Abstract
Background
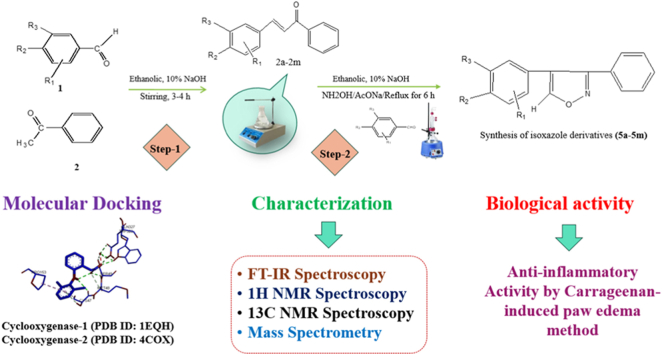
The present study is aimed to investigate the anti-inflammatory activities of thirteen substituted-isoxazole derivatives (5a–5m). Isoxazole is a key pharmacophore in medicinal chemistry, known for its broad range of pharmacological activities.This study explores the synthesis and anti-inflammatory potential of thirteen substituted-isoxazole derivatives (5a–5m), with 5c, 5d, 5e, and 5g being novel compounds.
Objectives
The primary objectives were to synthesize some novel substituted isoxazole derivatives, evaluate their interaction with cyclooxygenase (COX-1/2) enzymes through computational methods, and assess their anti-inflammatory effectiveness in laboratory animals.
Methods
Substituted chalcones (0.01 mol) (2a-2m), sodium ethoxide (0.01 mol), and hydroxylamine hydrochloride (0.01 mol) were dissolved in absolute ethanol (15 ml), and then the mixture was refluxed for 6 h in an oil bath and monitored by TLC (ethyl acetate:hexane 7:3 v/v as eluent; a UV lamp was used to visualize the plates). After the completion of the reaction, as per TLC, the contents of the reaction mixture were poured into ice-cold water (50 ml). The obtained precipitates were filtered, washed two times, dried for 2 h at room temperature, and then recrystallized with ethenol. The structures of these compounds were confirmed via Fourier transform infrared spectroscopy (FT-IR), proton nuclear magnetic resonance (1H NMR) spectroscopy, carbon-13 nuclear magnetic resonance (13C NMR) spectroscopy, and mass spectrometry. Anti-inflammatory activity was evaluated using the carrageenan-induced rat paw edema method.
Results
The results indicated that three compounds (5b, 5c, and 5d) demonstrated significant in vivo anti-inflammatory potential (% edema inhibition 75.68, 74.48, & 71.86 in 2 h and 76.71, 75.56, & 72.32 in 3 h) with modest effectiveness (0.83, 0.81 & 0.71), low toxicity, and minimal adverse effects. The molecular docking analyses further elucidated the interaction with the active site COX-2 enzyme (PDB ID: 4COX) using Autodock Vina. The compounds 5b, 5c, and 5d −8.7, −8.5, and −8.4 indicate good binding affinity (kcal/mol) and H-bond interaction with residues such as Cys41, Ala151, and Arg120 for COX-2, which also carried out RMSD values of 2.174, 41.13, and 22.25, which are decisive for the reported anti-inflammatory activity of diverse compounds.
Conclusions
The findings indicate that isoxazole derivatives have modest antiinflammatory potential, with compounds (5b, 5c, and 5d) acting as lead molecules to be studied further for pain relief with fewer adverse effects.
Keywords: Isoxazole, Molecular docking, Substituted chalcones, Cyclooxygenases, Diclofenac sodium, Anti-inflammatory activity
Graphical abstract
Highlights
-
•
The synthesis of total thirteen isoxazole derivatives from substituted chalcones and their structural confirmation through FT-IR, 1HNMR, 13C NMR spectroscopy, and Mass spectrometry.
-
•
Four novel isoxazole derivatives (5c, 5d, 5e, and 5g) synthesized and characterized.
-
•
Molecular docking analysis to study interactions of compounds 5b, 5c, and 5d with the anti-inflammatory enzyme (COX-2) active site.
-
•
Drug likeness studies and ADMET predictions of isoxazole derivatives exploring them as potential anti-inflammatory agents.
-
•
Compounds 5b, 5c, and 5d showed significant anti-inflammatory activity in laboratory tests, exhibiting low toxicity and minimal adverse effects.
1. Introduction
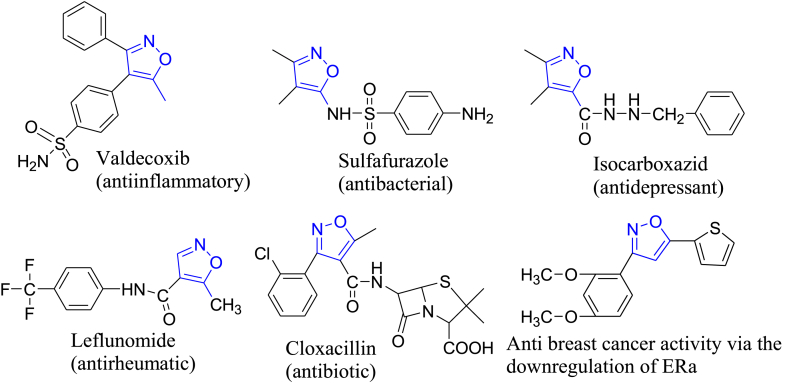
Non-steroidal anti-inflammatory drugs (NSAIDs), one of the most common OTC drugs, show variable selectivity for the inhibition of COX-1 and COX-2 enzymes. The pharmaceutical industry finds it challenging to develop new NSAIDs with an improved safety profile on the gastrointestinal tract since their clinical use as analgesics and anti-inflammatory drugs is invariably associated with adverse gastrointestinal problems. Since the discovery of celecoxib, scientists have concentrated on creating new derivatives of this class that lessen inflammation while having fewer adverse effects [1]. Inflammation is the immune system's physiologic response to potentially harmful stimuli such as infection and injuries [2]. It is important in the pathophysiology of many illnesses, including lupus erythematosus, autoimmune disease, neurological disorders, rheumatoid arthritis, cancer, and colitis [3]. Fig. 1 shows isoxazole-based pharmaceutically important molecules, including drugs, introduced in the late 1990s and commonly referred to as COXIBs, that selectively inhibit cyclooxygenase-2 (COX-2) with minimal gastrointestinal adverse effects [4,5]. However, allergic reactions have been reported with Celecoxib due to the presence of the sulfonamide moiety, while Valdecoxib has recently been withdrawn due to significant cardiovascular side effects [6,7] (see Fig. 2).
Fig. 1.
Isoxazole-based pharmaceutically important molecules, including drugs.
