Abstract
Colour is crucial for enhancing the appetizing value and consumer acceptance of food products. The commonly used food colourants and food preservatives such as Malachite Green (MG) and Copper Sulfate (CS) can cause severe health problems. This study investigates the toxicity of these food-grade colourants through acute exposure using in vivo cytotoxicity using the brine shrimp model including 3D surface analysis (3DSA) and in-silico studies Brine shrimp were treated with various concentrations of MG and CS. The cytotoxic effect was confirmed by brine shrimp lethality assay and 3DSA. Molecular docking and Molecular Dynamic simulation were done using hAChE binding cavity. Results showed that concentrations (2.5–10 µg/ml) of MG and CS significantly decreased locomotor behaviour within 1 h, while higher concentrations (10–100 µg/ml) caused high mortality rates. Morphological studies revealed that there is a significant reduction (p<0.05) in shrimp length treated with MG and CS. The 3DSA indicates that there is an inappropriate surface of the shrimp morphology. Interestingly, MG-treated shrimps had shown significant inhibition of AChE in homogenates, indicating cholinergic nerve-mediated toxicity. Computational studies showed MG confined active binding with human acetylcholinesterase (hAChE), with a binding energy MMGBSA of −51.3 kcal/mol. MD simulation confirmed reversible binding stability inside the hAChE pocket. It can be concluded that acute exposure to brine shrimps with MG and CS exhibited cytotoxicity as evidenced by the increase in mortality of the shrimps. This study further warrants the investigation of MG and CS residues from commonly used fruits and vegetables and their putative toxic effect using in-vivo studies.
Keywords: Artemia salina, Malachite Green (MG), Copper Sulphate (CS), 3-Dimensional Surface Analysis (3DSA), Acetylcholinesterase (AChE)
Graphical Abstract
Highlights
-
•
Acute Toxicity Assessment: Evaluated the cytotoxic effects of Malachite Green (MG) and Copper Sulfate (CS) on brine shrimp using various concentrations.
-
•
Behavioral Impact: Noted significant reduction in locomotor behavior of brine shrimp at MG and CS concentrations ranging from 2.5 to 10 µg/ml within one hour.
-
•
Mortality Rates: Observed high mortality rates in brine shrimp at higher concentrations (10–100 µg/ml) of MG and CS.
-
•
AChE Inhibition: Demonstrated inhibition of acetylcholinesterase (AChE) activity in MG-treated shrimp homogenates, indicating cholinergic nerve-mediated toxicity.
-
•
Computational Insights: Revealed strong and stable binding of MG with human acetylcholinesterase (hAChE) through molecular docking and molecular dynamic simulations.
1. Introduction
Food additives are various chemicals added to foods for specific purposes. They are used to preserve flavour, taste, appearance or sensory qualities [1]. The usage of food additives has been increasing in recent years in the modern food industry for the production and storage of foods. It is also used by food chain suppliers to prevent the degradation of food-related commodities and maintain their quality [2]. The commonly used food-grade additives are malachite green (MG) and copper sulfate (CS). They are commonly used for the preservation of freshness and also to prevent microbial growth. The MG has been used as a dye in the textile industry due to its attractive greenish colour. MG has been misused by vegetable vendors to dye green colour vegetables including green peas, lady’s fingers and greenish leaves to attract customers [3]. On the other hand, Copper Sulphate (CS) is used as a fungicide, algaecide, and herbicide in agricultural and non-agricultural settings. Daily consumption of these chemicals mixed with vegetables and fruits can lead to various harmful health hazards. The acute effect of additives may cause intense untoward signs and symptoms in the human body. The harmful effect of these chemicals is so detrimental to know the toxicodynamic effects of additives in the biological system [4]. The cytotoxicity testing of chemicals using in-vitro cell culture and in-vivo brine shrimps has been receiving attention as an alternative to animal models. The use of the shrimp model validates the lethal index of many toxins and chemicals upon acute exposure [5], [6].
On the contrary, there are challenges in studying the biochemical and morphological parameters of the shrimps with and without test substances. The use of molecular docking, molecular dynamics (MD) simulations, and Molecular Mechanics Generalized Born Surface Area (MMGBSA) has profoundly improved toxicology evaluations by offering detailed insights into the interactions of toxic compounds at the molecular level [7], [8]. Molecular docking predicts how molecules like Malachite Green (MG) and Copper Sulfate (CS) bind to target proteins such as human acetylcholinesterase (hAChE), which is essential for assessing potential neurotoxic effects. MD simulations build on these predictions by providing a dynamic view of how these interactions evolve, including changes in conformation and the stability of the binding [9]. Extending the simulation duration allows for a more comprehensive understanding of these interactions. The MMGBSA method complements these studies by quantifying binding free energies, taking into account solvation and entropy effects to provide accurate assessments of binding strength. Together, these computational techniques offer a robust framework for evaluating the toxicological profiles of compounds, enhancing our understanding of their potential effects and informing safer practices in the use of food additives [10], [11]. Scientific studies related to pesticide pollution on vegetables and fruits are crucial in understanding the toxic effects of pesticides on these foods. The present study aims to investigate the exposure of commonly used food-grade colourants, such as Malachite Green (MG) and Copper Sulfate (CS), using brine shrimp (Artemia salina). The objectives of this manuscript are to report the acute mechanistic interactions of MG and CS in brine shrimp, including their morphological structure and 3D structural analysis [12].
2. Materials and methods
2.1. Chemicals and reagents
Sodium Chloride (NaCl), Magnesium Chloride (MgCl2), Sodium Sulphate (Na2So4), Calcium Chloride (CaCl2), Potassium Chloride (KCl), Sodium Hydrogen Carbonate (NaHCO3), Potassium Bromide (KBr), Hydrogen Borate (H3BO3), Potassium Permanganate (Kmno4), Copper Sulphate (CuSo4) and Malachite green are procured from SRL chemicals. All other reagents and solvents are analytical grade.
2.2. Preparation of copper sulphate and malachite green solution
The stock solution of CS 1 mg/ml and MG 1 mg/ml was prepared by standard protocol. 100 mg copper sulphate and Malachite green were weighed separately and transferred into 100 ml volumetric standard flask with double distilled water. For in-vivo assay, 1–100 µg/ml /ml concentration of CS and MG were used.
