Highlights
-
•
Cannabis has transitioned from being stigmatized and illegal to gaining recognition for its medical benefits.
-
•
Shifting cannabis from a prohibited substance to a recognized medicine involves complex changes in science, public opinion, and regulations.
-
•
Scientific research, public perception, and safety considerations interrelate and impact the future of medical cannabis use and patient adherence.
Keywords: Medical cannabis, Therapeutic benefits, Public perception, Stigma, Safety strategies, Patient adherence
Abstract
The landscape of medical cannabis has evolved dramatically over the past few decades. Once stigmatized and illegal in most parts of the world, cannabis is now recognized for its potential therapeutic benefits, supported by an expanding body of scientific research. However, the transition from prohibition to medical recognition is shaped by complex interactions among scientific advancements, public perception and regulatory frameworks for its legalization. This review examines the recent breakthroughs in medical cannabis research, explores the shifting public perceptions and the stigma associated with its use and discusses strategies for enhancing the safety of medical cannabis. We also synthesize the connections between scientific research, public perception and safety considerations in the uses of medical cannabis, providing a comprehensive understanding of how these elements influence each other and shape the future of medical cannabis use for patient adherence.
Graphical abstract
1. Introduction
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most recognized components of cannabis, but the plant contains over 500 distinct compounds, including more than 80 naturally occurring cannabinoids.1 “Medical cannabis” refers to the therapeutic use of cannabis and its derivatives to alleviate symptoms of various medical conditions.2 Unlike recreational cannabis, medical cannabis is prescribed by healthcare providers to treat specific ailments or symptoms, such as chronic pain,3 particularly when other treatments are ineffective in certain conditions like arthritis, fibromyalgia, and multiple sclerosis. Among all phytocannabinoids in Cannabis, THC and CBD being extensively researched. THC is the psychoactive component of cannabis that produces a “high” and is used medically to relieve pain, reduce nausea, and increase appetite,4 especially in patients undergoing chemotherapy or living with conditions like AIDS.5,6 On the other hand, CBD is non-psychoactive and is known for its anti-inflammatory, anti-anxiety, and pain-relieving properties, commonly used to treat conditions like epilepsy, anxiety disorders, chronic pain, and inflammation.7,8 Notably, Epidiolex, a CBD-based medication, has been approved to treat severe forms of epilepsy like Dravet syndrome9 and Lennox-Gastaut syndrome.10
The legal status of medical cannabis varies across countries and regions. In some places, it is fully legal and regulated, while in others, it is restricted or not permitted at all. Where legal, patients typically need a prescription from a healthcare provider to obtain medical cannabis from licensed dispensaries. Despite its increasing acceptance and use worldwide, medical cannabis remains a complex and evolving field, with ongoing research into its safety and effectiveness. Public perception is shaped by historical, cultural, and legal factors, contributing to the stigma associated with its use. This stigma is shaped by societal attitudes, misconceptions, and varying degrees of acceptance within different medical communities11,12 and it creates significant barriers to the widespread acceptance and integration of cannabis into mainstream medical practice, impacting both patient experiences and treatment outcomes.
In this review, we aim to explore the connections between scientific research, public perception, and safety considerations in the context of medical cannabis. We trace the history of ancient use to current practices, examine how public attitudes and stigma have evolved, and discuss the implications for patient care. Finally, we explained the steps needed to ensure that cannabis becomes a safer and more accepted option for medical treatment.
2. Evolution of medical cannabis use
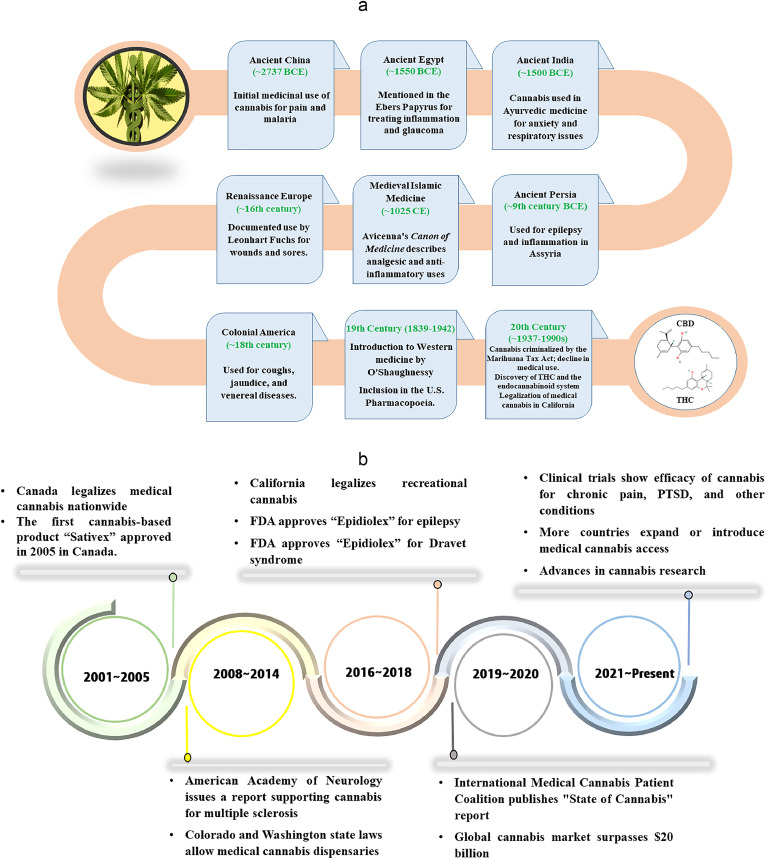
The use of cannabis for medicinal purposes has a rich and complex history that spans thousands of years and diverse cultures. From ancient herbal remedies to modern pharmaceuticals, cannabis has long been valued for its therapeutic properties.13 The use of cannabis in medicine can be traced to Fig. 1 (a). The earliest recorded use of cannabis in medicine dates back to ancient China, where it was mentioned in the “Pen Ts'ao Ching,” attributed to Emperor Shen Nong around 2737 BCE.14,15 In medieval times, Islamic scholars, like Avicenna, recognized the medicinal benefits of cannabis, incorporating it into treatments for pain and inflammation as described in his “Canon of Medicine” in 1025 CE.16 The modern era of medical cannabis began in the 19th century, when Western medicine started to acknowledge its therapeutic potential. By the late 1800s, cannabis was widely used in the United States and included in the U.S. Pharmacopoeia, prescribed for conditions like migraines, menstrual cramps, and sleep disorders.17 However, its use declined throughout much of the 20th century due to legal restrictions. The reappearance of interest in medical cannabis in the 1990s, particularly with California's legalization of medical cannabis in 1996 under Proposition 215,18 marked the beginning of a new era. The discovery of the endocannabinoid system19, 20, 21 further expanded the scientific research, leading to a growing acceptance of cannabis as a legitimate treatment option. Today, supported by an expanding body of scientific evidence, policy making for its legalization and market growth of medical cannabis mostly THC and CBD, and partly some minor cannabinoids (shown in Fig. 2), is also increasingly recognized for its potential to treat a variety of conditions which is shown in Fig. 1 (b).
