of diabetic kidney disease and efficacy of TCM intervention. Ren. Fail. 2023;45(1) doi: 10.1080/0886022X.2022.2146512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng Z., Zhang Y., Zhu Y., et al. BRD9 inhibition attenuates matrix degradation and pyroptosis in nucleus pulposus by modulating the NOX1/ROS/NF-kappaB axis. Inflammation. 2023;46(3):1002–1021. doi: 10.1007/s10753-023-01786-6. [DOI] [PubMed] [Google Scholar]
- 40.Kim S.O., Park J.Y., Jeon S.Y., et al. Saikosaponin a, an active compound of Radix Bupleuri, attenuates inflammation in hypertrophied 3T3-L1 adipocytes via ERK/NF-kappaB signaling pathways. Int. J. Mol. Med. 2015;35(4):1126–1132. doi: 10.3892/ijmm.2015.2093. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H., Li S., Zhang H., et al. Saikosaponin A protects against experimental sepsis via inhibition of NOD2-mediated NF-kappaB activation. Exp. Ther. Med. 2015;10(2):823–827. doi: 10.3892/etm.2015.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juneja L., Parmar H.S. Ovalbumin induced allergic rhinitis and development of prediabetes to rats: possible role of Th2 cytokines. Inflamm. Allergy - Drug Targets. 2013;12(3):199–205. doi: 10.2174/1871528111312030007. [DOI] [PubMed] [Google Scholar]
- 43.Jung D.H., Lee A., Hwang Y.H., et al. Therapeutic effects of Pulsatilla koreana Nakai extract on ovalbumin-induced allergic rhinitis by inhibition of Th2 cell activation and differentiation via the IL-4/STAT6/GATA3 pathway. Biomed. Pharmacother. 2023;162 doi: 10.1016/j.biopha.2023.114730. [DOI] [PubMed] [Google Scholar]
- 44.Piao C.H., Song C.H., Lee E.J., et al. Saikosaponin A ameliorates nasal inflammation by suppressing IL-6/ROR-gammat/STAT3/IL-17/NF-kappaB pathway in OVA-induced allergic rhinitis. Chem. Biol. Interact. 2020;315 doi: 10.1016/j.cbi.2019.108874. [DOI] [PubMed] [Google Scholar]
- 45.Zhao C., Li J., Cai H., et al. An injectable hydrogel scaffold with IL-1beta-activated MSC-derived exosomes for the treatment of endometritis. Biomater. Sci. 2023;11(4):1422–1436. doi: 10.1039/d2bm01586b. [DOI] [PubMed] [Google Scholar]
- 46.Zhou G., Shen P., Sun Y., et al. Transcriptome profiling of bovine endometrial epithelial cells induced by lipopolysaccharides in vitro. Anim. Biotechnol. 2023:1–12. doi: 10.1080/10495398.2023.2174876. [DOI] [PubMed] [Google Scholar]
- 47.Wang J., Wang W., Pang Y. Saikosaponin A inhibits LPS-induced endometritis in mice through activating Nrf2 signaling pathway. Inflammation. 2018;41(4):1508–1514. doi: 10.1007/s10753-018-0796-5. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y., Gao L., Zhao X., et al. Saikosaponin A protects from pressure overload-induced cardiac fibrosis via inhibiting fibroblast activation or endothelial cell EndMT. Int. J. Biol. Sci. 2018;14(13):1923–1934. doi: 10.7150/ijbs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang N., Che D., Zhang T., et al. Saikosaponin A inhibits compound 48/80-induced pseudo-allergy via the Mrgprx2 pathway in vitro and in vivo. Biochem. Pharmacol. 2018;148:147–154. doi: 10.1016/j.bcp.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Huang W., Zheng X., Yang X., et al. Stimulation of osteogenic differentiation by saikosaponin-A in bone marrow stromal cells via WNT/beta-Catenin pathway. Calcif. Tissue Int. 2017;100(4):392–401. doi: 10.1007/s00223-017-0242-y. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt J., Rattner D.W., Lewandrowski K., et al. A better model of acute pancreatitis for evaluating therapy. Ann. Surg. 1992;215(1):44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao X., Miao G., Tao X., et al. Saikosaponin a protects TBI rats after controlled cortical impact and the underlying mechanism. Am J Transl Res. 2016;8(1):133–141. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X., Cheng H., Xu D., et al. Attenuation of neuropathic pain by saikosaponin a in a rat model of chronic constriction injury. Neurochem. Res. 2014;39(11):2136–2142. doi: 10.1007/s11064-014-1407-y. [DOI] [PubMed] [Google Scholar]