2.3. Preparation and optimization of artificial seawater (ASW)
This protocol described the method for the preparation of artificial seawater for the hatching of brine shrimp eggs under ambient temperature [13]. The chemical recipe for the preparation of ASW was as follows Sodium Chloride (NaCl), Magnesium Chloride (MgCl2), Sodium Sulphate (Na2So4), Calcium Chloride (CaCl2), Potassium Chloride (KCl), Sodium Hydrogen Carbonate (NaHCO₃), Potassium Bromide (KBr) and Hydrogen borate (H₃BO₃). All chemicals were weighed and dissolved in 1 L of double distilled water and the pH was adjusted to 7.5–8.5 by using 0.01 M HCl/NaOH. The final solution was filtered with filter paper and the temperature was maintained at 28℃ for better hatching
2.4. Hatching capacity of brine shrimps in artificial sea water (ASW)
The hatching capacity of brine shrimp eggs was carried out at different pH (7.5, 8.0 and 8.5) using ASW. Eggs (75 numbers, approx. weight of 25 mg) were soaked in 250 ml ASW for 24–48 hrs. The temperature was maintained at 280 C and provided with appropriate oxygen using an aquarium pump. The setup was illuminated with a 60 W light. The capacity of hatching is defined as the number of brine shrimps hatched and actively swimming in the ASW after 24–48 hrs. The hatching capacity was found to be 89 % at 48 hrs [14].
2.5. Brine shrimp lethality test (BSLT)
In the BSLT assay, a suspension of 25 live active nauplii was taken from 76 hrs after hatching (100 µl) into 1.5 ml Eppendorf tube and treated with different concentrations of MG and CS (1–100 µg/ml) for 24 hrs at 370 C in an incubator. After 24 hrs exposure to MG and CS the number of dead/live nauplii in each tube was counted using a binocular microscope. The percentage of live, dead nauplii and LC50 of MG and CS were calculated [15].
2.6. Measurement of brine shrimp size
At the end of the BSLT assay, the live brine shrimps (n=5) from control, MG and CS treated groups were fixed in buffer neutral formalin solution for 48 hrs [16]. After 48 h of fixing, the microscopic slides were imaged by OMAX optical microscope (M82ES-SC100-LP, USA) using 40x magnification. Images of the brine shrimps were analysed using ToupView software.
2.7. General and locomotor behaviour of brine shrimps
The effect of MG and CS on live brine shrimps locomotor behaviour is assessed by the previous method [17]. Live shrimps (n=15, 72hrs after hatching) treated with 2.5, 5 & 10 µg /ml concentration of MG and CS for 1 hr at room temperature. After 1 h, the swimming speed (cm/sec) of the individual live shrimps was observed under a binocular microscope (Toupview, software). The shrimps swimming speed was captured for 90 s to assess the shrimp’s locomotor behaviour in artificial seawater. The swimming speed by the shrimps in cubic cm/sec is assessed from the live recording videos.
2.8. 3-Dimensional surface analysis (3DSA) analysis
3D surface of the control and MG and CS treated brine shrimps were constituted using brine shrimp images. The 3D surface analysis of the images was performed using OMAX ToupView 3D surface analysis software. The XYZ planer surface of the shrimps were analysed using Quads tricolour Polygon mode. The impression pattern of the shrimps in the 3D plane was observed using ambient lighting angles of X=283, Y=175 and Z=20 [18].
2.9. Estimation of acetylcholinesterase enzyme (AChE) in shrimp homogenate
AChE inhibition assay was carried out by the spectrophotometric method [19]. Briefly, 0.25 ml of control, MG and CS-treated brine shrimp homogenate and 0.25 ml of 3 mM of Ellman’s reagent was added. The above test solution was incubated for 15 min at room temperature. After incubation, 0.5 ml of 15 mM acetylthiocholine iodide (ATCI) was added. The absorbance was recorded at 405 nm and the percentage inhibition of AChE was calculated.
2.10. Computational molecular modelling and molecular dynamics simulation protocol setup (hAChE)
X-ray diffraction structure of the human acetylcholinesterase (hAChE) enzyme was downloaded from the RCSB portal (https//doi.org/10.2210/pdb7RB7/pdb) and the ligand molecules were downloaded from PubChem public domine. Protein preparation and ligprep (1) modules in Schrodinger suite algorithms assign bond orders of hAChE and hydrogen bonds were corrected using the force field's algorithm (OPLS4) at pH 7.0 +/- 2.0. The binding pocket of the 7RB7 protein was defined based on the coordinates of existing PDB ligands with a radius of 9 Å. These proteins and ligands were submitted to the glide docking (2) input and extra precision level interaction was analyzed. The final high affine molecule complex was submitted to 100 ns molecular dynamic (MD) simulation (3). In the first step 7rb7 - ligand complex was solvated using the pre-defined SPC solvent model, and the border condition was set up as an orthorhombic box shape. The volume was minimized based on complex protein surface occupation, and the system was neutralized through the addition of Na+ and salt-negative ions (Cl-). The trajectory recording interval was set to 100 ps, with a 1.2 energy gap during the 100 ns simulation with the generation of 1000 conformations [20], [21]. Malathion and Parathion were used as standard.
2.11. Statistical analysis
All the data are expressed as Mean ± SE. The log dose-response relationship versus % of the mortality graph was constructed in Microsoft Excel. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by post hoc (for parametric data), and Mann-Whitney U test (for non-parametric data). A probability value less than p<0.05 was considered statistically significant.
3. Results
3.1. Effect of different doses of MG in brine shrimps lethality assay (BSLT)
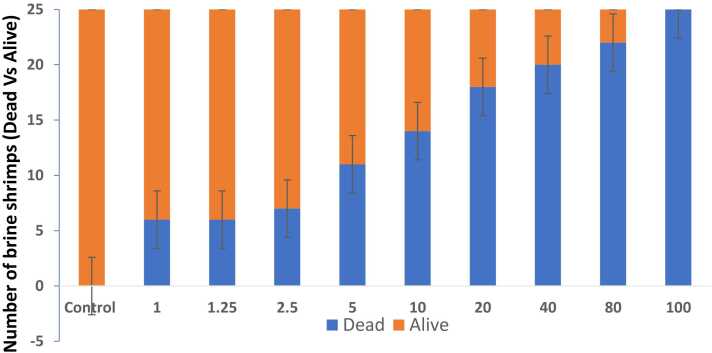
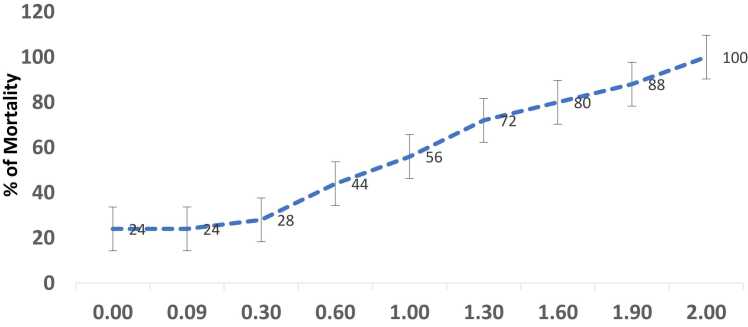
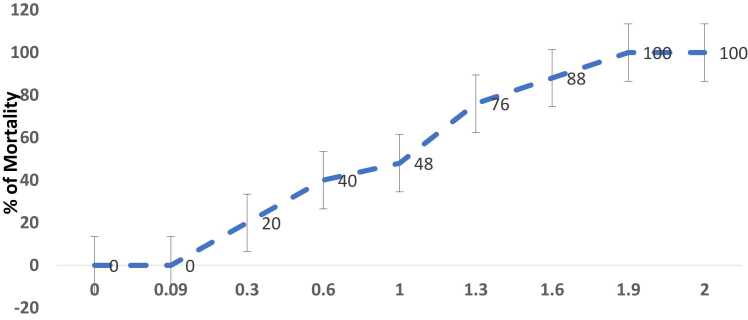
Fig. 1a represents the effect of MG on brine shrimp mortality number (dead versus live) and 1b represents the log dose of MG Vs % of mortality rate. As shown in Fig. 1a, the dose-dependent increase in the number of dead shrimps was found with increasing concentrations of MG exposure (1–100 μg/ml). The higher dose of MG (100 μg/ml) was shown to exhibit a 100 % mortality rate and the lowest concentration of 1 µg/ml was found to be 24 % (Fig. 1b). The lethal dose concentration (LC50) of MG on brine shrimps was found to be 12 μg/ml. However, there is no mortality was observed in the control group.