Fig. 1.
(a) The journey of medical cannabis from ancient herbal remedies to modern pharmaceutical applications. (b) Key milestones, including early documentation, major research breakthroughs, and legislative changes, highlight the significant developments in the understanding and utilization of cannabis for medical purposes.
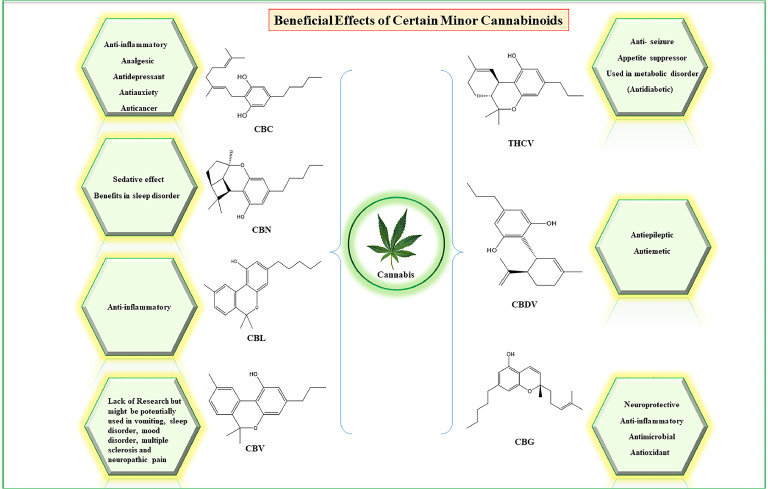
Fig. 2.
Therapeutic Potential of Minor Cannabinoids: The figure illustrates the potential of minor cannabinoids as promising therapeutic interventions for a range of diseases. Various cannabinoids, such as cannabichromene (CBC), cannabinol (CBN), cannabicyclol (CBL), cannabivarin (CBV), cannabigerol (CBG), cannabidivarin (CBDV), tetrahydrocannabivarin (THCV) and are depicted as emerging candidates for treating certain medical conditions.
In recent years, the use of cannabis for medicinal purposes has gained acceptance in various countries and regions around the globe. Nations like the Netherlands, Canada, France, Germany, and several U.S. states have embraced its therapeutic potential. Take the Netherlands as an example, where patients can access medicinal cannabis through a prescription from any physician. The Dutch Office of Medical Cannabis (OMC) oversees the cultivation of the plant under stringent regulations, ensuring both safety and consistency in the product.22 Recently, In the Netherlands, patients have access to five distinct types of medical cannabis, each with varying levels of THC and CBD. These range from Bedrocan, with a high THC content of 22 % and less than 1 % CBD, to Bedrolite, which contains less than 1 % THC and around 9 % CBD. The OMC emphasizes that the right type of cannabis is determined by the patient's specific medical condition. For instance, Bediol, which has a balanced ratio of 6 % THC and 8 % CBD, is frequently recommended as an initial treatment for chronic nerve pain. According to the OMC, THC plays a crucial role in alleviating pain, making it an essential component in the management of certain conditions.
As compassionate medicine gains momentum and public support for legalization grows, cannabis is finally receiving the scientific attention and now it is likely to play an even more significant role in medicine, offering new hope to patients worldwide.
3. Medical cannabis: evaluating its legitimacy as a treatment option
Medical cannabis is now recognized as a potential treatment for a wide range of conditions, including chronic pain, epilepsy, multiple sclerosis,23 and chemotherapy-induced nausea and vomiting. Rapid advancements in scientific research have deepened our understanding of the benefits and risks associated with cannabis use. The discovery of THC led to the identification of the endocannabinoid system (ECS) in the 1990s, which plays a crucial role in regulating pain, mood, appetite, and memory.24 This discovery provided a scientific basis for the therapeutic effects of cannabis and reignited interest in its medical applications. The concept of Clinical Endocannabinoid Deficiency (CECD), proposed by Dr. Ethan Russo, suggests that certain conditions, like migraines, fibromyalgia, and irritable bowel syndrome, multiple sclerosis, and posttraumatic stress disorder may be linked to deficiencies in the ECS.25 Research has also connected the ECS to neurodegenerative disorders, like Alzheimer's, Huntington's, and Parkinson's diseases, offering new perspectives on their origins. The approval of Sativex, a cannabis-based spray containing THC and CBD, in Canada in 2005 marked a significant breakthrough in the use of cannabinoids for treating conditions like multiple sclerosis and certain types of pain.26 Currently, oromucosal spray of this medication, has been approved in several countries for the treatment of spasticity in multiple sclerosis.
A 2017 report,2 from the National Academies of Sciences, Engineering, and Medicine provided strong evidence that cannabis is effective in treating chronic pain, leading to its use as an alternative to opioids in areas with high opioid addiction rates. Similarly, the Food and Drug Administration's (FDA) approval of Epidiolex, a CBD-based drug, in 201827 for treating certain types of epilepsy, such as Dravet syndrome9 and Lennox-Gastaut syndrome,10 was another milestone in medical cannabis. Some recent clinical trials have also shown that CBD can effectively reduce seizure frequency and manage symptoms related to cancer and its treatments, which makes it more promising future candidate in scientific community. However, a systematic review and meta-analysis published in Journal of the American Medical Association in 2015 found that while there is moderate-quality evidence supporting cannabinoids for chronic pain and spasticity, the evidence is weaker for other conditions like chemotherapy-induced nausea, sleep disorders, and Tourette syndrome. They also reported that cannabinoids were associated with a higher risk of short-term adverse effects.28 Despite these challenges, ongoing research is likely to expand the role of cannabis in medicine, offering new hope for patients with various diseases conditions.
