Summary
Background
Ambient pollution and non-optimal temperature are major risk factors for respiratory health. However, the relationships between short-term exposure to these factors and bronchiectasis mortality remain unknown.
Methods
A nationwide, time-stratified case-crossover study across Mainland China was conducted from 2013 to 2019. Records of bronchiectasis deaths were extracted from the National Death Registration Reporting Information System. Daily concentrations of fine particulate matter (PM2.5), coarse particulate matter (PM2.5–10), nitrogen dioxide (NO2), ozone (O3), and daily temperature were obtained from high-resolution prediction models. We utilized conditional logistic regression model and distributed lag nonlinear model to explore the associations of these exposures with bronchiectasis mortality.
Findings
We included a total of 19,320 bronchiectasis deaths. Air pollutant was associated with bronchiectasis mortality within the first 3 days after exposure and the exposure-response relationships were almost linear. An interquartile range increase in PM2.5, PM2.5–10, and O3 was associated with increments of 3.18%, 4.14%, and 4.36% in bronchiectasis mortality at lag 02 d, respectively. Additionally, lower temperature was associated with higher odds of bronchiectasis mortality. Compared to referent temperature (23.6 °C), the odds ratio for bronchiectasis mortality associated with extremely low temperature (P1: −13.4 °C) was 1.54 (95% CI: 1.05, 2.25).
Interpretation
This national study provides compelling evidence, and highlights the necessity and importance of reducing air pollution exposures and keeping warm for susceptible populations.
Funding
National Natural Science Foundation of China (81925001; 82330070); Innovation Program of Shanghai Municipal Education Commission (202101070007-E00097); Program of Shanghai Municipal Science and Technology Commission (21DZ2201800); Program of Shanghai Shenkang Development Center (SHDC12023110); and Major Project of National Health Commission of China.
Keywords: Air pollution, Ambient temperature, Bronchiectasis, Case-crossover study, Mortality
Research in context.
Evidence before this study
We searched PubMed and Web of Science up to June 10, 2024, using search terms “bronchiectasis AND (air pollution OR Pollutant OR Particulate Matter OR ambient temperature OR meteorological factors OR weather factors OR air temperature)” without language restrictions, to identify papers on the relationship between air pollution, non-optimum ambient temperature, and bronchiectasis. Exposure to air pollutants and non-optimal temperature are associated with respiratory health. Bronchiectasis is a chronic structural lung disease characterized by irreversible dilatation of the bronchi and is susceptible to external factors. It is reported that ambient pollution and non-optimal temperature have adverse relationships with bronchiectasis hospitalization and outpatient visits, while their relationships with bronchiectasis mortality remain unknown.
Added value of this study
Our study found that short-term exposure to air pollutants (PM2.5, PM2.5–10, O3) and low temperature were associated with bronchiectasis mortality. The exposure-response relationships of air pollution and low temperature with bronchiectasis mortality were approximately linear. The stratified analyses reveal that the relationships of air pollution and temperature with bronchiectasis mortality varied by subpopulation and region.
Implications of all the available evidence
This study provides convincing evidence and insights for bronchiectasis management, with significant implications for clinical practice and public health. Moreover, it also highlights the importance of devising effective preventative measures for susceptible populations.
Introduction
Bronchiectasis is a chronic structural lung disease characterized by irreversible dilatation of the bronchi and impaired mucus clearance, with primary clinical symptoms including persistent sputum production, cough, and recurrent respiratory infections.1,2 The recurring exacerbations linked to the disease usually result in decreased quality of life and increased healthcare costs,3 imposing a heavy burden on both patients and the society. Notably, although bronchiectasis death is not a primary clinical endpoint used in bronchiectasis-related studies,4 it was reported that the age-adjusted mortality rate of patients with bronchiectasis (895.2/100,000) is 2.14 times higher than that of general people,5 which highlights a critically overlooked concern on the mortality related to bronchiectasis.