Fig. 1.
a) Effect of various concentrations of MG on the number of dead and alive brine shrimps (n=25). Data are expressed as Mean ± SE. b) Log dose-response relationship of MG versus % mortality of brine shrimps (n=25). Data are expressed as Mean ± SE.
3.2. Effect of different doses of CS in brine shrimps lethality assay (BSLT)
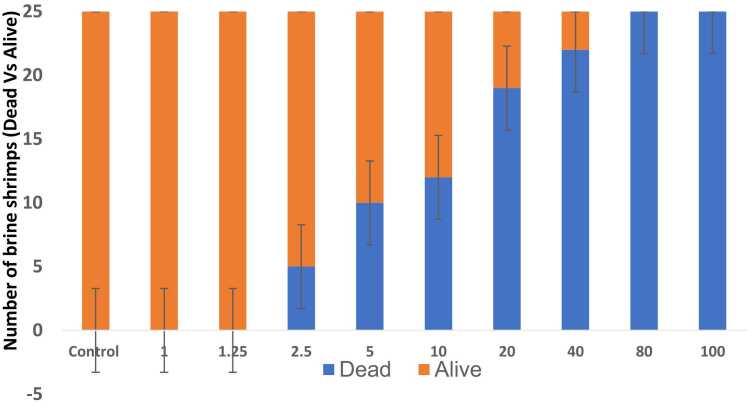
Fig. 2a represents the effect of CS on brine shrimp mortality number (dead versus live) and 1b represents the log dose of CS Vs % of mortality rate. As shown in Fig. 2a, the dose-dependent increase in the number of dead shrimps was found with increasing concentrations of CS from 2.5 to 100 μg/ml. The low dose of CS which is 1, 1.25 µg/ml did not show any lethality in brine shrimps. The lower dose of 2.5 µg/ml and higher dose of 100 µg/ml of CS showed % mortality of 20 and 100 % respectively. The lethal dose concentration (LC50) of CS on brine shrimps was found to be 17 µg/ml (Fig. 2b). However, there is no mortality was observed in the control group.
Fig. 2.
a) Effect of various concentrations of CS on the number of dead and alive brine shrimps (n=25). Data are expressed as Mean ± SE. b) Log dose-response relationship of CS versus % mortality of brine shrimps (n=25). Data are expressed as Mean ± SE.
3.3. Effect of MG and CS on locomotor behaviour of the brine shrimps
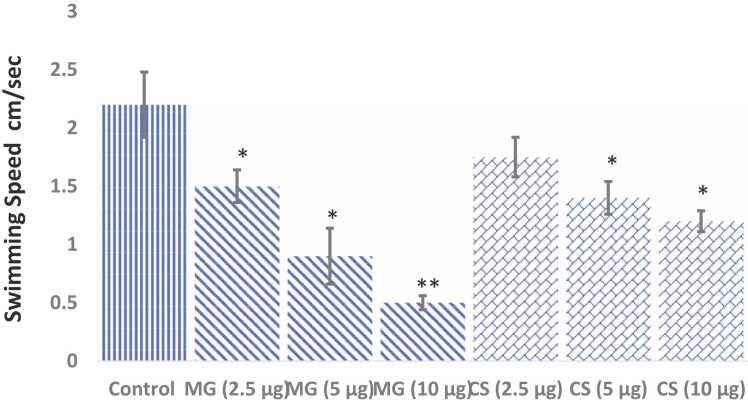
The locomotor behaviour of the brine shrimps upon acute exposure to MG and CS at different concentrations is shown in Fig. 3. Brine Shrimps treated with MG at different concentrations (2.5–10 µg/ml) were shown a significant decrease in locomotion as evidenced by the decrease in swimming speed by the shrimps. MG at the concentration of 2.5 and 5 µg/ml had shown a significant (p<0.05) decrease in swimming speed (cm/sec) [1.53 ± 0.15 vs 2.2 ± 0.24 and 0.9 ± 0.11 vs 2.2 ± 0.24 cm/sec respectively] as compared to that of control brine shrimps. The higher concentration of 10 µg/ml of MG had shown high significant (p<0.001) decrease in locomotion behaviour as evidenced by the decrease in swimming speed [0.5 ± 0.07 vs 2.2 ± 0.24]. The effect of different concentrations of MG in shrimp locomotor behaviour was dose-dependent. Acute exposure of 5–10 µg/ml of CS to brine shrimps showed a significant decrease (p<0.05) in swimming speed [1.4 ± vs 2.2 ± 0.24 and 1.24 ± 0.15 vs 2.2 ± 0.24 cm/sec respectively] in the ASW as compared to control brine shrimps.
Fig. 3.
The locomotor behaviour of the brine shrimps (n=15) upon acute exposure to MG and CS (n=15). Data are expressed as Mean ± SE. * p<0.05 compared with the control group.