- 54.Fu Y., Hu X., Cao Y., et al. Saikosaponin a inhibits lipopolysaccharide-oxidative stress and inflammation in Human umbilical vein endothelial cells via preventing TLR4 translocation into lipid rafts. Free Radic. Biol. Med. 2015;89:777–785. doi: 10.1016/j.freeradbiomed.2015.10.407. [DOI] [PubMed] [Google Scholar]
- 55.Ahn S.S., Lee Y.H., Yeo H., et al. Saikosaponin A and saikosaponin C reduce TNF-alpha-induced TSLP expression through inhibition of MAPK-mediated EGR1 expression in HaCaT keratinocytes. Int. J. Mol. Sci. 2022;23(9) doi: 10.3390/ijms23094857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim S.H., Lee H.S., Han H.K., et al. Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK signaling pathways in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui L., Li C., Zhuo Y., et al. Saikosaponin A inhibits the activation of pancreatic stellate cells by suppressing autophagy and the NLRP3 inflammasome via the AMPK/mTOR pathway. Biomed. Pharmacother. 2020;128 doi: 10.1016/j.biopha.2020.110216. [DOI] [PubMed] [Google Scholar]
- 58.Homayoonfal M., Gilasi H., Asemi Z., et al. Quercetin modulates signal transductions and targets non-coding RNAs against cancer development. Cell. Signal. 2023;107 doi: 10.1016/j.cellsig.2023.110667. [DOI] [PubMed] [Google Scholar]
- 59.Adorisio S., Muscari I., Fierabracci A., et al. Biological effects of bergamot and its potential therapeutic use as an anti-inflammatory, antioxidant, and anticancer agent. Pharm. Biol. 2023;61(1):639–646. doi: 10.1080/13880209.2023.2197010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang S.J., Lee Y.J., Kim B.M., et al. Effect of Bupleuri Radix extracts on the toxicity of 5-fluorouracil in HepG2 hepatoma cells and normal human lymphocytes. Basic Clin. Pharmacol. Toxicol. 2008;103(4):305–313. doi: 10.1111/j.1742-7843.2008.00280.x. [DOI] [PubMed] [Google Scholar]
- 61.Wen-Sheng W. ERK signaling pathway is involved in p15INK4b/p16INK4a expression and HepG2 growth inhibition triggered by TPA and Saikosaponin a. Oncogene. 2003;22(7):955–963. doi: 10.1038/sj.onc.1206237. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y.L., He S.X., Luo J.Y. [Progress in research on antitumor activity of saikosaponin and its mechanism] Zhong Xi Yi Jie He Xue Bao. 2006;4(1):98–100. doi: 10.3736/jcim20060129. [DOI] [PubMed] [Google Scholar]
- 63.Chen C.H., Chen M.F., Huang S.J., et al. Saikosaponin a induces apoptosis through mitochondria-dependent pathway in hepatic stellate cells. Am. J. Chin. Med. 2017;45(2):351–368. doi: 10.1142/S0192415X17500227. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Q., Wu W.W., Yu C.L., et al. Saikosaponin A inhibits growth of human bladder carcinoma T24 and 5637 cells both in vitro and in vivo. Biol. Pharm. Bull. 2022;45(7):863–871. doi: 10.1248/bpb.b21-01025. [DOI] [PubMed] [Google Scholar]
- 65.Kim T.W. Targeting ER stress with saikosaponin A to overcome resistance under radiation in gastric cancer cells. Int. J. Mol. Sci. 2023;24(6) doi: 10.3390/ijms24065661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J.C., Chang N.W., Chung J.G., et al. Saikosaponin-A induces apoptotic mechanism in human breast MDA-MB-231 and MCF-7 cancer cells. Am. J. Chin. Med. 2003;31(3):363–377. doi: 10.1142/S0192415X03001065. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Zhao L., Han X., et al. Saikosaponin A inhibits triple-negative breast cancer growth and metastasis through downregulation of CXCR4. Front. Oncol. 