Air pollutants have been extensively studied for their association with a wide range of respiratory diseases, including COPD,6,7 asthma,8 and pneumonia.9,10 Short-term exposure to these pollutants can significantly induce inflammatory responses and oxidative stress in the airways,11,12 impairing airway epithelial barrier13 and deteriorating lung function.11 These pathological changes may exacerbate the symptoms of bronchiectasis and accelerate the disease progression.14,15 On the other hand, non-optimal temperature, both excessively cold and hot, have adverse relationships with respiratory health, particularly in patients with pre-existing structural lung lesions, such as those with cystic fibrosis and COPD.16, 17, 18 Currently, although a growing number of studies have demonstrated the short-term exposure to air pollutants and temperature were associated with respiratory diseases, there is a lack of epidemiological evidence exploring the relationships of air pollutants and non-optimum temperature with bronchiectasis. Only a few former studies have addressed the relationships of these environmental risk factors with hospitalization, outpatient visits or medical care accessibility of bronchiectasis.15,18, 19, 20 Nevertheless, previous studies were limited by single region or center, small sample size, crude exposure assessment or ecological designs, thereby restricting the representativeness and validity of the estimates. Furthermore, the associations of air pollution and temperature with bronchiectasis mortality remain unknown. Consequently, there remains a key gap in more comprehensive and accurate understanding on the associations of air pollutants and temperature with medical services due to acute bronchiectasis exacerbation.
To improve public health management for patients with bronchiectasis, we conducted a national, time-stratified, case–crossover study utilizing a national death registry. We aimed to investigate the associations of transient exposures to air pollution and non-optimum ambient temperature with bronchiectasis mortality, and to identify potentially susceptible populations, thereby providing additional evidence and insights for bronchiectasis management.
Methods
Study region and design
We conducted a national, time-stratified, case–crossover study at the individual level among all registered deaths of bronchiectasis in the China's national death system from 2013 to 2019. This design has been widely used in epidemiological studies of air pollutants and temperature.21, 22, 23 It allows every patient to serve as his/her own controls, thus some potential confounders that basically remain stable over a short period, such as body mass index (BMI), age, sex, comorbidities, socioeconomic, and behavioral factors, can be automatically controlled. Specifically, we defined the case period as the date of bronchiectasis death, and the corresponding control periods for each death were selected to be the same days of the week, month and year to control for time trends such as seasonality. For example, if a patient died on Thursday, January 22, 2015 (case period), the corresponding control periods were Thursday, January 1, 8, 15, and 29, 2015. Finally, each case period would be matched for 3 or 4 control periods.
Data source and study population
The death data of bronchiectasis were derived from the National Death Registration and Reporting Information System (DRIS), which was supervised and maintained by the Centre for Chronic and Noncommunicable Disease Control and Prevention of China. This integrated system has strict criteria for data quality control, and collects and verifies the death records across all administrative regions of the Chinese mainland, covering 31 provincial-level and 2844 county-level jurisdictions. The system records detailed information for each death case, including sex, birth date, ID number, ethnicity, registration code, residential code, disease diagnosis, diagnosis time, and cause of death. It incorporates a hierarchical review function that analyses the underlying causes of death and disease classification codes, while also flagging cause-of-death codes that are unsuitable as primary causes. Additionally, the system includes a verification feature for death reports, which can identify, remove, and consolidate duplicate case information for the same disease and individual. Reporting institutions regularly perform statistical analyses and visualizations to manage the quality of death case data. All of these functions are implemented in accordance with the Basic Functional Specification for Population Death Registration Information System of China, as outlined in the standard WS/T 596-2018. Due to its rigorous reporting guidelines and standardized procedures for data registration, input, and reporting, the utilization of the DRIS dataset in scientific researches is deemed to have a high level of reliability.
For the ascertainment of underlying death cause, the diagnosis of in-hospital deaths was determined by clinicians, with completing the Medical Certificate of Death. For deaths occurring outside the hospital, community doctors confirmed the underlying death cause based on comprehensive information of patients and completed the Medical Inference of Death. Then, professionals from Chinese Centre for Disease Control and Prevention carefully reviewed all information of death case to ensure data quality. Therefore, each patient has a unique and independent primary cause of death. Furthermore, asthma and chronic obstructive pulmonary disease (COPD), closely related to bronchiectasis, had distinct ICD-10 codes in this system. Therefore, in this study, we included only patients whose underlying cause of death was bronchiectasis, and patients with bronchiectasis were identified using ICD-10 code J47.