3.4. Brine shrimp size measurement
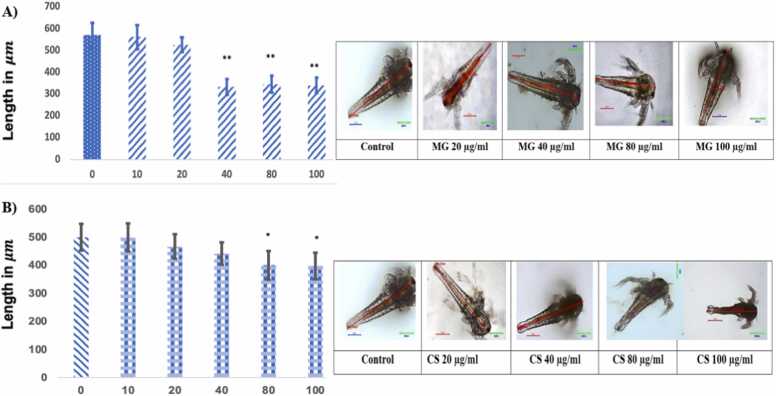
Fig. 4a & b represent the effect of MG and CS in the Brine shrimps length of the brine shrimps measured from head to tail after 24 hrs. There was a decrease in the length of the brine shrimps exposed to increased concentration of MG, suggesting MG plays a role in the growth retardation of brine shrimps. MG at the dose of 40–100 µg/ml had shown a significant (p<0.01) decrease in length as compared to control shrimps. The MG (40–100 µg/ml) treated brine shrimps mean length was found to be in the range of 333.5 and 345 µm whereas the control group mean length was found to be 570 µm. The CS-treated shrimps at the concentration of 80 & 100 µg/ml had shown a significant (p<0.05) growth retardation effect as evidenced by the decrease in length of the brine shrimps. The CS (80 and 100 µg/ml) treated brine shrimps mean length was found to be in the range of 526 and 340 µm whereas the control group shrimps and the mean length was found to be 570 µm.
Fig. 4.
a Effect of MG and CS on brine shrimp (n=5) Lenth & b. Data are expressed as Mean ± SE. * p<0.01 and **p<0.05 compared with the control group.
3.5. Effect of MG and CS on 3D surface analysis
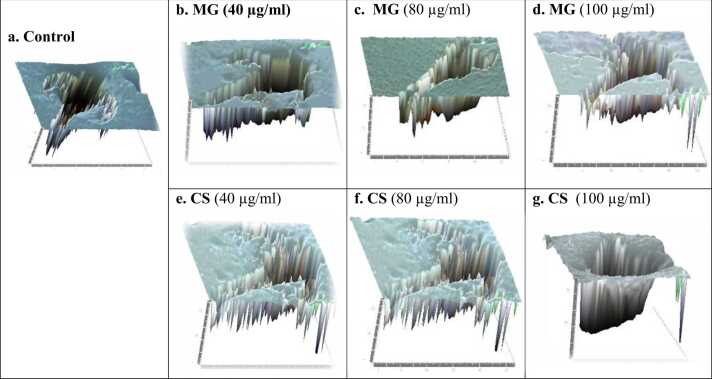
Fig. 5 (A-D) represents the 3D surface analysis of brine shrimp delineated from the microscopical picture of brine shrimps under 40x magnification. Brine shrimps were treated with different concentrations (40–100 µg/ml) of MG and CS showed an irregular surface pattern. It was observed that the control brine shrimps have shown equal longitudinal surface alignment in XYZ planner surface suggesting the normal growth pattern of the brine shrimps. The impression pattern of the head, fore, mid and hindgut including appendages on both sides are in normal alignment. However, the impression of MG and CS-treated brine shrimps were not in alignment as evidenced by an inappropriate impression of the longitudinal plane including swimming appendages on both sides suggesting retardation of the brine shrimp growth. The impression pattern of brine shrimps exposed with high concentration had shown squeezed and inappropriate planner impression.
Fig. 5.
represents the 3D surface analysis of brine shrimps exposed to various concentrations of MG (fig b-d) and CS (Fig e-g).
3.6. Effect of MG and CS in brine shrimps homogenate acetylcholinesterase
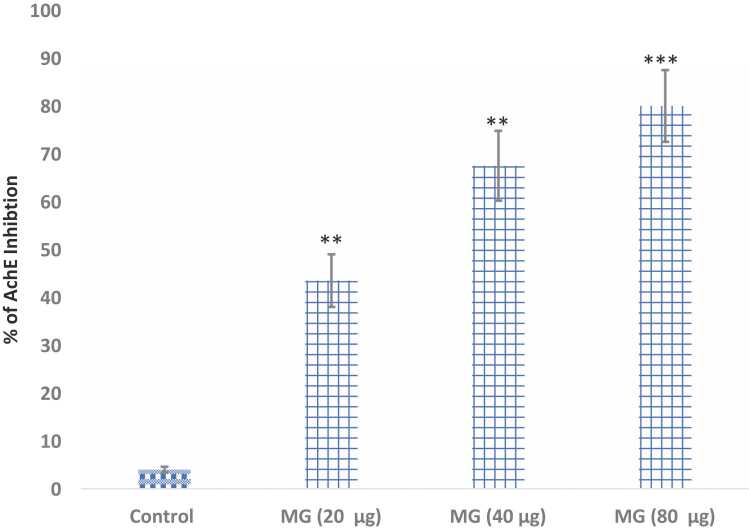
Fig. 6 represents the % inhibition of Acetylcholinesterase enzyme (AChE) of brine shrimp homogenates upon acute exposure to MG. MG at the concentrations of 10–40 µg/ml had shown a significant (P<0.01) increase in the % of AChE inhibition. The higher concentration (80 µg/ml) showed a higher % of AChE inhibition (p<0.0001). The mean % inhibition of AChE to MG (at the dose of 10, 20 & 100 µg/ml) treated brine shrimps was found to be 43.5 %, 67.5 % & 80 % respectively. CS-treated shrimps did not show any significant effect on AChE level (data not shown).
Fig. 6.
AChE inhibition effect of MG on Brine Shrimps (n=25) homogenates. Data are expressed as Mean ± SE. **p<0.01 and ***p<0.001 compared with the control group.