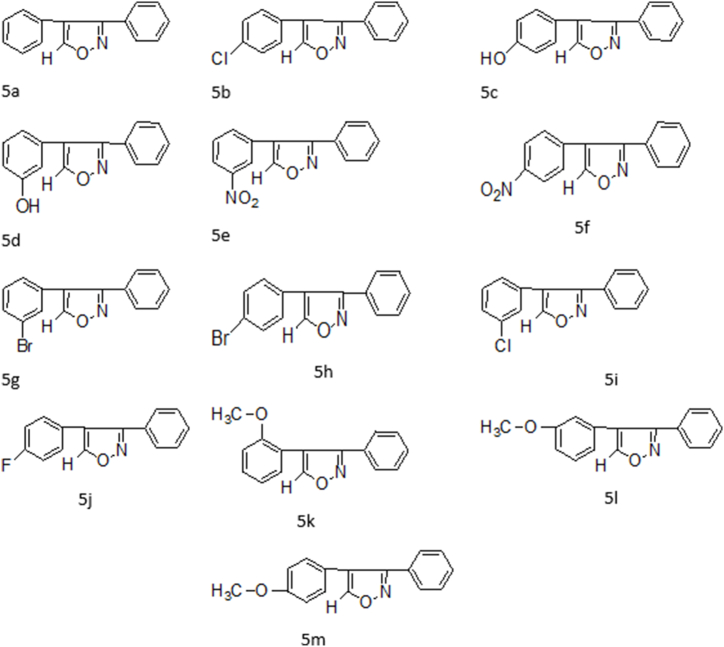
Fig. 2.
Synthesized compounds structure of isoxazole derivatives (5a-5m).
That's why developing new anti-inflammatory drugs with improved safety profiles still remains challenging. The structure of coxibs is similar to that of vicinal diaryl stilbenes, having a core ring that functions as isoxazole. The isoxazole ring is one of the heterocyclic rings known for good therapeutic responses [8], and because of its pharmacological relevance, the biological profile of isoxazole derivatives has received a lot of research over the years. They have shown diverse biological profiles, including anticancer [9,10], antidiabetic [11], antioxidant [12], anti-Alzheimer's agents [13], anti-inflammatory [14], anticonvulsant [15], antihyperlipidemic [16], anti-tubulin [17], antimycobacterial [18], and other biological activities. Isoxazole consists of a five-membered ring comprising nitrogen and oxygen atoms.
In order to create molecules that have low toxicity, high effectiveness, and no adverse reactions, such as cardiovascular side effects, gut irritation, or hemorrhaging, an isoxazole ring was selected to synthesize some novel isoxazole derivatives. In this paper, we describe the synthesis and characterization of thirteen isoxazole derivatives (including four novel compounds) along with their in silico and in vivo anti-inflammatory studies.
2. Materials and methods
2.1. General
Merck India Limited supplied all chemicals and reagents. The Mel-Temp tools were used to measure uncorrected melting points in an open capillary.TLC (silica gel H, BDH, ethyl acetate-hexane, 4:5) was used to monitor the progress of the reaction.The IR spectra were captured using a PerkinElmer analyzer and a 2400 FT-IR spectrometer.The wave numbers were stated in centimeters per second. NMR spectra were recorded by Bruker DRX-300 at 500 MHz for proton and 100 MHz for carbon NMR using TMS (tetramethylsilane) as an internal standard and CDCl3 as solvent.TMS is used as an internal standard for recording any chemical changes in ppm. A VG 7070H mass spectrometer was used to record the mass spectra.
2.2. Experimental
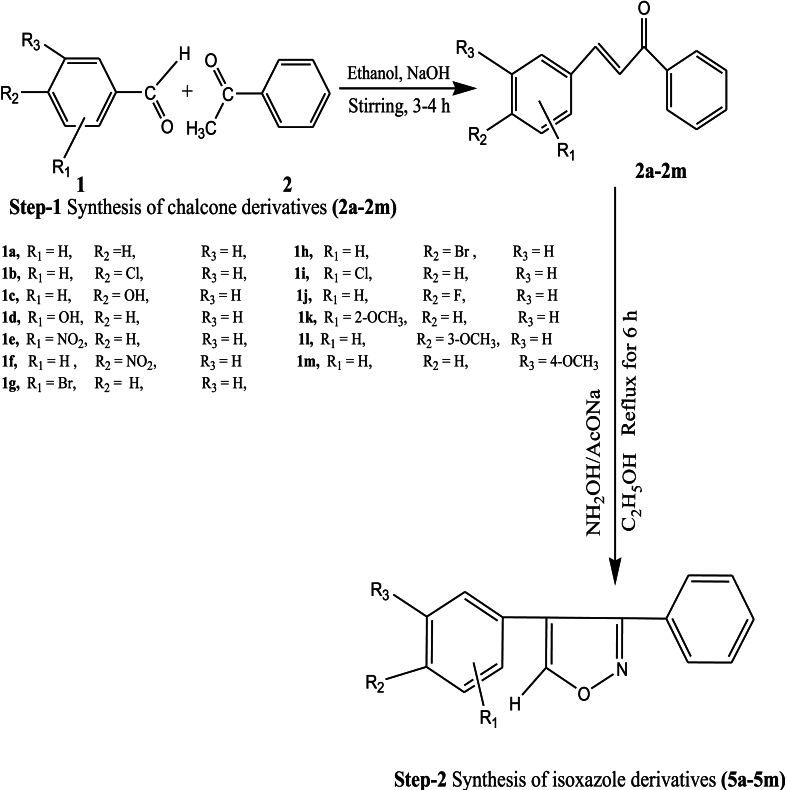
2.2.1. General procedure for substituted chalcones (2a–2m) synthesis
To 15 ml of absolute ethanol, acetophenone (4.66 ml, 0.04 mol) and substituted benzaldehyde (0.04 mol) were added. To the above reaction mixture, 10 ml of 10 %% sodium hydroxide (which is 10 g in 100 ml) ethanolic solution was added, and the reaction mixture was stirred at room temperature for 3–4 h. The completion of the reaction was monitored by thin layer chromatography (TLC) using ethyl acetate:hexane 2:5 v/v as eluent (a UV lamp was used to visualize the plates). After completion of the reaction, the reaction mixture was poured in cold water, and the final product was filtered, vacuum dried, and then recrystallized.
2.2.2. General procedure for isoxazole derivatives (5a–5m) synthesis
Substituted chalcones (0.01 mol) (2a-2m), sodium ethoxide (0.01 mol), and hydroxylamine hydrochloride (0.01 mol) were dissolved in absolute ethanol (15 ml), and then the mixture was refluxed for 6 h in an oil bath and monitored by TLC (ethyl acetate:hexane 7:3 v/v as eluent; a UV lamp was used to visualize the plates). After the completion of the reaction, as per TLC, the contents of the reaction mixture were poured into ice-cold water (50 mL). The obtained precipitates were filtered, washed two times with 2 h of air, and then recrystallized. All synthesized isoxazole derivatives (5a–5m) were characterized by melting point, FT-IR, 1H NMR, 13C NMR, and MS (ESI) (Scheme 1).
Scheme-1.
The synthetic route for isoxazole derivatives (5a-5m).
2.2.3. Characterization data of (5a-5m) isoxazole derivatives
2.2.3.1. 4-Diphenyl-isoxazole (compound 5a)
White solid (81.23 %), m.p. 177–179 °C. FT-IR (KBr, υmax/cm−1): 3234 (Aromatic C-H), 1474 (C=N), 1077 (C-O), 832 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 7.35–7.57 (m, 10H, Ar-H). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.69, 128.18, 126.85, 140.41, 129.89, 129.86, 129.60,112.62. MS (ESI): (M+1) 222.08.