4. Advances in medical cannabis: recent scientific developments
Recent scientific developments in preclinical and clinical use of medical cannabis have significantly advanced our understanding of its effects and therapeutic potential. The discovery of cannabinoid receptors CB1 and CB2, along with their natural ligands, the endocannabinoids, has been crucial in explaining how cannabis works in the body.21 For example, CB1 receptors play a key role in regulating neuroinflammation and pain perception, making them important targets for treating chronic pain and neurodegenerative diseases. As cannabis contains versatile medicinal compounds, the “entourage effect” refers to the enhanced therapeutic impact that occurs when cannabinoids interact with other cannabis compounds like terpenes and flavonoids.29 Some research supports this effect, showing that these combinations are more effective at modulating pain and inflammation than isolated compounds.30
Advances in genomics and pharmacogenetics are leading to more personalized medical cannabis therapies. A comprehensive study by Thorpe et al. has shown that genetic differences in cannabinoid receptors and metabolizing enzymes can influence how individuals respond to cannabis, allowing for more tailored and effective treatments.31 Additionally, research has explored the role of cannabinoids in cancer treatment, with evidence suggesting that cannabinoids can enhance the effectiveness of traditional therapies and reduce side effects. For instance, the involvement of specific pathways, such as the stress-regulated protein p8 (nupr1)/ tribbles-homologue 3 (p8/trib3) mediated autophagy, has been shown to contribute to the antitumor effects of cannabinoids in various cancer cells.32, 33, 34 Moreover, a recent study on the effects of CBD on anxiety conducted by L. Riley Gournay found that while a single dose of CBD did not reduce worry severity or anxiety symptoms, repeated administration of a higher dose (300 mg) over two weeks significantly reduced anxiety symptoms compared to a placebo. Their findings highlight the importance of dosage and duration in the effectiveness of CBD treatments.35 Overall, recent trends and scientific advances in cannabis research have opened up new opportunities for the safer use of cannabinoids in specific medical settings.
5. Nurturing curiosity and innovation on minor cannabinoids and synthetic analogue
The exploration of minor cannabinoids and synthetic analogues has opened new avenues for therapeutic applications, significantly impacting the cannabis industry. The increasing focus on synthetic cannabinoids and novel derivatives has not only expanded research but also driven economic growth in the cannabis market. The U.S. cannabinoid market, valued at USD 18.14 billion in 2022, is expected to grow at a compound annual growth rate (CAGR) of 15.3 % from 2023 to 2030, fueled by rising awareness of the health benefits associated with cannabinoids.36 However, the high cost of cannabinoid-based products, particularly minor cannabinoids such as cannabigerol (CBG), Cannabichromene (CBC), and cannabinol (CBN), remains a barrier to wider accessibility, despite their demonstrated efficacy and growing research interest. Among the minor cannabinoids being investigated, compounds like CBG, CBN, and cannabidivarin (CBDV) have shown particular promise.37, 38, 39, 40, 41, 42
For instance, cannabidivarin (CBDV), a non-psychoactive cannabinoid closely related to CBD, has shown potential in treating epilepsy and neurological disorders, with ongoing studies exploring its efficacy in conditions like autism spectrum disorder (ASD)43 and Rett syndrome.44 A phase 2 randomized controlled trial published in Cannabis and Cannabinoid Research showed that CBDV reduced seizures by 40.5 % in people with focal seizures, and was well tolerated, indicating a suitable pharmacological effect.45 CBG has demonstrated neuroprotective and anti-inflammatory properties, making it a potential candidate for managing neurodegenerative diseases as neuroprotective46 and anti-inflammatory agent, particularly in the context of inflammatory bowel disease (IBD).47 A study by Sara Valdeolivas and colleagues found that CBG had protective effects in a model of Huntington's disease. They reported that CBG helped improve movement issues and protected brain cells from damage in mice exposed to the toxin 3-nitropropionate (3NP). Additionally, it also reduced inflammation and boosted antioxidant levels, which were lowered by the toxin. These findings suggest that CBG is useful in treating neurodegenerative disorders.48 Additionally, CBC has shown potential in supporting brain cell viability, which could be beneficial in treating depression and anxiety,49 while CBN is being studied for its sedative effects and potential use in sleep disorders. A recent study found that a water-soluble nano form of CBN helped people with sleeplessness fall asleep quickly and improved both the duration and quality of their sleep.50 Furthermore, THCV has been investigated for its role in weight management and type 2 diabetes due to its ability to regulate appetite and blood sugar levels, highlighting its potential as a therapeutic agent in metabolic disorders.51 Currently, there is growing research interest in CBN due to its structural similarity to other cannabinoids. However, research on CBN remains limited. Given its structural resemblance, scientists suggest that CBN may have potential therapeutic uses for conditions such as nausea, sleep disorders, mood disorders, and neuropathic pain.
Taken together, minor cannabinoids could be a promising therapeutic intervention for certain diseases (depicted in Fig. 2). Despite the promising therapeutic potential of these minor cannabinoids, much of the research is still in its early stages, and more comprehensive studies are needed. As research progresses, we expect that these compounds are likely to play an increasingly important role in developing new treatments for various medical conditions.
6. Current clinical trials: emerging hope and new findings on medical cannabis
Recent clinical trials are exploring the therapeutic potential and safety of cannabinoids, including minor cannabinoids, in treating various diseases. These studies aim to determine how these compounds can be safely and effectively used in different treatment plans. One such trial, “Improving Pain Disability with the Use of Oral Cannabinoids”,52 is evaluating THC and CBD for chronic pain management, with results expected by late 2024. Early findings suggest that THC may significantly reduce pain and improve sleep in chronic pain patients, while CBD also helps with pain but to a lesser extent. Another ongoing study, “CBDV vs. Placebo in Children with Autism Spectrum Disorder (ASD)”53 is examining CBDV's effectiveness in reducing irritability in children with ASD, with results anticipated by late 2025. In addition, two Phase-2 trials54,55 are investigating how THC and CBD affect symptoms in people with Multiple Sclerosis (MS), addressing the current lack of strong scientific evidence for cannabis in treating MS symptoms. In the field of oncology, a trial is ongoing for comparing hemp-derived CBD with and without THC to a placebo, focusing on sleep, pain, mood, cognitive function, and quality of life in cancer patients.56 Another Phase-2 trial is testing different cannabis extract combinations (varying levels of THC and CBD) to determine which is most effective for cancer-related symptoms like nausea, pain, anxiety, and sleep disturbances.57 A very recent Phase-2 trial led by Francisney Nascimento and Taynara Silva is evaluating the effects of a CBD/THC compound on Alzheimer's Disease, involving patients in the mild and moderate stages.58
These ongoing trials highlight the growing interest in cannabinoids as potential treatments for a wide range of conditions. As these studies conclude, they are expected to provide valuable insights into the efficacy and safety of these compounds, potentially leading to new treatment options and better patient outcomes.