2019;9:1487. doi: 10.3389/fonc.2019.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao X., Liu J., Ge S., et al. Saikosaponin A inhibits breast cancer by regulating Th1/Th2 balance. Front. Pharmacol. 2019;10:624. doi: 10.3389/fphar.2019.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du J., Song D., Cao T., et al. Saikosaponin-A induces apoptosis of cervical cancer through mitochondria- and endoplasmic reticulum stress-dependent pathway in vitro and in vivo: involvement of PI3K/AKT signaling pathway. Cell Cycle. 2021;20(21):2221–2232. doi: 10.1080/15384101.2021.1974791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang L., Zeng Y., Ai L., et al. Decreased HMGB1 expression contributed to cutaneous toxicity caused by lapatinib. Biochem. Pharmacol. 2022;201 doi: 10.1016/j.bcp.2022.115105. [DOI] [PubMed] [Google Scholar]
- 71.Kim B.M., Hong S.H. Sequential caspase-2 and caspase-8 activation is essential for saikosaponin a-induced apoptosis of human colon carcinoma cell lines. Apoptosis. 2011;16(2):184–197. doi: 10.1007/s10495-010-0557-x. [DOI] [PubMed] [Google Scholar]
- 72.Shi C., Sun L., Fang R., et al. Saikosaponin-A exhibits antipancreatic cancer activity by targeting the EGFR/PI3K/Akt pathway. Curr Pharm Biotechnol. 2023;24(4):579–588. doi: 10.2174/1389201023666220610113514. [DOI] [PubMed] [Google Scholar]
- 73.Feng J., Xi Z., Jiang X., et al. Saikosaponin A enhances Docetaxel efficacy by selectively inducing death of dormant prostate cancer cells through excessive autophagy. Cancer Lett. 2023;554 doi: 10.1016/j.canlet.2022.216011. [DOI] [PubMed] [Google Scholar]
- 74.Tsai Y.J., Chen I.L., Horng L.Y., et al. Induction of differentiation in rat C6 glioma cells with Saikosaponins. Phytother Res. 2002;16(2):117–121. doi: 10.1002/ptr.752. [DOI] [PubMed] [Google Scholar]
- 75.Zhang P., Lai X., Zhu M.H., et al. Saikosaponin A, a triterpene saponin, suppresses angiogenesis and tumor growth by blocking VEGFR2-mediated signaling pathway. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng T., Ying M. Antitumor effect of saikosaponin A on human neuroblastoma cells. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/5845554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Q., Zheng X.L., Yang L., et al. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J. Exp. Clin. Cancer Res. 2010;29(1):159. doi: 10.1186/1756-9966-29-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ye R.P., Chen Z.D., Saikosaponin A. An active glycoside from Radix bupleuri, reverses P-glycoprotein-mediated multidrug resistance in MCF-7/ADR cells and HepG2/ADM cells. Xenobiotica. 2017;47(2):176–184. doi: 10.3109/00498254.2016.1171932. [DOI] [PubMed] [Google Scholar]
- 79.Lee J.E., Song B.K., Kim J.H., et al. Production of Prosaikogenin F., Prosaikogenin G., Saikogenin F., Saikogenin G. By the recombinant enzymatic hydrolysis of saikosaponin and their anti-cancer effect. Molecules. 2022;27(10) doi: 10.3390/molecules27103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vieira W.F., Iosifescu D.V., Mceachern K.M., et al. Photobiomodulation: an emerging treatment modality for depression. Psychiatr Clin North Am. 2023;46(2):331–348. doi: 10.1016/j.psc.2023.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Wang H.Q., Wang Z.Z., Chen N.H. The receptor hypothesis and the pathogenesis of depression: genetic bases and biological correlates. Pharmacol. Res. 2021;167 doi: 10.1016/j.phrs.2021.105542. [DOI] [PubMed] [Google Scholar]
- 82.Yang Y., Cui Y., Sang K., et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554(7692):317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 83.Zhou L., Wang T., Yu Y., et al. The etiology of poststroke-depression: a hypothesis involving HPA axis. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113146. [DOI] [PubMed] [Google Scholar]