3.7. Molecular docking of MG and CS in the catalytic site of human acetylcholinesterase (hAChE) enzyme
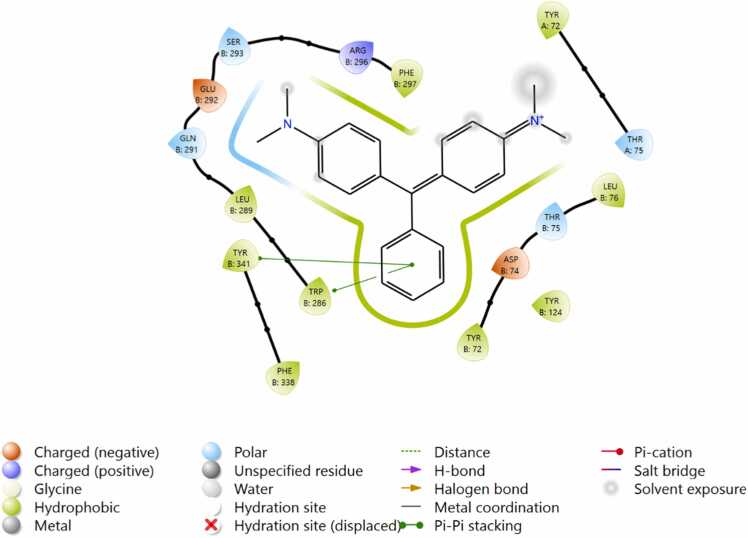
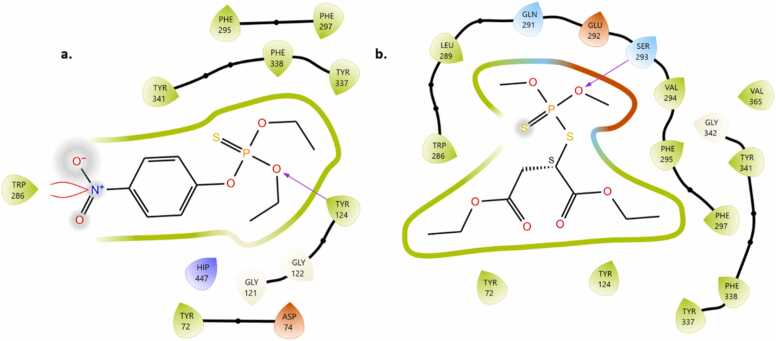
MG formed four different kinds of binding interaction with hAChE. Specifically, the two amino acid residues TyrB:341 and TRP B:286 are formed by π- π stacking interaction with the benzene ring p-orbitals of MG. Additionally, the 7 amino acid residues (green leaves) formed hydrophobic interaction, and four amino acids (Thr A:75 & B, Gln 291, Ser 293) networked by polar interaction with MG. Asp 74 and Glu 292 negatively interacted and one amino acid Arg 296 formed an interaction with the molecule. These interactions modify the secondary structure of the hAChE protein suggesting enzyme inhibition. The binding poses of CS interaction with active amino acid: TYR and LEU residues likely contribute to stabilization through hydrophobic or π-π stacking interactions, depending on their positions relative to the ligand. Parathion and malathion interact with hAChE protein through the hydrophobic amino acid residues. Also, parathion formed hydrogen bonds with Tyr124, Trp286 and HIP447 (positive), Asp74 (negative) amino acids to aid the binding of polar fragments of the molecule. Similarly, Ser293 developed a hydrogen bond with the phosphate oxygen atom of malathion (Fig. 7, Fig. 8, Fig. 9).
Table 1.
Docking Score and MMGBSA energy.
| Title | Structure | Docking score | MMGBSA dG Bind |
|---|---|---|---|
| 7RB7–5-removed waters | |||
| Malachite Green | 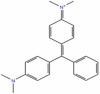 |
−7.932 | −51.3 |
| Copper Sulfate | 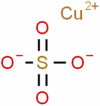 |
−6.177 | −14.97 |
| Malathion | 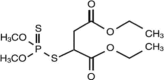 |
−4.677 | −29.30 |
| Parathion |  |
−3.458 | −20.33 |
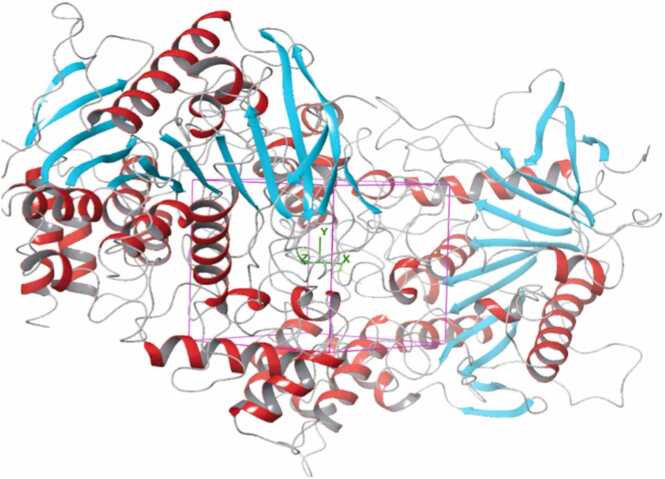
Fig. 7.
Structure of hAChE in complex with substrate analogy 4K-TMA and MMB4 oxime and the binding pocket grid of docking.
Fig. 8.
a Binding of Malachite green inside the catalytic site of hAChE protein. 8b Binding of copper sulfate inside the catalytic site of hAChE protein.
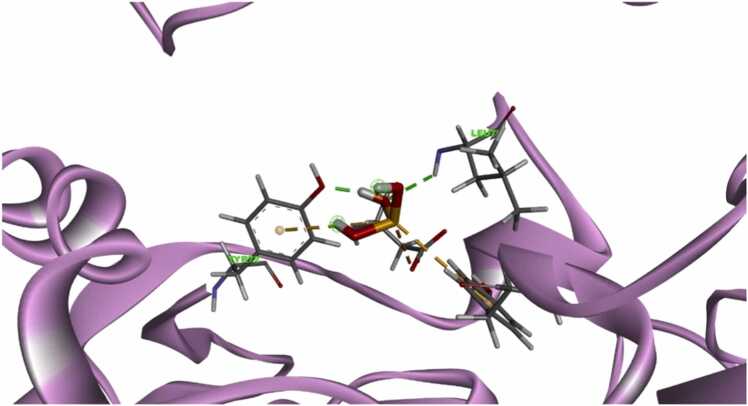
Fig. 9.
Binding of parathion(a) and malathion (b) inside the catalytic site of B hAChE protein.
3.8. Quantum model mechanics /molecular dynamics simulation
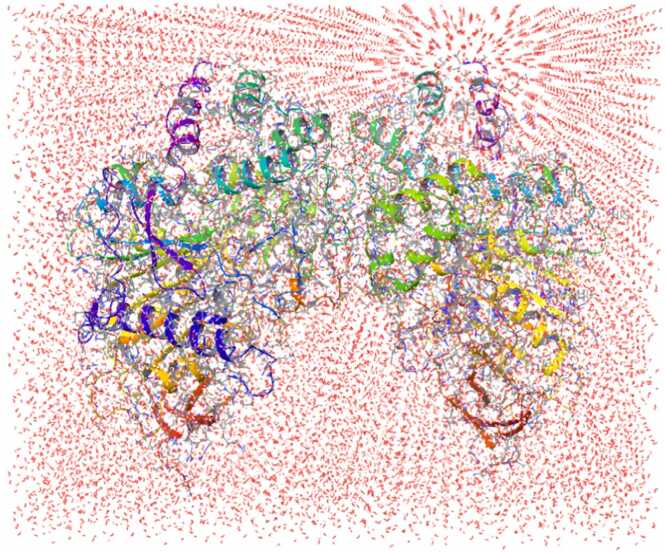
MD simulation mechanic runs aid to find the MG binding reversibly or irreversibly inside the hAChE. Fig. 10 depicts the solvation model which is generated with 22324 water molecules and mimics the human body realistic model in the Artificial [AI] environment.