2.2.3.2. 3-(4-Chloro-phenyl)-4-phenyl-isoxazole (compound 5b)
White solid (83.51 %), m.p.220-222 °C. FT-IR (υmax/cm−1): 3251 (Aromatic C-H), 1636 (C=N), 1084 (C-O), 852 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 7.35–7.56 (m, 9H, Ar-H). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.18, 126.87, 140.39, 130.91, 129.26. MS (ESI): (M+1) 256.02.
2.2.3.3. 4-(4-Phenyl-isoxazol-3-yl)-phenol (compound 5c)
Black solid (72.19 %), m.p: 289–291 °C. FT-IR (υmax/cm−1): 3235 (Aromatic C-H), 3417 (OH), 1619 (C=N), 1025 (C-O), 848 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 6.86–7.46 (m, 9H, Ar-H), 6.85 (2d, 1H, OH). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.18, 126.87, 140.39, 116.40, 130.96. MS (ESI): (M+1) 238.06.
2.2.3.4. 3-(4-Phenyl-isoxazol-3-yl)-phenol (compound 5d)
Black solid (77.28 %), m.p. 279–281 °C. FT-IR (υmax/cm−1): 3228 (Aromatic C-H), 3416 (OH), 1635 (C=N), 1050 (C-O), 832 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.68 (s, 1H), 6.86–7.47 (m, 9H, Ar-H), 5.85 (2d, 1H, OH). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.18, 126.87,155.34, 122.56, 130.96, 118.40, 115.12. MS (ESI): (M+1) 238.06.
2.2.3.5. 3-(3-Nitro-phenyl)-4-phenyl-isoxazole (compound 5e)
Yellow solid (68.43 %), m.p 260–265 °C. FT-IR (υmax/cm−1): 3237 (Aromatic C-H), 1617 (C=N), 1030 (C-O), 849 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 7.35–8.45 (m, 9H, Ar-H). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.18, 126.87, 155.20, 136.16, 130.29, 126.07, 125.27. MS (ESI): (M+1) 267.07.
2.2.3.6. 3-(4-Nitro-phenyl)-4-phenyl-isoxazole (compound 5f)
Yellow solid (79.68 %), m.p. 191–193 °C. FT-IR (υmax/cm−1): 3226 (Aromatic C-H), 1615 (C=N), 1090 (C-O), 832 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.62 (s, 1H), 7.35–8.81 (m, 9H, Ar-H). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.65, 128.18, 126.87, 140.39, 129.65, 125.54. MS (ESI): (M+1) 267.06.
2.2.3.7. 3-(3-Bromo-phenyl)-4-phenyl-isoxazole (compound 5g)
Yellow solid (68.19 %), m.p. 250–252 °C. FT-IR (υmax/cm−1): 3250 (Aromatic C-H), 1604 (C=N), 1086 (C-O), 846 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 7.34–7.76 (m, 9H, Ar-H). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.18, 126.87, 155.37, 128.59, 130.85, 133.08, 132.24. MS (ESI): (M+1) 399.97.
2.2.3.8. 3-(4-Bromo-phenyl)-4-phenyl-isoxazole (compound 5h)
Yellow solid (70.43 %), m.p. 255–257 °C. FT-IR (υmax/cm−1): 3272 (Aromatic C-H), 1600 (C=N), 1036 (C-O), 848 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 7.35–7.64 (m, 9H, Ar-H). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.18, 126.87,140.41, 130.56, 131.86. MS (ESI): (M+1) 399.98.
2.2.3.9. 3-(3-Chloro-phenyl)-4-phenyl-isoxazole (compound 5i)
White solid (78.62 %), m.p. 134-136 °C. FT-IR (υmax/cm−1): 3195 (Aromatic C-H), 1616 (C=N), 1040 (C-O), 942 (CH=CH).1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 7.35–7.64 (m, 9H, Ar-H). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.20, 126.87, 155.37, 128.19, 129.86, 130.67, 129.10. MS (ESI): (M+1) 256.02.
2.2.3.10. 3-(4-Fluoro-phenyl)-4-phenyl-isoxazole (compound 5j)
Brown solid (62.88 %), m.p. 221–223 °C. FT-IR (υmax/cm−1): 3417 (Aromatic C-H), 1638 (C=N), 1025 (C-O), 848 (CH=CH).1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 7.12–7.60 (m, 9H, Ar-H). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.20, 126.87, 140.39, 131.06,116.10. MS (ESI): (M+1) 240.02.
2.2.3.11. 3-(2-Methoxy-phenyl)-4-phenyl-isoxazole (compound 5k)
White solid (75.38 %), m.p. 223–225 °C. FT-IR (υmax/cm−1): 3414 (Aromatic C-H), 1615 (C=N), 1090 (C-O), 815 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.94 (s, 1H), 6.88–7.56 (m, 9H, Ar-H), 3.92 (s, OCH3). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.18, 126.90, 155.86, 111.96, 130.92, 122.26, 131.39, 56.4. MS (ESI): (M+1) 252.94.
2.2.3.12. 3-(3-Methoxy-phenyl)-4-phenyl-isoxazole (compound 5l)
White solid (81.59 %), m.p. 227–229 °C. FT-IR (υmax/cm−1): 3474 (Aromatic C-H), 1617 (C=N), 1026 (C-O), 873 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 6.85–7.47 (m, 9H, Ar-H), 3.83 (s,OCH3). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.18, 126.90, 155.86, 111.96, 130.92,122.26, 131.39, 55.33. MS (ESI): (M+1) 252.94.
2.2.3.13. 3-(4-Methoxy-phenyl)-4-phenyl-isoxazole (compound 5m)
White solid (77.25 %), m.p. 230–232 °C. FT-IR (υmax/cm−1): 3473 (Aromatic C-H), 1636 (C=N), 1171 (C-O), 848 (CH=CH). 1H NMR (500 MHz, CDCl3) δ (ppm); 8.69 (s, 1H), 7.04–7.52 (m, 9H, Ar-H), 3.70 (s, 3H, OCH3). 13C NMR (100 MHz, CDCl3) δ (ppm); 129.86, 128.18,126.87, 140.39, 114.64,130.56, 55.34. MS (ESI): (M+1) 252.94.
2.2.4. Docking studies
Drug discovery and design have become increasingly complex since medication research has grown so expensive and time-consuming. Computational methods are expanding rapidly because of the time, money, and effort they save. Docking is a method that can be used to make predictions about the shape and orientation of ligands within the active site of a target.The goal of docking research is to create precise models of structures and gain insight into the activities of compounds [19]. In this study, the synthesized compounds (5a–5m) and diclofenac sodium were docked with the proteins Cyclooxygenase-1 (PDB ID: 1EQH) and Cyclooxygenase-2 (PDB ID: 4COX) as target proteins [20]. Using ChemDraw Ultra (7.0), the chemical structures were built and AutoDock Vina 4.2 (MGL Tools 1.5.7), which was used to calculate and provide scores, and compared with the docking results of diclofenac sodium that have been reported to have Cyclooxygenase-1/2 inhibitory activity [21,22]. The Cyclooxygenase search grid was determined to be center_x:168.223, center_y:166.521, and center_z:158.372 using size_x 25, size_y 25, and size_z 25. The value for exhaustiveness was set at 8. The protein-ligand complexes generated during the molecular docking studies were visualized with the help of Discovery Studio Visualizer (DSV) [[23], [24], [25]].