- 84.Spellman T., Liston C. Toward circuit mechanisms of pathophysiology in depression. Am J Psychiatry. 2020;177(5):381–390. doi: 10.1176/appi.ajp.2020.20030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhatt S., Devadoss T., Manjula S.N., et al. 5-HT(3) receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr. Neuropharmacol. 2021;19(9):1545–1559. doi: 10.2174/1570159X18666201015155816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y.S., Shen C.Y., Jiang J.G. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and prospects for drug discovery. Pharmacol. Res. 2019;150 doi: 10.1016/j.phrs.2019.104520. [DOI] [PubMed] [Google Scholar]
- 87.Leite J.A., Orellana A.M., Andreotti D.Z., et al. Ouabain reverts CUS-induced disruption of the HPA Axis and avoids long-term spatial memory deficits. Biomedicines. 2023;11(4) doi: 10.3390/biomedicines11041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gordon J.L., Girdler S.S., Meltzer-Brody S.E., et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. 2015;172(3):227–236. doi: 10.1176/appi.ajp.2014.14070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang H., He L., Li S., et al. Cerebral iron deficiency may induce depression through downregulation of the hippocampal glucocorticoid-glucocorticoid receptor signaling pathway. J. Affect. Disord. 2023;332:125–135. doi: 10.1016/j.jad.2023.03.085. [DOI] [PubMed] [Google Scholar]
- 90.Du F., Yu Q., Swerdlow R.H., et al. Glucocorticoid-driven mitochondrial damage stimulates Tau pathology. Brain. 2023;146(10):4378–4394. doi: 10.1093/brain/awad127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang A.-R., Mi L.-F., Zhang Z.-L., et al. Saikosaponin A improved depression-like behavior and inhibited hippocampal neuronal apoptosis after cerebral ischemia through p-CREB/BDNF pathway. Behav. Brain Res. 2021;403 doi: 10.1016/j.bbr.2021.113138. [DOI] [PubMed] [Google Scholar]
- 92.Wei K., Xu Y., Zhao Z., et al. Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int. J. Mol. Med. 2016;38(1):337–344. doi: 10.3892/ijmm.2016.2591. [DOI] [PubMed] [Google Scholar]
- 93.Frank F., Ortlund E.A., Liu X. Structural insights into glucocorticoid receptor function. Biochem. Soc. Trans. 2021;49(5):2333–2343. doi: 10.1042/BST20210419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen X.Q., Chen S.J., Liang W.N., et al. Saikosaponin A attenuates perimenopausal depression-like symptoms by chronic unpredictable mild stress. Neurosci. Lett. 2018;662:283–289. doi: 10.1016/j.neulet.2017.09.046. [DOI] [PubMed] [Google Scholar]
- 95.Guo J., Zhang F., Gao J., et al. Proteomics-based screening of the target proteins associated with antidepressant-like effect and mechanism of Saikosaponin A. J. Cell Mol. Med. 2020;24(1):174–188. doi: 10.1111/jcmm.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y., Yuan S., Pu J., et al. Integrated metabolomics and proteomics analysis of Hippocampus in a rat model of depression. Neuroscience. 2018;371:207–220. doi: 10.1016/j.neuroscience.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 97.Keyes K.M., Kreski N.T., Joseph V.A., et al. What is not measured cannot Be counted: sample characteristics reported in studies of hippocampal volume and depression in neuroimaging studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8(5):492–494. doi: 10.1016/j.bpsc.2023.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alemu J.L., Elberling F., Azam B., et al. Electroconvulsive treatment prevents chronic restraint stress-induced atrophy of the hippocampal formation-A stereological study. Brain Behav. 