Fig. 10.
Solvated MD simulation protein complex.
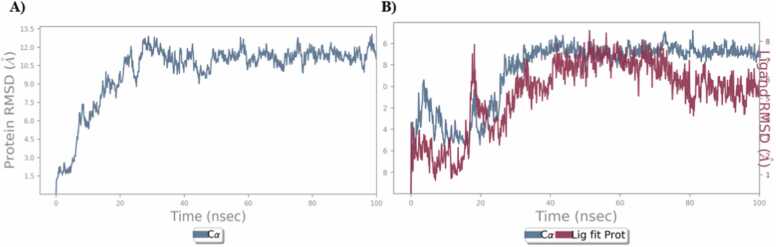
The RMSD graph revealed that the hAChE protein and protein-ligand complex were stable up to 100 ns. The plain protein deviation was found to be 9–12 Å (Fig. 11A) and the MG hAChE complexed protein deviation was found to be 8–11 Å (Fig. 11 B) due to the interaction of the compound.
Fig. 11.
RMSD Protein and protein-ligand complex QMM/MD A) protein B) MG.
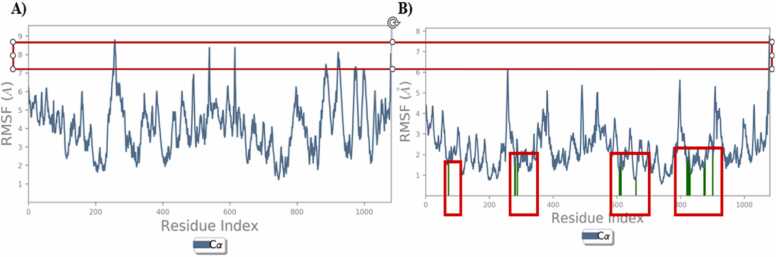
Fig. 12 A illustrates the amino acids fluctuation of hAChE protein was found to be 8–9 Å, After the complex formation the fluctuations of amino acids were reduced to less than 6 Å (Fig. 12 B). The reduction of RMSA due to the formation of hydrogen bonds between the amino acid residue and the MG atoms.
Fig. 12.
RMSF AChE protein & Protein-ligand complex QMM/MD A) protein B) MG complex.
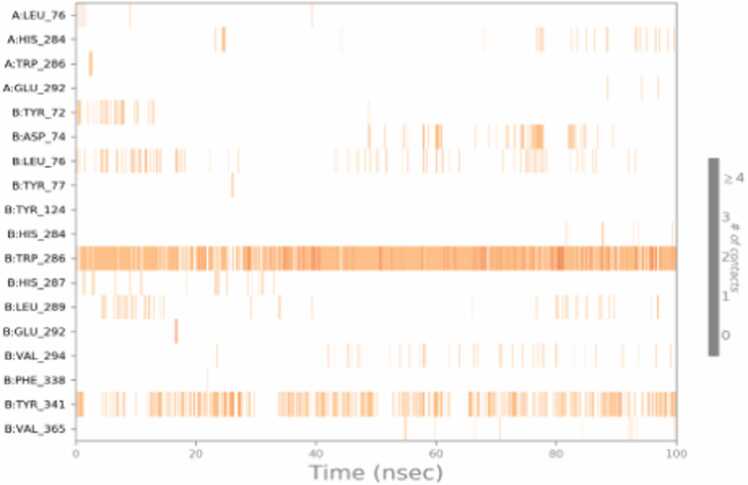
The complex contact heat map shown in Fig. 13 illustrates the interaction with 18 active site amino acids of A chain & B chain. Specifically, more interaction was found with hydrophobic amino acids and they are breakable during the 100 ns simulation. Among the amino acids, TRP 286 and TYR 341 interacted
Fig. 13.
Heat map Malachite green and hAChE protein amino acid Interaction of 100 ns period throughout the simulation without cleaving. This work of the Quantum Model Mechanics / Molecular Dynamics simulation studies confirmed that the malachite green binds with the hAChE protein well and reversibly.
4. Discussion
The present study demonstrates the acute toxic effect of food-grade additives namely MG and CS in live Artemia salina nauplii in artificial seawater preparation (ASW). Acute exposure to MG and CS affects the normal physiological parameters like development and locomotion behaviour of the shrimps tested 72 hrs after hatching, the lethal effect of additives were observed after 3 days of exposure. The brine shrimp locomotion is facilitated by the rhythmic beating of their trunk appendages from upside down which propels the brine shrimp to move forward in the ASW. The rhythmic beating and synchronization of the swimming behaviour of the shrimp is controlled by the local nervous system integrated with the ganglia and drugs like d-Tubocurarine inhibit the locomotion behaviour in shrimps [22]. The impaired locomotor effect can be explained by the fact that the MG and CS could affect the shrimp’s motor neuron neurotransmitter system. The inhibitory effect of copper ions on nerve action potential and neurotransmitter release has been studied by various researchers. In the previous research have been shown that acute exposure to Cu+ retards the growth, and development by inhibiting neurotransmitter release at the local nerve terminal of c. elegans [23], [24], fish and in rat hippocampal neuronal slices [25] Conversely, the neurotoxic effect of MG has also been shown in C. carpio through behavioural and histochemical analysis [26]. We observed the diverging effect of MG on the locomotor effect as compared to that of CS. The irregular effect shown by CS and MG at the same concentrations may also be due to different uptake or penetration of MG or CS to shrimps and also the degradation potential of these additives in the test medium during different periods. Conversely, CS and MG exposure decreased the growth of shrimps measured at the end of the experiment. The normal development and growth pattern of the shrimps depends on various factors including temperature[27].The total protein concentration (Data not shown) was significantly decreased in the shrimp group exposed to MS and CS this explains the growth retardation effect of these additives on shrimps. The 3D structure modelling analysis of brine shrimps exposed to MG and CS have shown inappropriate planner orientation of the shrimps further confirming the toxic effect of MG and CS on shrimps’ growth. It has been shown the 20 amino acids including essential amino acids and heat shock protein p26 play a vital role in the ontogeny of the shrimps [28], [29] The growth retardation in the shrimps may be due to the negative effect of MG and CS on amino acid turnover or the heat shock proteins in the shrimps.