2.2.4.1. In silico drug-likeness and ADMET prediction
In this research, the total surface area as well as the physiological characteristics of each of the most active compounds (5b, 5c, and 5d) were calculated using computational tools pkCSM online and the SwissADME application [26], and the druglikeness was evaluated in accordance with Lipinski's rule [27]. Lipinski proposed that a compound's absorption is more likely to improve if the molecule meets a minimum of three of the following four rules: (i) M. Wt. 500; (ii) LogP 5; (iii) hydrogen bond acceptor groups 10; and (iv) hydrogen bond donor groups 5 (tabel 5). The reference anti-inflammatory drug diclofenac sodium follows all of Lipinski's principles in this investigation. Our derivatives 5b, 5c, and 5d also follow all of Lipinski's instructions. One hydrogen-bonding donor group and two, three, and four hydrogen-bonding acceptor groups are present in all of the most active variations. Furthermore, molecular weights less than 500 and log P less than 5 had a hepatotoxicity drawback similar to that of diclofenac sodium, which displayed a hepatotoxicity drawback. Finally, the LD50 oral acute risky dosages of the novel isoxazole derivatives 5b, 5c, and 5d are nearly as low as the reference medication (2.231, 2.213, and 2.096, respectively, compared to 2.620 for diclofenac sodium).
2.2.5. Biological activity
2.2.5.1. Animals
Female and male albino rats weighing 160–196 g had been used in the biological experiment. The rats have been obtained from the IFTM University's Institutional Animal House in Moradabad, India. The animals were housed in a research facility setting for seven days before the experiment began. The animals were kept in a 25 ± 10C environment with a light/dark cycle of 12/12 h. The animals were randomly allocated to one of two or more groups during the acclimatization phase, control or experimental. The rats were housed individually in polyacrylic cages with sterile rice-husk bedding. The animals were all fed the same pellet diet and provided unlimited access to clean water.
2.2.5.2. Acute toxicity studies
In this study, we evaluated the toxicity of the target compounds (5b, 5c, and 5d) using the acute toxic class method, as outlined by the OECD 423 guidelines [28,29]. Adult, healthy male and female albino rats weighing between 160 and 196 g were used and monitored for 14 days, then for 72 h to evaluate for mortality. However, on day 7, the animals die, so the dose is decreased from 2000 mg/kg to 500 mg/kg, and they remain under observation for another 14 days. LD50 values were verified on additional groups of animals in the same condition.The effective dose is one-tenth of the LD50 [30,31]. Six rats (3 males and 3 females) were split up into the five subsequent groups of animals.Group 1: Consisted of healthy rats and received normal food and water for 10 days; Group 2: Inflammatited rats received diclofenac sodium (10 mg/kg, s.c.) injection; Group 3–5: Inflammatited rats orally fed with synthesized compounds (5b, 5c, and 5d) (as 0.25 % CMC suspension) at a dose of 100 mg/kg, b.w.p.o [32,33].
3. Results and discussion
3.1. Chemistry
Using the synthetic approach shown in Scheme 1, isoxazole derivatives (5a-5m) have been synthesized from the chalcones (2a-2m) in excellent yields.
3.2. Biological in vivo anti-inflammatory activity
Carrageenan-induced paw edema was used to investigate the anti-inflammatory efficacy of the synthesized compounds [34]. The test compounds (100 mg/kg), vehicle, or reference medication diclofenac sodium (10 mg/kg) were given orally shortly before the inflammation was induced, which was done by injecting 6 % carrageenan solution subcutaneously into the left hindpaw's plantar area. Using a plethysmometer, the anti-inflammatory activity was then determined based on paw-volume changes at 2h and 3h following carrageenan injection. The right hindpaw acted as a point of reference when evaluating the opposing limb. The results were expressed as a percentage of paw-volume change (ml) or % edema inhibition. Diclofenac sodium was used as a reference medication, while the carrageenan-induced rat paw edema assay was used to assess the in vivo anti-inflammatory activity of all target compounds. When the tested compounds and
diclofenac sodium (10 mg/kg) were compared for paw-volume change (% edema inhibition) after 2 h and 3 h of subcutaneous carrageenan injection-induced inflammation. Table 1 revealed a wide range of anti-inflammatory activity (7.36–75.68 %; 2 h) and (7.06–76.71 %; 3 h) in comparison to the reference drug diclofenac sodium (74.22 %; 2 h, 73.62 %; 3 h). These three compounds showed superior anti-inflammatory activities and were more powerful than diclofenac sodium, while other isoxazole derivatives possessed substantial efficacy. The percentage inhibition of the inflammatory effect of the title compounds, compared to control, was calculated using the following expression.
Table 1.
Results of in vivo anti-inflammatory activity of isoxazole derivative (5a-5m) using a carrageenan-induced paw edema model.
|
Treatments |
Dose (mg/kg) | Increase in Paw Volume (mm) |
% Inhibition |
Effectiveness |
||
|---|---|---|---|---|---|---|
| upon 2h | upon 3h | upon 2h | upon 3h | |||
| 5a | 100 | 0.54 ± 0.03 | 0.54 ± 0.03 ∗∗∗ | 45.92 | 46.32 | 0.56 |
| 5b | 100 | 0.35 ± 0.02 ∗∗∗ | 0.34 ± 0.02 ∗∗∗ | 75.68 | 76.71 | 0.83 |
| 5c | 100 | 0.36 ± 0.02 ∗∗∗ | 0.37 ± 0.02 ∗∗∗ | 74.48 | 75.56 | 0.81 |
| 5d | 100 | 0.43 ± 0.01 ∗∗∗ | 0.43 ± 0.02 ∗∗∗ | 71.86 | 72.32 | 0.71 |
| 5e | 100 | 0.82 ± 0.07 | 0.85 ± 0.06 | 13.48 | 11.89 | 0.16 |
| 5f | 100 | 0.46 ± 0.04 ∗∗∗ | 0.46 ± 0.05 ∗∗∗ | 51.31 | 51.89 | 0.66 |
| 5g | 100 | 0.78 ± 0.04 ∗ | 0.79 ± 0.04 ∗ | 17.68 | 17.41 | 0.22 |
| 5h | 100 | 0.91 ± 0.04 | 0.95 ± 0.03 | 12.30 | 11.92 | 0.02 |
| 5i | 100 | 0.57 ± 0.02 ∗∗∗ | 0.51 ± 0.03 ∗∗∗ | 39.4 | 46.37 | 0.55 |
| 5j | 100 | 0.36 ± 0.02 ∗∗∗ | 0.36 ± 02 ∗∗∗ | 61.34 | 62.24 | 0.80 |
| 5k | 100 | 0.88 ± 0.01 | 0.89 ± 0.09 | 7.36 | 7.06 | 0.09 |
| 5l | 100 | 0.53 ± 0.03 | 0.53 ± 0.03 ∗∗∗ | 43.94 | 44.32 | 0.57 |
| 5m | 100 | 0.49 ± 0.02 ∗∗∗ | 0.48 ± 0.02 ∗∗∗ | 47.81 | 50.34 | 0.63 |
| Diclofenac sodium | 10 | 0.21 ± 0.02 ∗∗∗ | 0.23 ± 0.02 ∗∗∗ | 74.22 | 73.62 | 1 |
| Control Group | 100 | 0.94 ± 0.02 | 0.95 ± 0.02 | |||
All results are mean SEM. ∗p 0.05, ∗∗p 0.01, ∗∗∗p 0.001; ANOVA, followed by Dunnett, s multiple comparion test (n = 6). All results were compared to that of the control group.