2019;9(2) doi: 10.1002/brb3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yin Q., Du T., Yang C., et al. Gadd45b is a novel mediator of depression-like behaviors and neuroinflammation after cerebral ischemia. Biochem. Biophys. Res. Commun. 2021;554:107–113. doi: 10.1016/j.bbrc.2021.03.104. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y., Peng J., Leng Q., et al. Effects of aloe-emodin on the expression of brain aquaporins and secretion of neurotrophic factors in a rat model of post-stroke depression. Int. J. Mol. Sci. 2023;24(6) doi: 10.3390/ijms24065206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saral S., Topcu A., Alkanat M., et al. Agomelatine attenuates cisplatin-induced cognitive impairment via modulation of BDNF/TrkB signaling in rat hippocampus. J. Chem. Neuroanat. 2023;130 doi: 10.1016/j.jchemneu.2023.102269. [DOI] [PubMed] [Google Scholar]
- 102.Di-Bonaventura S., Fernandez-Carnero J., Matesanz-Garcia L., et al. Effect of different physical therapy interventions on brain-derived neurotrophic factor levels in chronic musculoskeletal pain patients: a systematic review. Life. 2023;13(1) doi: 10.3390/life13010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Permoda-Pachuta A., Malewska-Kasprzak M., Skibinska M., et al. Changes in adipokine, resitin, and BDNF concentrations in treatment-resistant depression after electroconvulsive therapy. Brain Sci. 2023;13(10) doi: 10.3390/brainsci13101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carniel B.P., Da Rocha N.S. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: perspectives for the management of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;108 doi: 10.1016/j.pnpbp.2020.110151. [DOI] [PubMed] [Google Scholar]
- 105.Chong P.S., Poon C.H., Roy J., et al. Neurogenesis-dependent antidepressant-like activity of Hericium erinaceus in an animal model of depression. Chin. Med. 2021;16(1):132. doi: 10.1186/s13020-021-00546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duman R.S., Deyama S., Fogaca M.V. Role of BDNF in the pathophysiology and treatment of depression: activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2021;53(1):126–139. doi: 10.1111/ejn.14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li M., Han L., Xiao J., et al. IL-1ra treatment prevents chronic social defeat stress-induced depression-like behaviors and glutamatergic dysfunction via the upregulation of CREB-BDNF. J. Affect. Disord. 2023;335:358–370. doi: 10.1016/j.jad.2023.05.049. [DOI] [PubMed] [Google Scholar]
- 108.Wei G., Chen Y.B., Chen D.F., et al. beta-Asarone inhibits neuronal apoptosis via the CaMKII/CREB/Bcl-2 signaling pathway in an in vitro model and AbetaPP/PS1 mice. J Alzheimers Dis. 2013;33(3):863–880. doi: 10.3233/JAD-2012-120865. [DOI] [PubMed] [Google Scholar]
- 109.Liang H., Tang L.Y., Ge H.Y., et al. Neuronal survival factor TAFA2 suppresses apoptosis through binding to ADGRL1 and activating cAMP/PKA/CREB/BCL2 signaling pathway. Life Sci. 2023;334 doi: 10.1016/j.lfs.2023.122241. [DOI] [PubMed] [Google Scholar]
- 110.Wang A.R., Mi L.F., Zhang Z.L., et al. Saikosaponin A improved depression-like behavior and inhibited hippocampal neuronal apoptosis after cerebral ischemia through p-CREB/BDNF pathway. Behav. Brain Res. 2021;403 doi: 10.1016/j.bbr.2021.113138. [DOI] [PubMed] [Google Scholar]
- 111.Wu S., Li H.M., Bing Y.F., et al. Bupleurum scorzonerifolium: systematic research through pharmacodynamics and serum pharmacochemistry on screening antidepressant Q-markers for quality control. J. Pharm. Biomed. Anal. 2023;225 doi: 10.1016/j.jpba.2022.115202. [DOI] [PubMed] [Google Scholar]