In our study, acute exposure to MG at different concentrations inhibited cholinesterase levels in the shrimp homogenate preparation suggesting cholinergic toxicity. However, the hermetic effect of MG has been shown in two different studies using Drosophila larvae and rat brain AChE levels. In the former study, Drosophila larvae exposed to different concentrations of textile-grade dye enhanced the cholinesterase enzyme activity [30] and a later study found that MG administered at the dose of 13.5 mg/kg in rats to reduce the transfer latency as an indicator for assessing the spatial memory task tested in Morris Water Maze behavioural paradigm the results infer that the memory impairment of the rats fed with MG was associated with an imbalance in AChE and stress markers in the rat hippocampus and frontal cortex. This AChE inhibitory effect of MG may also explain the decrease in the locomotion effect of shrimps treated with MG due to AChE inhibition-mediated cholinergic toxicity.
Further, the in-silico molecular studies confirmed the AChE inhibitory effect of MG using human AChE as a target. The docking and MMGBSA scores suggest that MG has high-affinity binding towards hAChE enzyme pockets through 2 π-π stack interactions as compared to that of a few standard pesticides such as parathion and malathion. There is a conformational change in the B chain secondary structure of the hAChE enzyme in protein-ligand interaction suggesting the possible inhibition of hAChE by MG. Further, the stability of the MG and hAChE enzyme was confirmed by molecular dynamic simulation (MD-simulation) studies [31]. The results of MDS of MG and hAChE suggest that the MG reversibly binds through 2 amino acids residues of hAChE enzymes namely Try 286 and Tyr 341 that last for 100 nanoseconds. Previous molecular docking reports have confirmed that the formation of a hydrogen bond with Tyr341 in the binding site canyon of 4K-TMA: hAChE enzyme is essential for the inhibitory effect of the ligand and protein structure. However, this canyon with molecular hydrogen bonding interaction with Tyr341 was not observed in the apo-hAChE protein structure. Refer the peripheral anionic site (PAS) binding of ligands makes π-π stacking interactions with Trp286 and does not obtrude outside the Tyr124 choke point in the active site gorge. This reveals the structural changes in the cavities of the active site and is enlightened by the docking of the covalent reversible inhibitor [32].
It can be concluded that acute exposure to MG and CS which are commonly used food-grade additives are toxic to brine shrimp growth and the toxicity is mediated by affecting acetylcholinesterase enzyme at the cholinergic nerve ending. Further, the in-silico studies with human AChE as the target also point toward its inhibition with exposure to MG and opens up a plethora of domains for study exposure to MG in the nervous system of humans. This study warrants the further investigation of MG and CS residues extracted from commonly used fruits and vegetables and their putative toxic effect using in-vivo studies.
CRediT authorship contribution statement
Irfan N: Software, Methodology. Ismail Y: Data curation. Haja Nazeer Ahamed: Visualization, Funding acquisition, Conceptualization. Thameemul Ansari LH: Validation. Ilham Jaleel: Writing – original draft. Shanmugarajan TS: Project administration. Mohammad Zaidh S: Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The first author acknowledges B.S Abdur Rahman Crescent Institute of Science and Technology for providing University Seed Money grant support Lr no. 1255 / Dean (R) /2019.
Handling Editor: Dr. L.H. Lash
Data Availability
Data will be made available on request.
References
- 1.Wang D., Saleh N.B., Byro A., Zepp R., Sahle-Demessie E., Luxton T.P.…Su C. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022;17(4):347–360. doi: 10.1038/s41565-022-01082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabzevari S., Hofman J. A worldwide review of currently used pesticides' monitoring in agricultural soils. Sci. Total Environ. 2022;812 doi: 10.1016/j.scitotenv.2021.152344. [DOI] [PubMed] [Google Scholar]
- 4.Gómez-Sierra T., Hernández-Cruz E.Y., Ortega-Lozano A.J., Jiménez-Uribe A.P., Chaverri J.P., Medina-Reyes E.I. In: Natural Additives in Foods. Valencia G.A., editor. Springer; Cham: 2023. Toxicological aspects of natural food additives. [DOI] [Google Scholar]
- 3.EFSA Panel on Contaminants in the Food Chain (CONTAM Malachite green in food. EFSA J. 2016;14(7) doi: 10.2903/j.efsa.2016.4530. [DOI] [Google Scholar]
- 5.Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobsen L.B., Nichols D.E.J., McLaughlin J.L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45(05):31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- 6.Ullah M.O., Haque M., Urmi K.F., Zulfiker A.H.M., Anita E.S., Begum M., Hamid K. Anti–bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac. J. Trop. Biomed. 2013;3(1):1–7. doi: 10.1016/S2221-1691(13)60015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva W.J.L., de Freitas R.F. Correction to: Assessing the performance of docking, FEP, and MM/GBSA methods on a series of KLK6 inhibitors. J. Comput. - Aided Mol. Des. 2023;37(10) doi: 10.1007/s10822-023-00521-5. 505-505. [DOI] [PubMed] [Google Scholar]
- 8.Zaidh S.M., Aher K.B., Bhavar G.B., Irfan N., Ahmed H.N., Ismail Y. Genes adaptability and NOL6 protein inhibition studies of fabricated flavan-3-ols lead skeleton intended to treat breast carcinoma. Int. J. Biol. Macromol. 2024;258 doi: 10.1016/j.ijbiomac.2023.127661. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X., Li S., Zhang H., Wang L.L. Discovery of potentially biased agonists of mu-opioid receptor (MOR) through molecular docking, pharmacophore modeling, and MD simulation. Comput. Biol. Chem. 2021;90 doi: 10.1016/j.compbiolchem.2020.107405. [DOI] [PubMed] [Google Scholar]