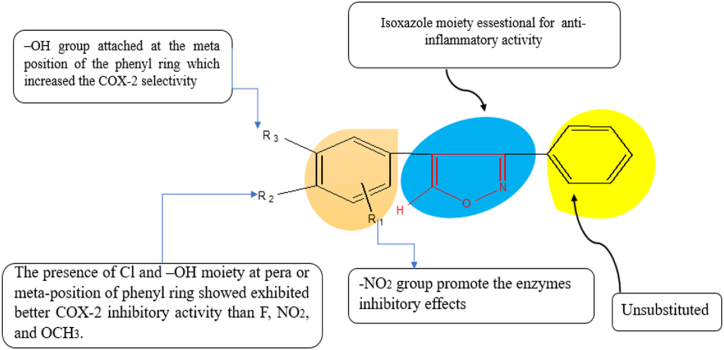
3.2.1. Structure-activity relationship (SAR)
Anti-inflammatory activities of the synthesized novel isoxazole derivatives increasing order (5b, 5c, and 5d) and all functional groups were discussed in SAR (Fig. 3).
Fig. 3.
Structure activity relationship of the novel synthesized 5b, 5c, and 5d compounds.
3.3. Docking study
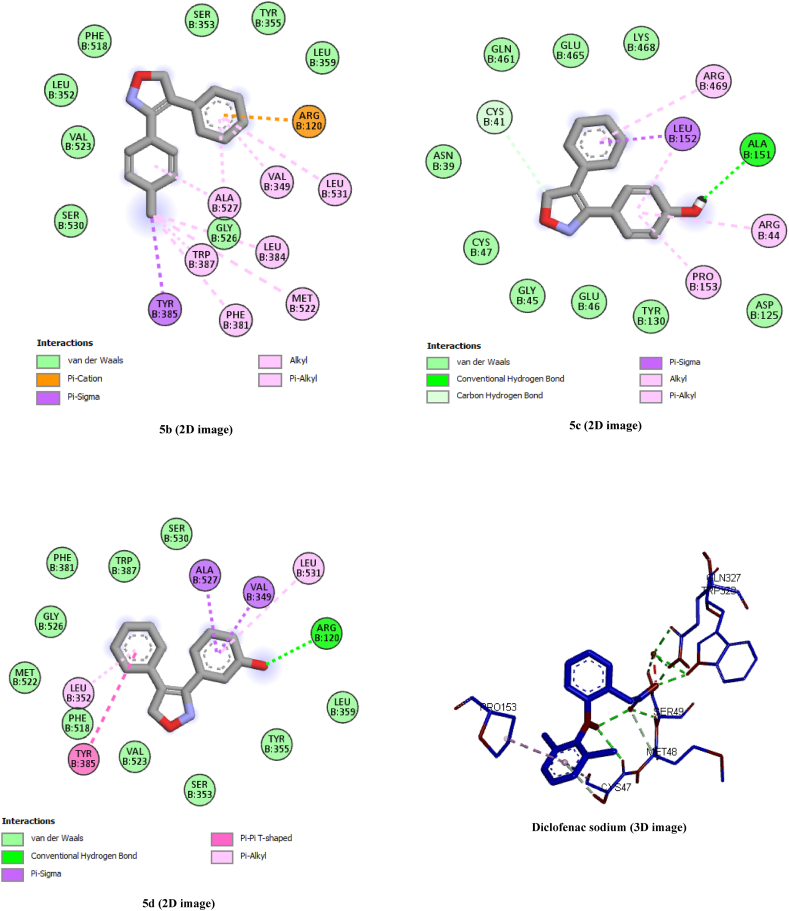
Molecular docking studies were carried out to demonstrate the plausible mechanism of interactions between the COX-2 enzyme and the synthesized drugs. The protein data bank provided the X-ray and crystal structural information for the enzyme along with a code (PDB ID: 4COX). For the docking procedure, Autodock Vina 4.2.6 (MGLtools 1.5.7) was utilized. The synthesized compounds and diclofenac sodium docking results showed a varied binding manner of interactions inside the COX-2 enzyme's active domain, with an energy score ranging from −7.8 to −8.7. Together with the least active molecule, the binding mechanism of interactions between the COX-2 active site and some of the most active compounds (5b, 5c, and 5d) is shown in Table 2 (Fig. 4). In Table 2, the docking findings were compiled, along with their energy scores and hydrogen bonding contact between amino acid residues and functional groups of docked molecules.
Table 2.
Results of molecular docking studies of compounds (5a-5m) and Diclofenac sodium into COX-1/COX-2.
| C.C | Cyclooxygenase-1 (PDB ID: 1EQH) |
Binding affinity | RMSD | Cyclooxygenase-2 (PDB ID: 4COX) |
Binding affinity | RMSD |
|---|---|---|---|---|---|---|
| Amino acid residues are implicated | Amino acid residues are implicated | |||||
| 5a | His207, Phe210,Thr212, His386,Val451 | −7 | 47.654 | Cys41, Tyr130, Leu152, Pro153, Arg469 | −8.1 | 40.842 |
| 5b | Ile124, Lys532, Pro542 | −7.6 | 2.569 | Arg120,Val349,Phe381,Leu384,Tyr385,Trp387, Met522, Ala527 | −8.7 | 2.174 |
| 5c | Phe142, Arg376, Arg374, Asn375 | −7.2 | 34.832 | Asn39,Cys41, Ala151, Leu152,Pro153, Gln461, Arg469 | −8.5 | 41.132 |
| 5d | Val116, Arg120, Leu352, Tyr355, Gly526, Ala527, Leu531 | −7.4 | 23.631 |
Arg120,Val349, Leu352,Tyr385, Ala527, Leu531 |
−8.4 | 22.256 |
| 5e | Phe142, Ser143,Val145, Arg374, Arg376, Arg374 | −7.9 | 2.882 | Arg44, Cys47, Leu152, Lys546 | −8.3 | 2.521 |
| 5f | Ile46, Leu152, Pro153, Glu465, Lys468, Arg469 | −8.7 | 5.873 | Leu145,Val 228, Tyr373, Asn375, Asn537 | −8.1 | 4.008 |
| 5g | Gln44, Ile46, Pro153,Glu465, Lys468, Arg469 | −8.4 | 2.371 | Cys41, Cys47, Tyr130, Leu152, Pro153, Arg469 | −8.2 | 2.094 |
| 5h | Val116, Leu352, Ala527 | −7.8 | 26.574 | Cys47, Leu152, Pro153 | −8 | 2.216 |
| 5i | Thr206, His207, His386, His388, Met391,Val447 | −7.8 | 27.057 | Cys41, Cys47, Tyr130, Leu152, Pro153, Arg469 | −8.1 | 15.159 |
| 5j | Phe142,Val145, Arg374, Arg376, Pro538 | −7.4 | 3.655 | Val349, Leu352, Leu531, Ala 527 | −8.2 | 25.085 |
| 5k | Ile46,Tyr130, Leu152,Gln461 | −8 | 1.799 | Ser121, Tyr 122, Ser126, Gln372, Lys532 | −7.8 | 1.746 |
| 5l | Phe142, Ser143,Val145, Arg374, Asn375 | −7.6 | 15.805 | Asn39, Cys41, Leu152, Pro153, Lys468,Arg469 | −8.1 | 45.125 |
| 5m | His207, His386, His388 | −8 | 26.334 | Ser121, Tyr 122, Ser126, Leu366, Phe367, Gln370 | −8.0 | 28.242 |
| Diclofenac sodium | Ile46,Cys47,Pro153,Pro156,Trp323,Gln327 | −7.3 | 32.019 | Trp323,Gln327,Cys47, Met48,Ser49, Pro153 | −7.9 | 2.386 |
C.C- Compound Code, In Bold: H- bonding interaction, RMSD- Root Mean Square Deviation.