7. Public perception and stigma in medical cannabis use: addressing and overcoming barriers
Public perception of medical cannabis has long been influenced by its association with recreational use and illegal activities, leading to significant stigma. This stigma can cause patients to feel embarrassed or ashamed, affecting their willingness to seek help or follow treatment plans, which can lead to poorer health outcomes. This negative view, rooted in decades of prohibition and media portrayals of cannabis users as criminals, continues to be a major barrier to the acceptance of medical cannabis. Despite advances in research, many people, especially those in conservative areas, still see cannabis use as morally wrong or socially unacceptable.59 This stigma can prevent patients from discussing cannabis with their healthcare providers or considering it as a treatment option.60 A study in Harm Reduction Journal interviewed 23 people using cannabis for medical reasons and found that their experiences of stigma were linked to negative views of cannabis as a recreational drug, criminal penalties, and the context of their health issues.61 Studies also have shown that patients using cannabis for medical purposes often face judgment and fear being stigmatized by family, friends, and even healthcare professionals, which can lead to underutilization of beneficial treatments.61 Concurrently, healthcare providers also feel the effects of this stigma, with some hesitating to prescribe or recommend cannabis due to concerns about their professional reputation, legal issues, or lack of knowledge about its use.62 To identify barriers to prescribing cannabis-based products on the National Health Service (NHS) where it was deemed clinically appropriate, a review conducted by NHS England and NHS Improvement in 2019, which revealed that a significant number of clinicians viewed the lack of robust Randomized Controlled Trial (RCT) data as a major obstacle, particularly concerning products containing THC. This report also highlighted a range of perspectives among clinicians regarding their willingness to prescribe these products on a case-by-case basis. For severe treatment-resistant epilepsy, in particular, unique considerations were noted when assessing the use of cannabis-based products. Among the recommendations were the creation of a UK-wide pediatric specialist clinical network, clear guidance for patients on the prescribing process, and further research by the National Institute for Health and Care Research (NIHR) into priority areas such as treatment-resistant epilepsy. Additionally, it was suggested that access to reliable information on high-quality products should be improved.63
However, as research on cannabis has grown, attitudes are slowly changing, especially in areas where medical cannabis is legal.64 Transparent communication about both the benefits and risks of cannabis can build public confidence and reduce skepticism. Public awareness of the medical benefits of cannabis, supported by scientific evidence, has helped shift opinions. High-profile cases, such as that of Charlotte Figi, a young girl whose severe epilepsy was improved by CBD oil,65 have also played a role in changing public perception. Experts in the field of medical cannabis argue that education and open dialogue are essential to overcoming this stigma and they emphasize the importance of educating both healthcare professionals and the public about the endocannabinoid system and the scientific evidence supporting the use of medical cannabis.66 Legalization of medical cannabis in various regions has also positively influenced public perception, with over 40 countries and 38 U.S. states having legalized it by 2024.67 This growing acceptance has helped to normalize medical cannabis use.
To further reduce stigma, aligning views with the FDA's approval process is crucial. The FDA's programs, such as Fast Track, Breakthrough Therapy, Accelerated Approval, and Priority Review are specifically designed to speed up the creation and approval of drug products. Additionally, the FDA's expanded access programs, also known as “compassionate use,” are intended to make investigational products available to patients with serious conditions when no other satisfactory therapy exists. While the FDA monitors approved drugs for safety, unapproved cannabis products carry risks due to the lack of rigorous testing. Therefore, it's important to rely on FDA-approved drugs for confidence in their safety and effectiveness. Furthermore, the FDA has not reviewed clinical trial data to support the safety and effectiveness.68 So we need to align our perception with the FDA approval process, as this will strengthen trust in medical cannabis use and ultimately improve its therapeutic outcomes by increasing patient adherence to cannabis treatments.
8. Enhancing cannabis safety: strategies for safer medical use and patients adherence
As the use of cannabis for medical purposes becomes increasingly accepted and widespread, ensuring its safety and effectiveness is crucial. However, the path to making cannabis a safer option for medical use involves addressing several challenges, including standardization of products, understanding pharmacological interactions, exploring safer methods of consumption, and implementing rigorous regulatory frameworks. One major challenge is the variability in cannabis products. The effects of cannabinoids, terpenes, and flavonoids depend on their ratios, which can differ greatly between strains, leading to varying therapeutic results and side effects.69, 70, 71 To mitigate these risks, it's important to standardize cannabis products to ensure consistent cannabinoid profiles and potency.72 This includes ensuring consistent cannabinoid profiles and potency across batches. Advances in cultivation, such as selective breeding and genetic engineering,73 could help produce plants with stable cannabinoid profiles suited for medical use. The cannabis industry is still in its relative infancy, leading to inconsistencies in product quality, labeling, and safety. Regulatory oversight is also essential to ensure product quality and clear labeling, including information on cannabinoid content and potential side effects. Implementing Good Manufacturing Practices (GMP)74 and developing pharmaceutical-grade formulations75 could further enhance safety.
Another important issue is that medical cannabis is often used alongside other medications, which raises concerns about potential drug interactions. Cannabinoids are metabolized by the cytochrome P450 enzyme system,76 which is also responsible for the metabolism of many prescription drugs. This can lead to altered blood levels of either cannabis or the co-administered drugs, affecting their efficacy or causing adverse effects. For example, using cannabis with blood thinners like warfarin can increase bleeding risk, while combining it with certain antiepileptic drugs can cause excessive sedation.77 More research is needed to understand these interactions fully, so patients should consult healthcare providers. Similarly, UDP- glucuronosyltransferase-1 family, like- UGT1A1, UGT1A3, UGT1A9 enzymes and the enzyme UDP- glucuronosyltransferase-2, mainly UGT2B7 are involved in metabolization and detoxification of phytocannabinoids.78 Recently, it has been shown that phytocannabinoids, especially CBD, are able to inhibit many of UGT enzymes (mainly UGTs1A6, 1A9, 2B4 and 2B7), suggesting that deleterious effects of cannabis may be more likely to occur in patients with reduced renal or hepatic function.79
The method of consumption impacts safety as well. Smoking cannabis, while traditional, can cause respiratory issues, particlarly as it modulates pulmonary immune function,80 poses a risk of lung cancer and chronic bronchitis. Alternatives like vaporization, edibles, tinctures, and topical applications are being explored for safer use, though they also have challenges such as delayed onset or dose control difficulties. Studies have shown that vaporizing cannabis is associated with fewer respiratory symptoms compared to smoking.81,82 However, for right dose selection, clinician may require some trial and error, under resourceful medical setup.