- 112.Li X., Hou R., Qin X., et al. Synergistic neuroprotective effect of saikosaponin A and albiflorin on corticosterone-induced apoptosis in PC12 cells via regulation of metabolic disorders and neuroinflammation. Mol. Biol. Rep. 2022;49(9):8801–8813. doi: 10.1007/s11033-022-07730-5. [DOI] [PubMed] [Google Scholar]
- 113.Jichao S., Xinmin H., Xianguo R., et al. Saikosaponin A alleviates symptoms of attention deficit hyperactivity disorder through downregulation of dat and enhancing BDNF expression in spontaneous hypertensive rats. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/2695903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X.H., Xu D.Q., Chen Y.Y., et al. Traditional Chinese Medicine: a promising strategy to regulate inflammation, intestinal disorders and impaired immune function due to sepsis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.952938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cui S., Zhang X., Li Y., et al. UGCG modulates heart hypertrophy through B4GalT5-mediated mitochondrial oxidative stress and the ERK signaling pathway. Cell. Mol. Biol. Lett. 2023;28(1):71. doi: 10.1186/s11658-023-00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang H.W., Wang Q., Mei H.X., et al. RvD1 ameliorates LPS-induced acute lung injury via the suppression of neutrophil infiltration by reducing CXCL2 expression and release from resident alveolar macrophages. Int Immunopharmacol. 2019;76 doi: 10.1016/j.intimp.2019.105877. [DOI] [PubMed] [Google Scholar]
- 117.Zheng Z., Li X., Chen P., et al. Design and synthesis optimization of novel diimide indoles derivatives for ameliorating acute lung injury through modulation of NF-kappaB signaling pathway. Bioorg. Chem. 2023;136 doi: 10.1016/j.bioorg.2023.106557. [DOI] [PubMed] [Google Scholar]
- 118.Du Z.A., Sun M.N., Hu Z.S. Saikosaponin a ameliorates LPS-induced acute lung injury in mice. Inflammation. 2018;41(1):193–198. doi: 10.1007/s10753-017-0677-3. [DOI] [PubMed] [Google Scholar]
- 119.Peng D., Chen Y., Sun Y., et al. Saikosaponin A and its epimers alleviate LPS-induced acute lung injury in mice. Molecules. 2023;28(3) doi: 10.3390/molecules28030967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu B., Zhang H., Tan X., et al. GSPE reduces lead-induced oxidative stress by activating the Nrf2 pathway and suppressing miR153 and GSK-3beta in rat kidney. Oncotarget. 2017;8(26):42226–42237. doi: 10.18632/oncotarget.15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Song Y., Sun H., Gao S., et al. Saikosaponin a attenuates lead-induced kidney injury through activating Nrf2 signaling pathway. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021;242 doi: 10.1016/j.cbpc.2020.108945. [DOI] [PubMed] [Google Scholar]
- 122.Zhang L., Li D., Yin L., et al. Neuroglobin protects against cerebral ischemia/reperfusion injury in rats by suppressing mitochondrial dysfunction and endoplasmic reticulum stress-mediated neuronal apoptosis through synaptotagmin-1. Environ. Toxicol. 2023;38(8):1891–1904. doi: 10.1002/tox.23815. [DOI] [PubMed] [Google Scholar]
- 123.Wang X., Yang G. Saikosaponin A attenuates neural injury caused by ischemia/reperfusion. Transl. Neurosci. 2020;11(1):227–235. doi: 10.1515/tnsci-2020-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chiu C.H., Lin Y.J., Ramesh S., et al. Gemcitabine resistance in non-small cell lung cancer is mediated through activation of the PI3K/AKT/NF-kappaB pathway and suppression of ERK signaling by reactive oxygen species. J. Biochem. Mol. Toxicol. 2023 doi: 10.1002/jbt.23497. [DOI] [PubMed] [Google Scholar]
- 125.Zhu Y., Chen X., Rao X., et al. Saikosaponin a ameliorates lipopolysaccharide and d-galactosamine-induced liver injury via activating LXRalpha. Int Immunopharmacol. 2019;72:131–137. doi: 10.1016/j.intimp.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 126.Wu S.J., Lin Y.H., Chu C.C., et al. Curcumin or saikosaponin a improves hepatic antioxidant capacity and protects against CCl4-induced liver injury in rats. J. Med. Food. 2008;11(2):224–229. doi: 10.1089/jmf.2007.555. [DOI] [PubMed] [Google Scholar]