Fig. 4.
2D, 3D Interactions of compounds 5b, 5c, 5d and diclofenac sodium within the binding pocket of Cyclooxygenase-2.
3.3.1. ADMET study
The ligand chemical structures were submitted to the pkCSM web server (http://biosig.unimelb.edu.au/pkcsm/prediction) and SwissADME (www.swissadme.ch/) for in silico analysis. This analysis assessed various pharmacokinetic parameters, including gastrointestinal absorption, skin permeation, blood-brain barrier penetration, synthetic associability, interaction with cytochromes P450 (CYP), bioavailability score, and drug-likeness prediction based on Lipinski rules. Additionally, the PkCSM web server provided predictions for toxicity values, AMES toxicity, and skin sensitization. This comprehensive in silico evaluation offers valuable insights for drug development and safety assessment. Most active synthetic compounds (5b, 5c, and 5d) would interact with human metabolism. Moreover, it assesses the impact of these chemical compounds on pharmacological activity when taken orally. Numerous factors, including physicochemical characteristics, water solubility, lipophilicity, pharmacokinetics, and drug-likeness, were found and are shown in Table 3.
Tabel 3.
ADMET profile of the three (5b, 5c, 5d) most active compounds and diclofenac sodium.
| Parameter | 5b | 5c | 5d | Diclofenac Sodium |
|---|---|---|---|---|
| Molecular properties | ||||
| Molecular Weight | 255.704 | 237.258 | 237.258 | 318.135 |
| LogP | 4.662 | 3.7142 | 3.7142 | 3.9062 |
| Rotatable Bonds | 2 | 2 | 2 | 4 |
| Acceptors | 2 | 3 | 3 | 3 |
| Donors | 0 | 1 | 1 | 1 |
| Surface Area | 109.814 | 104.305 | 104.305 | 144.12 |
| Absorption | ||||
| Water solubility | −4.694 | −3.174 | −3.264 | −4.497 |
| Caco2 permeability | 1.043 | 1.323 | 1.301 | 1.121 |
| Intestinal absorption (human) | 95.242 | 94.292 | 94.444 | 94.028 |
| Skin Permeability | −2.592 | −2.733 | −2.736 | −2.846 |
| P-glycoprotein substrate | No | No | No | No |
| P-glycoprotein I inhibitor | No | No | No | No |
| P-glycoprotein II inhibitor | Yes | Yes | Yes | No |
| Distribution | ||||
| VDss (human) | 0.221 | 0.019 | −0.111 | 0.224 |
| Fraction unbound (human) | 0.183 | 0.174 | 0.161 | 0.102 |
| BBB permeability | 0.184 | 0.016 | 0.355 | 0.254 |
| CNS permeability | −1.365 | −1.366 | −1.437 | −1.115 |
| Metabolism | ||||
| CYP2D6 substrate | No | No | No | No |
| CYP3A4 substrate | Yes | No | No | No |
| CYP1A2 inhibitior | Yes | Yes | Yes | Yes |
| CYP2C19 inhibitior | Yes | Yes | Yes | Yes |
| CYP2C9 inhibitior | Yes | Yes | Yes | Yes |
| CYP2D6 inhibitior | No | No | No | No |
| CYP3A4 inhibitior | No | No | No | Yes |
| Excretion | ||||
| Total Clearance | 0.23 | 0.357 | 0.343 | 1.116 |
| Renal OCT2 substrate | No | No | No | No |
| Toxicity | ||||
| AMES toxicity | No | Yes | Yes | No |
| Max. tolerated dose (human) | 0.577 | 0.724 | 0.697 | No |
| hERG I inhibitor | No | No | No | 0.521 |
| hERG II inhibitor | Yes | Yes | Yes | No |
| Oral Rat Acute Toxicity (LD50) | 2.231 | 2.213 | 2.096 | 2.620 |
| Oral Rat Chronic Toxicity (LOAEL) | 0.885 | 1.708 | 1.768 | 0.966 |
| Hepatotoxicity | No | No | No | No |
| Skin Sensitization | No | No | No | No |
| T.Pyriformis toxicity | 0.493 | 0.466 | 0.469 | 1.397 |
| Minnow toxicity | −0.606 | 0.466 | 0.605 | −2.43 |
3.3.1.1. Discussion
Scheme 1 depicts the reaction method for some novel synthesized isoxazole derivatives (5a–5m) (see Table 4). The key intermediary substituted chalcone derivatives (2a–2m) were synthesized by the reaction of substituted benzaldehyde with acetophenone in a 10 percent sodium hydroxide alcoholic solution. Finally, substituted chalcone derivatives (2a–2m) reacted with hydroxylamine hydrochloride; sodium ethoxide was added to ethanol (25 ml), and the mixture was combined, then refluxed for 6 h in an oil bath. The structures of the isoxazole derivatives (5a–5m) were determined using FTIR, 1H NMR, 13C NMR, and MS. 13C NMR spectra indicate the existence of all carbon atoms in compounds (5a–5m). The molecular weights of substances were validated by mass spectra. All of the compounds had a molecular ion peak.
Tabel 4.
The druglikeness studies of synthesized compounds (5a-5m) and diclofenac sodium based on Lipinski's rule of five.