Several pieces of evidence suggests that long-term cannabis use, especially in adolescents, may affect cognitive development and increase the risk of psychiatric disorders, potentially leading to long-term deficits in memory, attention, and executive function.83, 84, 85 Additionally, early cannabis use is associated with an increased risk of developing psychiatric disorders, such as schizophrenia,86,87 especially in individuals with a predisposition to these conditions. To protect vulnerable populations, age restrictions and education on the risks of cannabis use are important for ensuring that medical cannabis is prescribed only when the benefits clearly outweigh the risks.
Another important issues is that Cannabis treatment is very different from an allopathic therapy, as finding the right dose of cannabis for a patient is challenging, due to factors like age, disease type, and individual genetics and metabolism. This complexity was highlighted by a prospective epidemiological study conducted by Bar-Lev Schleide et al.88 As the CNR1 gene encodes the CB1 receptor, and polymorphisms in this gene have been linked to differences in the psychoactive effects of cannabis. To investigate whether CNR1 variation predicts CNR1 prefrontal mRNA expression in postmortem prefrontal human tissue, a study conducted by Colizzi, M. et al found that the deleterious effects of cannabis use are more evident on a specific genetic background related to its receptor expression89 which can affect susceptibility to cannabis use disorders and response to cannabis treatment. Similarly, genetic variations in the genes responsible for encoding enzymes that metabolize cannabinoids have been studied in relation to changes in enzyme activity. For instance, numerous functional haplotypes have been discovered in cytochrome P450 genes, leading to phenotypes that differ in frequency across ethnic groups. A single nucleotide polymorphism (SNP) in the CYP 2C9 enzyme affects one of the steps involved in THC conversion, potentially lowering THC metabolism by as much as 70 % compared to individuals carrying the wild-type allele.90 Thus, genetic tests could help tailor treatments and improve adherence to cannabis therapy. For example, THC and CBD genetic tests are emerging as a valuable tool for personalizing cannabis use based on an individual's genetic profile. These tests analyze genetic variants that influence how a person metabolizes and responds to cannabinoids, providing insights into tolerance, risk of adverse effects, and potential therapeutic benefits.
9. Concluding remarks and future directions
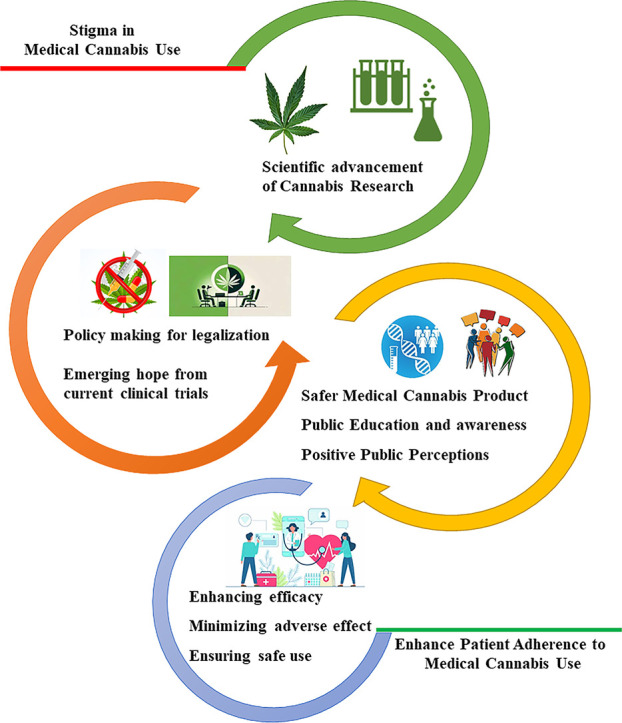
The future of medical cannabis is shaped by the interplay between scientific research, public perception, and safety considerations which forms a feedback loop (shown in the graphical abstract). These interconnected elements influence the ongoing development and use of cannabis for medical purposes. Scientific research lays the groundwork by uncovering the therapeutic benefits and potential risks of cannabinoids, which in turn inform regulatory policies and public opinion. Positive research outcomes can reduce stigma and increase acceptance of legalization, while findings of risks may lead to more cautious approaches. we propose that extensive research on medical cannabis has the potential to drive legislative changes that standardize dosage, strain selection, and delivery methods (such as oils, edibles, and vaporizers). This standardization is essential for ensuring consistency and predictability in treatment outcomes. Additionally, safety-related research can inform the establishment of regulations regarding product testing, labeling, and quality control, ultimately protecting patients from contaminants, improper dosing, and mislabeled products. By aligning research with legislative initiatives, we can create a more effective and safe framework for the use of medical cannabis in clinical practice. Public education, supported by research, is crucial for changing perceptions and promoting informed decisions. Addressing stigma is key to improving patient access to medical cannabis. Ongoing research must continue to evaluate the safety and efficacy of cannabis, ensuring that healthcare providers can make informed recommendations to patients. In this context, our narrative review recommends that research-backed guidelines be utilized to train healthcare providers, thereby enhancing patient trust and adherence to treatment regimens through knowledgeable and clear instructions from physicians.
Looking forward, a balanced approach that integrates scientific evidence, public education, and safety considerations will be essential. This approach will help to maximize the therapeutic potential of medical cannabis while minimizing risks, ultimately leading to its wider acceptance and integration into mainstream medical practice.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00335573) and National Research Council of Science & Technology (NST) grant by the Korea government (MSIT) (No. CAP 23052-000). This study is also supported by Jeonbuk National University in 2023.
CRediT authorship contribution statement
Muhammad Kamal Hossain: Conceptualization, Writing – original draft. Han Jung Chae: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
Acknowledgments
Acknowledgments
We are highly acknowledged to our funding authority- National Research Foundation of Korea (NRF) and Jeonbuk National University. We also extend our utmost gratitude to Professor Hyung-Ryong Kim, from Department of Pharmacology, College of Dentistry, Jeonbuk National University, Dr. Geum-Hwa Lee and Dr. Hwa-Young Lee from the Non-Clinical Evaluation Center (N-CEC), at Jeonbuk National University Medical School for their invaluable insights and feedback on this manuscript. This study is also supported by Jeonbuk National University in 2023.
Ethical statement
Not applicable.
Data availability
The data used for this review are from other literature sources and included as references.