- 10.Maraf M.B., Mountessou B.Y.G., Merlin T.F.H., Ariane P., Fekoua J.N.N., Yves T.B.J.…Ramasami P. Virtual screening, MMGBSA, and molecular dynamics approaches for identification of natural products from South African biodiversity as potential Onchocerca volvulus pi-class glutathione S-transferase inhibitors. Heliyon. 2024;10(9) doi: 10.1016/J.HELIYON.2024.E29560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X., Zhang H., Zhu R., Ren Q., Zhang L. Classification with noisy labels through tree-based models and semi-supervised learning: a case study of lithology identification. Expert Syst. Appl. 2024;240 doi: 10.1016/j.eswa.2023.122506. [DOI] [Google Scholar]
- 12.Zhu B., Zhu S., Li J., Hui X., Wang G.X. The developmental toxicity, bioaccumulation and distribution of oxidized single walled carbon nanotubes in Artemia salina. Toxicol. Res. 2018;7(5):897–906. doi: 10.1039/c8tx00084k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartl M., Humpf H.U. Toxicity assessment of fumonisins using the brine shrimp (Artemia salina) bioassay. Food Chem. Toxicol. 2000;38(12):1097–1102. doi: 10.1016/S0278-6915(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 14.Neumeyer C.H., Gerlach J.L., Ruggiero K.M., Covi J.A. A novel model of early development in the brine shrimp, Artemia franciscana, and its use in assessing the effects of environmental variables on development, emergence, and hatching. J. Morphol. 2015;276(3):342–360. doi: 10.1002/jmor.20344. [DOI] [PubMed] [Google Scholar]
- 15.Ahamed K.F.H., Kumar V., Manikandan L., Wahile A.M., Mukherjee K., Saha B.P., Mukherjee P.K. Brine shrimp lethality and cytotoxicity assay of Araucaria bidwillii Hook in human carcinoma cell lines. Adv. Tradit. Med. 2005;5(1):21–28. [Google Scholar]
- 16.Ragavendran C., Srinivasan R., Kim M., Natarajan D. Aspergillus terreus (Trichocomaceae): a natural, eco-friendly mycoinsecticide for control of malaria, filariasis, dengue vectors and its toxicity assessment against an aquatic model organism Artemia nauplii. Front. Pharmacol. 2018;9:1355. doi: 10.3389/fphar.2018.01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkateswara Rao J., Kavitha P., Jakka N.M., Sridhar V., Usman P.K. Toxicity of organophosphates on morphology and locomotor behavior in brine shrimp, Artemia salina. Arch. Environ. Contam. Toxicol. 2007;53:227–232. doi: 10.1007/s00244-006-0226-9. [DOI] [PubMed] [Google Scholar]
- 18.Sivagnanam S., Krishnakumar V., Munuswamy N. Morphology and ultrastructure of cysts in different species of the brine shrimp, Artemia from Southern India. Int. J.Aquatic Biol. 2013;1(6):266–272. doi: 10.22034/ijab.v1i6.142. [DOI] [Google Scholar]
- 19.Worek F., Eyer P., Thiermann H. Determination of acetylcholinesterase activity by the Ellman assay: a versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug Test. Anal. 2012;4(3-4):282–291. doi: 10.1002/dta.337. [DOI] [PubMed] [Google Scholar]
- 20.Irfan N., Vaithyanathan P., Anandaram H., Mohammed Zaidh S., Priya Varshini S., Puratchikody A. Active and allosteric site binding MM-QM studies of Methylidene tetracyclo derivative in PCSK9 protein intended to make a safe antilipidemic agent. J. Biomol. Struct. Dyn. 2024;42(13):6813–6822. doi: 10.1080/07391102.2023.2239928. [DOI] [PubMed] [Google Scholar]
- 21.Navabshan I., Sakthivel B., Pandiyan R., Antoniraj M.G., Dharmaraj S., Ashokkumar V.…Show P.L. Computational lock and key and dynamic trajectory analysis of natural biophors against COVID-19 spike protein to identify effective lead molecules. Mol. Biotechnol. 2021;63(10):898–908. doi: 10.1007/s12033-021-00358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cong Y., Wang Y., Zhang M., Jin F., Mu J., Li Z., Wang J. Lethal, behavioral, growth and developmental toxicities of alkyl-PAHs and non-alkyl PAHs to early-life stage of brine shrimp, Artemia parthenogenetica. Ecotoxicol. Environ. Saf. 2021;220 doi: 10.1016/j.ecoenv.2021.112302. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Zhao C., Zhang H., Lu Q., Zhou J., Liu R.…Yin L. Trans-generational effects of copper on nerve damage in Caenorhabditis elegans. Chemosphere. 2021;284 doi: 10.1016/j.chemosphere.2021.131324. [DOI] [PubMed] [Google Scholar]
- 24.Baatrup E. Structural and functional effects of heavy metals on the nervous system, including sense organs, of fish. Comp. Biochem. Physiol. Part C: Comp. Pharmacol. 1991;100(1-2):253–257. doi: 10.1016/0742-8413(91)90163-N. [DOI] [PubMed] [Google Scholar]
- 25.Peters C., Muñoz B., Sepúlveda F.J., Urrutia J., Quiroz M., Luza S.…Opazo C. Biphasic effects of copper on neurotransmission in rat hippocampal neurons. J. Neurochem. 2011;119(1):78–88. doi: 10.1111/j.1471-4159.2011.07417.x. [DOI] [PubMed] [Google Scholar]
- 26.Sinha R., Jindal R. Elucidation of malachite green induced behavioural, biochemical, and histo-architectural defects in Cyprinus carpio, as piscine model. Environ. Sustain. Indic. 2020;8 doi: 10.1016/j.indic.2020.100055. [DOI] [Google Scholar]
- 27.Helland S., Triantaphyllidis G.V., Fyhn H.J., Evjen M.S., Lavens P., Sorgeloos P. Modulation of the free amino acid pool and protein content in populations of the brine shrimp Artemia spp. Mar. Biol. 2000;137:1005–1016. doi: 10.1007/s002270000409. [DOI] [Google Scholar]
- 28.Jackson S.A., Clegg J.S. Ontogeny of low molecular weight stress protein p26 during early development of the brine shrimp, Artemia franciscana. Dev., Growth Differ. 1996;38(2):153–160. doi: 10.1046/j.1440-169X.1996.t01-1-00004.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang W., Meng B., Chen W., Ge X., Liu S., Yu J. A proteomic study on postdiapaused embryonic development of brine shrimp (Artemia franciscana) Proteomics. 2007;7(19):3580–3591. doi: 10.1002/pmic.200700259. [DOI] [PubMed] [Google Scholar]
- 30.Rahimi S., Singh M.P., Gupta J. Adverse effects of textile dyes on antioxidant enzymes and cholinesterase activities in Drosophila melanogaster (Oregon R+) Drug Chem. Toxicol. 2022;45(3):1131–1139. doi: 10.1080/01480545.2020.1809671. [DOI] [PubMed] [Google Scholar]
- 31.Islam M.R., Islam Sovon M.S., Amena U., Rahman M., Hosen M.E., Kumer A.…Wondmie G.F. Ligand-based drug design against Herpes Simplex Virus-1 capsid protein by modification of limonene through in silico approaches. Sci. Rep. 2024;14(1):9828. doi: 10.1038/S41598-024-59577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babaoglu K., Shoichet B.K. Deconstructing fragment-based inhibitor discovery. Nat. Chem. Biol. 2006;2(12):720–723. doi: 10.1038/nchembio831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.