| Compounds Code | Property |
Lipinski violation | |||||
|---|---|---|---|---|---|---|---|
| LogP | H-bond acceptor | H-bond donor | Polar surface area (A2) | Rotatable Bonds | Molecular weight | ||
| 5a | 4.0086 | 2 | 0 | 99.511 | 2 | 221.259 | 0 |
| 5b | 4.662 | 2 | 0 | 109.814 | 2 | 255.704 | 0 |
| 5c | 3.7142 | 3 | 1 | 104.305 | 2 | 237.258 | 0 |
| 5d | 3.7142 | 3 | 1 | 104.305 | 2 | 237.258 | 0 |
| 5e | 3.9168 | 4 | 0 | 114.164 | 3 | 266.256 | 0 |
| 5f | 4.7711 | 2 | 0 | 113.378 | 2 | 300.155 | 0 |
| 5g | 4.7711 | 2 | 0 | 113.378 | 2 | 300.155 | 0 |
| 5h | 4.7711 | 2 | 0 | 113.378 | 2 | 300.155 | 0 |
| 5i | 4.662 | 2 | 0 | 109.814 | 2 | 255.704 | 0 |
| 5j | 4.1477 | 2 | 0 | 103.676 | 2 | 239.249 | 0 |
| 5k | 4.0172 | 3 | 0 | 110.989 | 3 | 251.285 | 0 |
| 5l | 4.0172 | 3 | 0 | 110.989 | 3 | 251.285 | 0 |
| 5m | 4.0172 | 3 | 0 | 110.989 | 3 | 251.285 | 0 |
| Diclofenac Sodium | 3.9062 | 3 | 1 | 144.912 | 4 | 318.13 | 0 |
4. Conclusion
In the present work, a series of isoxazole derivatives (5a–5m) were efficiently synthesized using efficient methodology (5c, 5d, 5e, and 5g) four of which were novel derivatives, and characterized using Fourier transform infrared spectroscopy (FT-IR), proton nuclear magnetic resonance (1H NMR) spectroscopy, carbon-13 nuclear magnetic resonance (13C NMR) spectroscopy, and mass spectrometry. The molecular docking study helped in supporting the observed COX-1 and COX-2 activity profiles. The anti-inflammatory activity was evaluated using the carrageenan-induced rat paw edema method. In vivo studies corroborate that test compounds have good anti-inflammatory activity. Moreover, compounds 5b, 5c, and 5d are more potent than diclofenac sodium. According to the results obtained from the pkCSM platform, most active compounds displayed good oral absorption, solubility, and lipophilicity when evaluated for ADMET properties in silico. Compounds 5b, 5c, and 5d demonstrated a good ADMET profile similar to diclofenac sodium with the least probability of hepatotoxicity. Aforementioned findings inferred 5b as a potential COX inhibitor and anti-inflammatory agent and thus render it a lead molecule for further development of new anti-inflammatory agents with better pharmacokinetic properties.
CRediT authorship contribution statement
Sonu: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis. Kamal YT: Funding acquisition. Girendra Kumar Gautam: Data curation, Formal analysis. Arun Kumar Mishra: Conceptualization. Baby Rabiya Parveen: Conceptualization, Writing – review & editing. Arvind Kumar: Writing – review & editing. Mhaveer Singh: Conceptualization, Investigation. Harpreet Singh: Supervision, Writing – original draft, Writing – review & editing.
Informed consent statement
Not applicable.
Institutional review board statement
The IFTM University Institutional Animal Ethics Committee reviewed and approved the animal study.
Ethics statement
The experimental protocols were approved by the Institutional Animal Ethics Committee of IFTM University, constituted under the Committee for the Purpose of Control and Supervision of Experimental Animals guidelines. The ethics approval number is IAEC/2022/33, and the resolution number is 837/PO/ReBiBt/S/04/CPCSEA. The study complies with all regulations.
Data availability
Data included in the article is referenced in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through the Large Research Project under grant number RGP2/580/45.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40300.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ragab F.A., Heiba H.I., El-Gazzar M.G., Abou-Seri S.M., El-Sabbagh W.A., El-Hazek R.M. Anti-inflammatory, analgesic and COX-2 inhibitory activity of novel thiadiazoles in irradiated rats. J. Photochem. Photobiol. B Biol. 2017;166:285–300. doi: 10.1016/j.jphotobiol.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Mallavadhani U.V., Chandrashekhar M., Shailaja K., Ramakrishna S. Design, synthesis, anti-inflammatory, cytotoxic and cell-based studies of some novel side chain analogues of myrrhanones A & B isolated from the gum resin of Commiphora mukul. Bioorg. Chem. 2018;82:306–323. doi: 10.1016/j.bioorg.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 3.hen L.Z., Yao L., Jiao M.M., Shi J.B., Tan Y., Ruan B.F., Liu X.h. Novel resveratr ol-based flavonol derivatives: synthesis and anti-inflammatory activity in vitro and in vivo. Eur. J. Med. Chem. 2019;175:114–128. doi: 10.1016/j.ejmech.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein F.E., Faich G., Goldstein J.L., Simon L.S., Pincus T., Whelton A., Makuch R., Eisen G., Agrawal N.M., Stenson W.F., Burr A.M., Zhao W.W., Kent J.D., Lefkowith J.B., Verburg K.M., Geis G.S. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial Celecoxib Long-term Arthritis Safety Study. J. Am. Med. Assoc. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 5.Bombardier C., Laine L., Reicin A., Shapiro D., Vargas R.B., Davis B., Day R., Ferraz M.B., Hawkey C.J., Hochberg M.C., Kvien T.K., Schnitzer T.J., VIGOR Study Group Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N. Engl. J. Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 6.Schneider F., Meziani F., Chartier C., Alt M., Jaeger A. Fatal allergic vasculitis associated with celecoxib. Lancet. 2002;359:852–853. doi: 10.1016/S0140-6736(02)07922-9. [DOI] [PubMed] [Google Scholar]
- 7.Dogné J.M., Supuran C.T., Pratico D. Adverse cardiovascular effects of the coxibs. J. Med. Chem. 2005;48:2251–2257. doi: 10.1021/jm0402059. [DOI] [PubMed] [Google Scholar]
- 8.Esquivel E.C.C., Rufino V.C., Nogueira M.H.T., Souza A.C.C., Júnior J.R., Valle M.S. Synthesis and characterization of 1,3,5-Triarylpyrazol-4-ols and 3,5- diarylisoxazol-4-ols from chalcones and theoretical studies of the stability of pyrazol-4-ol toward acid dehydration. J. Mol. Stru. 2019;1204 [Google Scholar]
- 9.Duan K., Tan F., Xie H., Liu H., Zhang Y., Jiang H., Wu W. Design, synthesis and antitumor activity of 3,4,5-Trisubstituted Isoxazoles. Eur. J. Med. Chem. Reports. 2024;12 [Google Scholar]
- 10.Shaik K.S., Saritha N., Reddy G.N. Design, synthesis and biological evaluation of aryl isoxazole incorporated pyrimidin-2-yl)oxazolo[4,5-b]pyridine derivatives as anticancer agents. Results in Chemistry. 2024;9 [Google Scholar]
- 11.Rashidi E.A., Ghannay S., Albadri A.E.A.E., Abid M., Kadri A., Aouadi K. Design, synthesis, biological evaluation, kinetic studies and molecular modeling of imidazo-isoxazole derivatives targeting both α-amylase and α-glucosidase inhibitors. Heliyon. 2024;10(20) [Google Scholar]