References
- 1.Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. Journal of natural products. 2016;79(2):324–331. doi: 10.1021/acs.jnatprod.5b00949. [DOI] [PubMed] [Google Scholar]
- 2.Sciences NAo, Division M, Health BoP, Practice PH, Marijuana CotHEo, Review AE, et al. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. 2017. [PubMed]
- 3.Xu DH, Cullen BD, Tang M, Fang Y. The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Current pharmaceutical biotechnology. 2020;21(5):390–402. doi: 10.2174/1389201020666191202111534. [DOI] [PubMed] [Google Scholar]
- 4.Russo EB. Cannabinoids in the management of difficult to treat pain. Therapeutics and clinical risk management. 2008;4(1):245–259. doi: 10.2147/tcrm.s1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrams DI. Cannabis, cannabinoids and cannabis-based medicines in cancer care. Integrative cancer therapies. 2022;21 doi: 10.1177/15347354221081772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortimer TL, Mabin T, Engelbrecht A-M. Cannabinoids: the lows and the highs of chemotherapy-induced nausea and vomiting. Future Oncology. 2019;15(9):1035–1049. doi: 10.2217/fon-2018-0530. [DOI] [PubMed] [Google Scholar]
- 7.Organization WH. Dependence WECoD . World Health Organization; 2018. WHO Expert Committee on Drug Dependence: thirty-ninth report. [Google Scholar]
- 8.Fiani B, Sarhadi KJ, Soula M, Zafar A, Quadri SA. Current application of cannabidiol (CBD) in the management and treatment of neurological disorders. Neurological Sciences. 2020;41:3085–3098. doi: 10.1007/s10072-020-04514-2. [DOI] [PubMed] [Google Scholar]
- 9.Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204–e1e11. doi: 10.1212/WNL.0000000000005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel AD, Mazurkiewicz-Bełdzińska M, Chin RF, Gil-Nagel A, Gunning B, Halford JJ, et al. Long-term safety and efficacy of add-on cannabidiol in patients with Lennox–Gastaut syndrome: Results of a long-term open-label extension trial. Epilepsia. 2021;62(9):2228–2239. doi: 10.1111/epi.17000. [DOI] [PubMed] [Google Scholar]
- 11.Hathaway AD, Comeau NC, Erickson PG. Cannabis normalization and stigma: Contemporary practices of moral regulation. Criminology & Criminal Justice. 2011;11(5):451–469. [Google Scholar]
- 12.Reid M. A qualitative review of cannabis stigmas at the twilight of prohibition. Journal of Cannabis Research. 2020;2:1–12. doi: 10.1186/s42238-020-00056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chemistry & biodiversity. 2007;4(8):1614–1648. doi: 10.1002/cbdv.200790144. [DOI] [PubMed] [Google Scholar]
- 14.Spicer L. Historical and cultural uses of cannabis and the canadian ‘marijuana clash’. Library of Parliament. 2002 [Google Scholar]
- 15.Warf B. High points: an historical geography of cannabis. Geographical Review. 2014;104(4):414–438. [Google Scholar]
- 16.Abu-Asab M, Amri H, Micozzi MS. Simon and Schuster; 2013. Avicenna's medicine: a new translation of the 11th-century canon with practical applications for integrative health care. [Google Scholar]
- 17.Russo EB. The pharmacological history of Cannabis. Handbook of cannabis. 2014;1 [Google Scholar]
- 18.Takakuwa KM, Schears RM. A history of the US medical cannabis movement and its importance to pediatricians: science versus politics in medicine's greatest catch-22. Clinical Pediatrics. 2019;58(14):1473–1477. doi: 10.1177/0009922819875550. [DOI] [PubMed] [Google Scholar]
- 19.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, et al. Cannabinoid receptor localization in brain. Proceedings of the national Academy of sciences. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devane WA, Hanuš L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 21.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annual review of psychology. 2013;64(1):21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 22.Cannabis OoM. Medicinal Cannabis Information Brochure for Patients. 2019.
- 23.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65(6):812–819. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Cañas M, Rodríguez-Cueto C, Satta V, Hernández-Fisac I, Navarro E, Fernández-Ruiz J. Endocannabinoid-binding receptors as drug targets. Endocannabinoid Signaling: Methods and Protocols. 2022:67–94. doi: 10.1007/978-1-0716-2728-0_6. [DOI] [PubMed] [Google Scholar]
- 25.Russo EB. Clinical endocannabinoid deficiency reconsidered: current research supports the theory in migraine, fibromyalgia, irritable bowel, and other treatment-resistant syndromes. Cannabis and cannabinoid research. 2016;1(1):154–165. doi: 10.1089/can.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dykukha I, Malessa R, Essner U, Überall MA. Nabiximols in chronic neuropathic pain: a meta-analysis of randomized placebo-controlled trials. Pain Medicine. 2021;22(4):861–874. doi: 10.1093/pm/pnab050. [DOI] [PubMed] [Google Scholar]
- 27.Rubin R. The path to the first FDA-approved cannabis-derived treatment and what comes next. Jama. 2018;320(12):1227–1229. doi: 10.1001/jama.2018.11914. [DOI] [PubMed] [Google Scholar]
- 28.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015;313(24):2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 29.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British journal of pharmacology. 2011;163(7):1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferber SG, Namdar D, Hen-Shoval D, Eger G, Koltai H, Shoval G, et al. The “entourage effect”: terpenes coupled with cannabinoids for the treatment of mood disorders and anxiety disorders. Current neuropharmacology. 2020;18(2):87–96. doi: 10.2174/1570159X17666190903103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorpe HH, Talhat MA, Khokhar JY. High genes: Genetic underpinnings of cannabis use phenotypes. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2021;106 doi: 10.1016/j.pnpbp.2020.110164. [DOI] [PubMed] [Google Scholar]
- 32.Salazar M, Carracedo A, Salanueva ÍJ, Hernández-Tiedra S, Lorente M, Egia A, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. The Journal of clinical investigation. 2009;119(5):1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carracedo A, Lorente M, Egia A, Blázquez C, García S, Giroux V, et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer cell. 2006;9(4):301–312. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Vara D, Salazar M, Olea-Herrero N, Guzmán M, Velasco G. Díaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death & Differentiation. 2011;18(7):1099–1111. doi: 10.1038/cdd.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gournay LR, Ferretti ML, Bilsky S, Vance E, Nguyen AM, Mann E, et al. The effects of cannabidiol on worry and anxiety among high trait worriers: a double-blind, randomized placebo controlled trial. Psychopharmacology. 2023;240(10):2147–2161. doi: 10.1007/s00213-023-06437-0. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Cannabinoids Market Size, Share & Trends Analysis Report By Product Type (Tetrahydrocannabinol (THC), Cannabidiol (CBD), Cannabigerol (CBG)), By Application (Inflammation, Pain Management), And Segment Forecasts, 2023 - 2030, <https://www.grandviewresearch.com/industry-analysis/us-cannabinoids-market>; Published 2023.