- 127.Huang M.Q., Cao X.Y., Chen X.Y., et al. Saikosaponin a increases interleukin-10 expression and inhibits scar formation after sciatic nerve injury. Neural Regen Res. 2018;13(9):1650–1656. doi: 10.4103/1673-5374.237139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y.F., Ma R.X., Zou B., et al. Endoplasmic reticulum stress regulates autophagic response that is involved in Saikosaponin a-induced liver cell damage. Toxicol. Vitro. 2023;88 doi: 10.1016/j.tiv.2022.105534. [DOI] [PubMed] [Google Scholar]
- 129.Zhong Y.H., Jiang S., Qu W.M., et al. Saikosaponin a promotes sleep by decreasing neuronal activities in the lateral hypothalamus. J. Sleep Res. 2022;31(2) doi: 10.1111/jsr.13484. [DOI] [PubMed] [Google Scholar]
- 130.Lee S.Y., Lee A.R., Choi J.W., et al. IL-17 induces autophagy dysfunction to promote inflammatory cell death and fibrosis in keloid fibroblasts via the STAT3 and HIF-1alpha dependent signaling pathways. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.888719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harsono A.D., Prasetyono T.O.H., Dilogo I.H. The role of interleukin 10 in keloid therapy: a literature review. Ann. Plast. Surg. 2022;88(6):617–621. doi: 10.1097/SAP.0000000000003044. [DOI] [PubMed] [Google Scholar]
- 132.Ngeow W.C., Atkins S., Morgan C.R., et al. A comparison between the effects of three potential scar-reducing agents applied at a site of sciatic nerve repair. Neuroscience. 2011;181:271–277. doi: 10.1016/j.neuroscience.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 133.Haroon E., Miller A.H., Sanacora G. Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42(1):193–215. doi: 10.1038/npp.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perin-Martins A., Teixeira J.M., Tambeli C.H., et al. Mechanisms underlying transient receptor potential ankyrin 1 (TRPA1)-mediated hyperalgesia and edema. J. Peripher. Nerv. Syst. 2013;18(1):62–74. doi: 10.1111/jns5.12010. [DOI] [PubMed] [Google Scholar]
- 135.Xu Y., Yu Y., Wang Q., et al. Active components of Bupleurum chinense and Angelica biserrata showed analgesic effects in formalin induced pain by acting on Nav1.7. J. Ethnopharmacol. 2021;269 doi: 10.1016/j.jep.2020.113736. [DOI] [PubMed] [Google Scholar]
- 136.Hogri R., Baltov B., Drdla-Schutting R., et al. Probing pain aversion in rats with the "Heat Escape Threshold" paradigm. Mol. Pain. 2023;19 doi: 10.1177/17448069231156657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li P., Gong Y., Zu N., et al. Therapeutic mechanism of Saikosaponin-d in anti-Thy1 mAb 1-22-3-induced rat model of glomerulonephritis. Nephron Exp. Nephrol. 2005;101(4):e111–e118. doi: 10.1159/000087437. [DOI] [PubMed] [Google Scholar]
- 138.Zhou C., Liu W., He W., et al. Saikosaponin a inhibits RANKL-induced osteoclastogenesis by suppressing NF-kappaB and MAPK pathways. Int Immunopharmacol. 2015;25(1):49–54. doi: 10.1016/j.intimp.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 139.Shin J.E., Kim H.J., Kim K.R., et al. Type I saikosaponins a and d inhibit osteoclastogenesis in bone marrow-derived macrophages and osteolytic activity of metastatic breast cancer cells. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/582437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Birnbaum S.G., Varga A.W., Yuan L.L., et al. Structure and function of Kv4-family transient potassium channels. Physiol. Rev. 2004;84(3):803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- 141.Tsaur M.L., Sheng M., Lowenstein D.H., et al. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992;8(6):1055–1067. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- 142.Hong Y., Deng N., Jin H.N., et al. Saikosaponin A modulates remodeling of Kv4.2-mediated A-type voltage-gated potassium currents in rat chronic temporal lobe epilepsy. Drug Des Devel Ther. 2018;12:2945–2958. doi: 10.2147/DDDT.S166408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xie W., Yu Y.H., Du Y.P., et al. Saikosaponin a enhances transient inactivating potassium current in rat hippocampal CA1 neurons. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/413092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ye M., Bi Y.F., Ding L., et al. Saikosaponin a functions as anti-epileptic effect in pentylenetetrazol induced rats through inhibiting mTOR signaling pathway. Biomed. Pharmacother. 2016;81:281–287. doi: 10.1016/j.biopha.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 145.Zhu D., Li A., Lv Y., et al. Traditional Chinese medicine: a class of potentially reliable epigenetic drugs. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.907031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu X., Latkolik S., Atanasov A.G., et al. Bupleurum chinense roots: a bioactivity-guided approach toward saponin-type NF-kappaB inhibitors. Planta Med. 2017;83(14–15):1242–1250. doi: 10.1055/s-0043-118226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen C., Gong W., Tian J., et al. Radix Paeoniae Alba attenuates Radix Bupleuri-induced hepatotoxicity by modulating gut microbiota to alleviate the inhibition of saikosaponins on glutathione synthetase. J Pharm Anal. 2023;13(6):640–659. doi: 10.1016/j.jpha.2023.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bak S.B., Song Y.R., Bae S.J., et al. Integrative approach to uncover antioxidant properties of Bupleuri Radix and its active compounds: multiscale interactome-level analysis with experimental validation. Free Radic. Biol. Med. 2023;199:141–153. doi: 10.1016/j.freeradbiomed.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 149.Sun J., Ma M., Zhong X., et al. Investigating the molecular mechanism of Qizhu anticancer prescription in inhibiting hepatocellular carcinoma based on high-resolution mass spectrometry and network pharmacology. J. Ethnopharmacol. 2024;328 doi: 10.1016/j.jep.2024.117985. [DOI] [PubMed] [Google Scholar]
- 150.Lan T., Wang W., Zeng X.X., et al. Saikosaponin A triggers cell ferroptosis in hepatocellular carcinoma by inducing endoplasmic reticulum stress-stimulated ATF3 expression. Biochem. Biophys. Res. Commun. 2023;674:10–18. doi: 10.1016/j.bbrc.2023.06.086. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Y., Dai K., Xu D., et al. Saikosaponin A alleviates glycolysis of breast cancer cells through repression of Akt/STAT3 pathway. Chem. Biol. Drug Des. 2023;102(1):115–125. doi: 10.1111/cbdd.14259. [DOI] [PubMed] [Google Scholar]
- 152.Wang C., Zhang R., Chen X., et al. The potential effect and mechanism of Saikosaponin A against gastric cancer. BMC Complement Med Ther. 2023;23(1):295. doi: 10.1186/s12906-023-04108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gao Z., Hu Y., Fu H., et al. Dasatinib causes keratinocyte apoptosis via inhibiting high mobility group Box 1-mediated mitophagy. Toxicol. Lett. 2023;373:22–32. doi: 10.1016/j.toxlet.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 154.Huang C., Qiu S., Fan X., et al. Evaluation of the effect of Shengxian Decoction on doxorubicin-induced chronic heart failure model rats and a multicomponent comparative pharmacokinetic study after oral administration in normal and model rats. Biomed. Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported are included and represented in the manuscript.