- 12.Nezhad S.M., Pourmousavi S.A., Zare E.N., Heidari G., Hosseini S., Peyvandtalab M. Magnetic poly(1,8-diaminonaphthalene)-nickel nanocatalyst for the synthesis of antioxidant and antibacterial isoxazole-5(4H)-ones derivatives. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e15886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vafadarnejad F., Razkenari E.K., Sameem B., Saeedi M., Firuzi O., Edraki N., Mahdavi M., Akbarzadeh T. Novel N-benzyl pyridinium moiety linked to aryl isoxazole derivatives as selective butyrylcholinesterase inhibitors: synthesis, biological evaluation, and docking study. Bioorg. Chem. 2019;92 doi: 10.1016/j.bioorg.2019.103192. [DOI] [PubMed] [Google Scholar]
- 14.Pallavi H.M., Al-Ostoot F.H., Vivek H.K., Khanum S.A. Synthesis, characterization, DFT, docking studies and molecular dynamics of some 3-phenyl-5-furan isoxazole derivatives as anti-inflammatory and anti-ulcer agents. J. Mol. Struct. 2022;1250 [Google Scholar]
- 15.Huang X., Dong S., Liu H., Wan P., Wang T., Quan H., Wang Z. Design, synthesis, and evaluation of novel benzo[d]isoxazole derivatives as anticonvulsants by selectively blocking the voltage-gated sodium channel NaV1.1, A.C.S. Chem. Neur. 2022;13(6) doi: 10.1021/acschemneuro.1c00846. [DOI] [PubMed] [Google Scholar]
- 16.Qiu R., Luo G., Li X., Zheng F., Li H., Zhang J., You Q. Lipid accumulation inhibitory activities of novel isoxazole-based chenodeoxycholic acids: design, synthesis and preliminary mechanism study. Bioorg. Med. Chem. Lett. 2018;28(17):2879–2884. doi: 10.1016/j.bmcl.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Wang G., Liu W., Huang Y., Li Y., Peng Z. Design, synthesis and biological evaluation of isoxazole-naphthalene derivatives as anti-tubulin agents. Arab. J. Chem. 2020;13(6):5765–5775. [Google Scholar]
- 18.Ravisankar N., Sarathi N., Maruthavanan T., Ramasundaram S., Ramesh M., Sankar C., Umamatheswari S., Kanthimathi G., Oh T.H. Synthesis, antimycobacterial screening, molecular docking, ADMET prediction and pharmacological evaluation on novel pyran-4-one bearing hydrazone, triazole and isoxazole moieties: potential inhibitors of SARS CoV-2. J. Mol. Stru. 2023;1285 doi: 10.1016/j.molstruc.2023.135461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das A.K., Paul P., Pranto M.P., Hassan M.J., Saha K., Hossain M.E. Design, synthesis, characterization, antimicrobial activity, cytotoxicity,molecular docking, and in-silico ADMET analysis of the novel cefuroxime derivatives. Eur. J. Med. Chem. Reports. 2024;10 [Google Scholar]
- 20.Degfie T., Endale M., Tafese T., Dekebo A., Shenkute K. In vitro antibacterial, antioxidant activities,molecular docking, and ADMET analysis of phytochemicals from roots of Hydnora johannis. Applied Bio. Chem. 2022:65–76. [Google Scholar]
- 21.Lahyaoui M., Filali M., Benamar K., Sghyar R., Benbrahim K.F., Haoudi A., Mazzah A., El khattabi S., Hadrami E., K Rodi Y., Sebbar N.K. Development of novel antibiotics derived from pyridazine: synthesis, spectroscopic characterization, in vitro antimicrobial activity and molecular docking studies. Results in Chemistry. 2024;10 [Google Scholar]
- 22.Bhat M.A., Al-Omar M.A., Raish M., Ansari M.A., Abuelizz H.A., Bakheit A.H., Naglah A.M. Indole derivatives as cyclooxygenase inhibitors: synthesis, biological evaluation and docking studies. Molecules. 2018;23(6):1250. doi: 10.3390/molecules23061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parveen S., Khan S., Iqbal T., Dera A.A., Hussain R., Khan Y. Synthesis, spectroscopy and biological investigation via DFT, ADMET and molecular docking of Thiadiazole/Oxadiazole based bis-Schiff bases: a potential towards diabetes and microbes. Results in Chemistry. 2024;11 [Google Scholar]
- 24.Korol N., Kamoshenkova O.H., Mariychuk R., Slivka M. Insights into bacterial interactions: comparing fluorine-containing 1,2,4-triazoles to antibiotics using molecular docking and molecular dynamics approaches. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e37538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Othman I.M.M., Mahross M.H., Gad-Elkareem M.A.M., Rudrapal M., Gogoi N., Chetia D., Aouadi K., Snoussi M., Kadri A. Toward a treatment of antibacterial and antifungal infections: design, synthesis and in vitro activity of novel arylhydrazothiazolylsulfonamides analogues and their insight of DFT, docking and molecular dynamic simulations. J. Mol. Stru. 2021;1243 [Google Scholar]
- 26.Kalirajan R., Rafick M.H.M., Sankar, Jubie S. Docking studies, synthesis, characterization and evaluation of their antioxidant and cytotoxic activities of some novel isoxazole-substituted 9-anilinoacridine derivatives. The. Scie. W. Jou. 2012 doi: 10.1100/2012/165258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abolhasani H., Zarghi A., Movahhed T.K., Abolhasani A., Daraei B., Dastmalchi S. Design, synthesis and biological evaluation of novel indanone containing spiroisoxazoline derivatives with selective COX-2 inhibition as anticancer agents. Bioorg. Med. Chem. 2021;32 doi: 10.1016/j.bmc.2020.115960. [DOI] [PubMed] [Google Scholar]
- 28.Podila N., Penddinti N.K., Rudrapal M., Rakshit G., Konidala S.K., Pulusu V.S., Bhandare R.R., Shaik A.B. Design, synthesis, biological and computational screening of novel pyridine-based thiadiazole derivatives as prospective anti-inflammatory agents. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e29390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarapula R., Gangarapu K., Manda S., Rekulapally S. Synthesis, in vivo anti-inflammatory activity, and molecular docking studies of new isatin derivatives. Int. J. Med. Chem. 2016 doi: 10.1155/2016/2181027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung W.Y., Jadhav S., Hsu P.K., Kuan C.M. Evaluation of acute and sub-chronic toxicity of bitter melon seed extract in Wistar rats. Toxicol Rep. 2022;9:1024–1034. doi: 10.1016/j.toxrep.2022.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Othman F.A., Zin A.M., Zakaria Y., H Salleh N., Tan S.C. Dataset of acute oral toxicity and subacute neurotoxicity risk assessments of flavonoid-enriched fraction extracted from Oroxylum Indicum on Sprague Dawley rats. Data Brief. 2023;49 doi: 10.1016/j.dib.2023.109411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gharge S., Alegaon S.G., Jadhav S., Ranade S.D., Kavalapure R.S. Design, synthesis, characterization and antidiabetic evaluation of 3,5-substituted thiazolidinediones: evidenced by network pharmacology, Molecular docking, dynamic simulation, in vitro and in vivo assessment. Eur. J. Med. Chem. Rep. 2024;12 [Google Scholar]
- 33.Kyhoiesh H.A.K., Al-Hussainawy M.K., Waheeb A.S., Al-Adilee K.J. Synthesis, spectral characterization, lethal dose (LD50) and acute toxicity studies of 1,4-Bis(imidazolylazo)benzene (BIAB) Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Z.Y., Jin Q.H., Qu Y.L., Guan L.P. Chalcone derivatives bearing chromen or benzo[f]chromen moieties: design, synthesis, and evaluations of anti-inflammatory, analgesic, selective COX-2 inhibitory activities. Bioorg Medi Chem Lett. 2019;29:1909–1912. doi: 10.1016/j.bmcl.2019.05.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in the article is referenced in the article.