- 37.Zagzoog A, Mohamed KA, Kim HJJ, Kim ED, Frank CS, Black T, et al. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Scientific Reports. 2020;10(1):20405. doi: 10.1038/s41598-020-77175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh KB, McKinney AE, Holmes AE. Minor cannabinoids: biosynthesis, molecular pharmacology and potential therapeutic uses. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.777804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas FJ, Kayser O. Minor cannabinoids of Cannabis sativa L. Journal of Medical Science. 2019;88(3):141–149. [Google Scholar]
- 40.Kwiecień E, Kowalczuk D. Therapeutic Potential of Minor Cannabinoids in Dermatological Diseases—A Synthetic Review. Molecules. 2023;28(16):6149. doi: 10.3390/molecules28166149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulpa J, Henderson RG, Schwotzer D, Dye W, Trexler KR, McDonald J, et al. Toxicological evaluation and pain assessment of four minor cannabinoids following 14-day oral administration in rats. Cannabis and Cannabinoid Research. 2023;8(S1):S25–S41. doi: 10.1089/can.2023.0049. [DOI] [PubMed] [Google Scholar]
- 42.Moore CF, Weerts EM, Kulpa J, Schwotzer D, Dye W, Jantzi J, et al. Pharmacokinetics of oral minor cannabinoids in blood and brain. Cannabis and cannabinoid research. 2023;8(S1):S51–S61. doi: 10.1089/can.2023.0066. [DOI] [PubMed] [Google Scholar]
- 43.Zamberletti E, Gabaglio M, Woolley-Roberts M, Bingham S, Rubino T, Parolaro D. Cannabidivarin treatment ameliorates autism-like behaviors and restores hippocampal endocannabinoid system and glia alterations induced by prenatal valproic acid exposure in rats. Frontiers in cellular neuroscience. 2019;13:367. doi: 10.3389/fncel.2019.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurley EN, Ellaway CJ, Johnson AM, Truong L, Gordon R, Galettis P, et al. Efficacy and safety of cannabidivarin treatment of epilepsy in girls with Rett syndrome: A phase 1 clinical trial. Epilepsia. 2022;63(7):1736–1747. doi: 10.1111/epi.17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brodie MJ, Czapinski P, Pazdera L, Sander JW, Toledo M, Napoles M, et al. A phase 2 randomized controlled trial of the efficacy and safety of cannabidivarin as add-on therapy in participants with inadequately controlled focal seizures. Cannabis and Cannabinoid Research. 2021;6(6):528–536. doi: 10.1089/can.2020.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Echeverry C, Prunell G, Narbondo C, de Medina VS, Nadal X, Reyes-Parada M, et al. A comparative in vitro study of the neuroprotective effect induced by cannabidiol, cannabigerol, and their respective acid forms: Relevance of the 5-HT 1A receptors. Neurotoxicity Research. 2021;39:335–348. doi: 10.1007/s12640-020-00277-y. [DOI] [PubMed] [Google Scholar]
- 47.Borrelli F, Fasolino I, Romano B, Capasso R, Maiello F, Coppola D, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochemical pharmacology. 2013;85(9):1306–1316. doi: 10.1016/j.bcp.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Valdeolivas S, Navarrete C, Cantarero I, Bellido ML, Muñoz E, Sagredo O. Neuroprotective properties of cannabigerol in Huntington's disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics. 2015;12(1):185–199. doi: 10.1007/s13311-014-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shinjyo N, Di Marzo V. The effect of cannabichromene on adult neural stem/progenitor cells. Neurochemistry international. 2013;63(5):432–437. doi: 10.1016/j.neuint.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Kaufmann R. Use of a water-soluble form of cannabinol for the treatment of sleeplessness. Int J Complement Alt Med. 2021;14(4):186–190. [Google Scholar]
- 51.Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes care. 2016;39(10):1777–1786. doi: 10.2337/dc16-0650. [DOI] [PubMed] [Google Scholar]
- 52.Carroll J. Improving Pain Disability With the Use of Oral Cannabinoids, <https://clinicaltrials.gov/study/NCT05351905?cond=NCT05351905&rank=1>; Published 2022.
- 53.Ferretti C. Cannabidivarin (CBDV) vs. Placebo in Children With Autism Spectrum Disorder (ASD), <https://clinicaltrials.gov/study/NCT03202303?cond=NCT03202303&rank=1>; Published 2019.
- 54.Duquette P. Cannabis as a Complementary Treatment in Multiple Sclerosis, <https://clinicaltrials.gov/study/NCT05092191?cond=NCT05092191&rank=1>; Published 2022.
- 55.Johnson D. Mechanisms of Cannabidiol in Persons With MS: the Role of Sleep and Pain Phenotype, <https://clinicaltrials.gov/study/NCT05269628?cond=NCT05269628&rank=1>; Published 2022.
- 56.Chrystal K. Cannabis for Palliative Care in Cancer; Published 2024.
- 57.Sharma A. Cannabis For Cancer-Related Symptoms <https://clinicaltrials.gov/study/NCT03948074?cond=NCT03948074&rank=1>; Published 2021.
- 58.Nascimento F. Use of a Cannabinoids as a Treatment Strategy for Alzheimer's Disease, <https://clinicaltrials.gov/study/NCT06570928?cond=NCT06570928&rank=1>; Published 2024.
- 59.Dahlke S, Butler JI, Hunter KF, Toubiana M, Kalogirou MR, Shrestha S, et al. The Effects of Stigma: Older Persons and Medicinal Cannabis. Qualitative Health Research. 2024 doi: 10.1177/10497323241227419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isaac S, Saini B, Chaar BB. The role of medicinal cannabis in clinical therapy: pharmacists' perspectives. PLoS one. 2016;11(5) doi: 10.1371/journal.pone.0155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bottorff JL, Bissell LJ, Balneaves LG, Oliffe JL, Capler NR, Buxton J. Perceptions of cannabis as a stigmatized medicine: a qualitative descriptive study. Harm reduction journal. 2013;10:1–10. doi: 10.1186/1477-7517-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weisman JM, Rodríguez M. A systematic review of medical students’ and professionals’ attitudes and knowledge regarding medical cannabis. Journal of Cannabis Research. 2021;3:1–20. doi: 10.1186/s42238-021-00100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.England N, Improvement N. Barriers to accessing cannabis-based products for medicinal use on NHS prescription. NHS England and NHS Improvement: London, UK 2019.
- 64.Resko S, Ellis J, Early TJ, Szechy KA, Rodriguez B, Agius E. Understanding public attitudes toward cannabis legalization: qualitative findings from a statewide survey. Substance use & misuse. 2019;54(8):1247–1259. doi: 10.1080/10826084.2018.1543327. [DOI] [PubMed] [Google Scholar]
- 65.Young S. Marijuana stops child's severe seizures. CNN International. 2013 [Google Scholar]
- 66.Russo EB. CRC Press; 2016. The solution to the medicinal cannabis problem. Ethical issues in chronic pain management; pp. 165–194. [Google Scholar]
- 67.Hong G, Sideris A, Waldman S, Stauffer J, Wu CL. Legal and regulatory aspects of medical cannabis in the United States. Anesthesia & Analgesia. 2024;138(1):31–41. doi: 10.1213/ANE.0000000000006301. [DOI] [PubMed] [Google Scholar]
- 68.Marcu JP, Schechter JB. The role of FDA in defining Cannabis and hemp: A brief perspective on the future of Cannabis regulation in the United States. Regulated Cannabis and Hemp Market Navigation: Business. Public Health, and Research Challenges. 2024;1:15. [Google Scholar]
- 69.Baron EP. Medicinal properties of cannabinoids, terpenes, and flavonoids in cannabis, and benefits in migraine, headache, and pain: an update on current evidence and cannabis science. Headache: The Journal of Head and Face Pain. 2018;58(7):1139–1186. doi: 10.1111/head.13345. [DOI] [PubMed] [Google Scholar]
- 70.Procaccia S, Lewitus GM, Lipson Feder C, Shapira A, Berman P, Meiri D. Cannabis for medical use: versatile plant rather than a single drug. Frontiers in Pharmacology. 2022;13 doi: 10.3389/fphar.2022.894960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nahler G, Jones T. Pure cannabidiol versus cannabidiol-containing extracts: Distinctly different multi-target modulators. Journal of Alternative Complementary & Integrative Medicine. 2018;4(048):1–11. [Google Scholar]
- 72.Thomas BF, ElSohly MA. Elsevier; 2015. The analytical chemistry of cannabis: Quality assessment, assurance, and regulation of medicinal marijuana and cannabinoid preparations. [Google Scholar]
- 73.Small E. Genetics and plant breeding of Cannabis sativa for controlled environment production. Handbook of Cannabis Production in Controlled Environments; Zheng, Y, Ed 2022:41-90.
- 74.Ilikj M, Brchina I, Ugrinova L, Karcev V, Grozdanova A. LAP LAMBERT Academic Publishing; 2021. GMP/GACP-new standards for quality assurance of cannabis. [Google Scholar]
- 75.Stella B, Baratta F, Della Pepa C, Arpicco S, Gastaldi D, Dosio F. Cannabinoid formulations and delivery systems: Current and future options to treat pain. Drugs. 2021;81:1513–1557. doi: 10.1007/s40265-021-01579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life sciences. 2007;80(15):1415–1419. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 77.Ho JJY, Goh C, Leong CSA, Ng KY, Bakhtiar A. Evaluation of potential drug–drug interactions with medical cannabis. Clinical and Translational Science. 2024;17(5):e13812. doi: 10.1111/cts.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hryhorowicz S, Walczak M, Zakerska-Banaszak O, Słomski R, Skrzypczak-Zielińska M. Pharmacogenetics of cannabinoids. European journal of drug metabolism and pharmacokinetics. 2018;43(1):1–12. doi: 10.1007/s13318-017-0416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nasrin S, Watson CJ, Bardhi K, Fort G, Chen G, Lazarus P. Inhibition of UDP-glucuronosyltransferase enzymes by major cannabinoids and their metabolites. Drug Metabolism and Disposition. 2021;49(12):1081–1089. doi: 10.1124/dmd.121.000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Preteroti M, Wilson ET, Eidelman DH, Baglole CJ. Modulation of pulmonary immune function by inhaled cannabis products and consequences for lung disease. Respiratory Research. 2023;24(1):95. doi: 10.1186/s12931-023-02399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Earleywine M, Barnwell SS. Decreased respiratory symptoms in cannabis users who vaporize. Harm Reduction Journal. 2007;4:1–4. doi: 10.1186/1477-7517-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tashkin DP. How beneficial is vaping cannabis to respiratory health compared to smoking? Addiction. 2015;110(11) doi: 10.1111/add.13075. [DOI] [PubMed] [Google Scholar]
- 83.Ganzer F, Bröning S, Kraft S, Sack P-M, Thomasius R. Weighing the evidence: a systematic review on long-term neurocognitive effects of cannabis use in abstinent adolescents and adults. Neuropsychology review. 2016;26:186–222. doi: 10.1007/s11065-016-9316-2. [DOI] [PubMed] [Google Scholar]
- 84.Solowij N, Pesa N. Cognitive abnormalities and cannabis use. Brazilian Journal of Psychiatry. 2010;32:531–540. [PubMed] [Google Scholar]
- 85.Cyrus E, Coudray MS, Kiplagat S, Mariano Y, Noel I, Galea JT, et al. A review investigating the relationship between cannabis use and adolescent cognitive functioning. Current opinion in psychology. 2021;38:38–48. doi: 10.1016/j.copsyc.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chadwick B, Miller ML, Hurd YL. Cannabis use during adolescent development: susceptibility to psychiatric illness. Frontiers in psychiatry. 2013;4:129. doi: 10.3389/fpsyt.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blanco C, Hasin DS, Wall MM, Flórez-Salamanca L, Hoertel N, Wang S, et al. Cannabis use and risk of psychiatric disorders: prospective evidence from a US national longitudinal study. JAMA psychiatry. 2016;73(4):388–395. doi: 10.1001/jamapsychiatry.2015.3229. [DOI] [PubMed] [Google Scholar]
- 88.Bar-Lev Schleider L, Mechoulam R, Sikorin I, Naftali T, Novack V. Adherence, safety, and effectiveness of medical cannabis and epidemiological characteristics of the patient population: a prospective study. Frontiers in medicine. 2022;9 doi: 10.3389/fmed.2022.827849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colizzi M, Fazio L, Ferranti L, Porcelli A, Masellis R, Marvulli D, et al. Functional genetic variation of the cannabinoid receptor 1 and cannabis use interact on prefrontal connectivity and related working memory behavior. Neuropsychopharmacology. 2015;40(3):640–649. doi: 10.1038/npp.2014.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gasse A, Vennemann M, Köhler H, Schürenkamp J. Toxicogenetic analysis of Δ9-THC-metabolizing enzymes. International Journal of Legal Medicine. 2020;134:2095–2103. doi: 10.1007/s00414-020-02380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this review are from other literature sources and included as references.