Additionally, we also extracted the information on sex (biological sex at birth), age, the date of death, race (Han and minorities, minorities were the other 55 ethnicities in China except Han), marriage status, education level, profession and residential address of patients from the DRIS. This system is automatically linked to the Household Registration System, allowing for the retrieval of basic demographic information through the identification of each individual's unique ID number. All data were anonymized to protect patient privacy and confidentiality.
Exposure assessment
Daily concentrations of fine particles (PM2.5, μg/m3), inhaled particles (PM10, μg/m3), nitrogen dioxide (NO2, μg/m3), and ozone (O3, μg/m3) were assessed using high-resolution (1 km × 1 km at a daily basis) prediction models.24, 25, 26, 27 Specifically, the daily concentrations of PM2.5 and PM10 were estimated based on the random forest models, which incorporated multi-angle implementation of atmospheric correction aerosol optical depth (MAIAC AOD), fixed-site measurements, meteorological parameters, altitude, and population density. Daily NO2 concentration was estimated using a random forest algorithm. Ground NO2 measurements served as the dependent variable, and meteorological parameters, NO2 simulations from the Community Multiscale Air Quality (CMAQ) model, and other variables were used as predictive variables. Besides, we constructed a random forest model to evaluate the daily maximum 8-h average concentrations of O3. This model integrated various predictor variables, including O3 simulations generated by the CMAQ model, meteorological parameters, road length, ground measurements, population density, and altitude. The ten-fold cross-validated R2 and root-mean-square error (RMSE) of PM2.5 model were 0.86 and 14.46 μg/m3; PM10 model were 0.88 and 20.92 μg/m3; NO2 model were 0.76 and 9.27 μg/m3; O3 model were 0.91 and 9.22 μg/m3, respectively.
Moreover, the daily concentration of coarse particulate (PM2.5–10) was calculated by subtracting the concentrations of PM2.5 from PM10. Daily mean temperature and relative humidity were obtained from the European Centre for Medium-Range Weather Forecasts (ECMWF) reanalysis version 5 (ERA5) (https://www.ecmwf.int/en/forecasts/dataset/ecmwf-reanalysis-v5) product with 10 × 10 km spatial resolutions. Then, we matched daily average concentrations of air pollutants and levels of temperature and humidity within the grid cells of each participant's residential address.
Statistical analyses
For the analyses of air pollutants, we applied the conditional logistic regression model to explore the associations with bronchiectasis mortality. Former studies have observed the lagged relationships of air pollutants with respiratory diseases within a few days after exposure.28,29 Thus, we empirically considered single-day lags, including 0 day (lag 0 d), 1 day (lag 1 d), 2 day (lag 2 d), and 3 day (lag 3 d). We also considered the potential associations of multiple-day averaged exposures, including lag 01 d, lag 02 d, and lag 03 d. We did not employ the distributed lag model as the relationships of air pollution are always transient. We introduced natural cubic splines with a degree of freedom (df) of 6 and 3 for air temperature and relative humidity on the same lag days of air pollutants into the conditional logistic regression model, respectively. Besides, a binary variable of public holiday was also introduced into the model. The detailed model formulation for the analyses of air pollution is shown below:
where P (Y = 1) was the probability of death; β0 was the intercept term; ns() meant the natural cubic splines for temperature and humidity; β1, β2, β3 were the coefficients for corresponding terms.
Furthermore, we plotted exposure-response relationship curves by replacing linear terms for air pollutants with natural cubic splines with 3 df. The results were presented as percent changes and 95% confidence intervals (CIs) for the odds of bronchiectasis mortality associated with an interquartile range (IQR) increase in concentrations of air pollutants.
For the analysis of non-optimum temperature, we also used the conditional logistic regression model to investigate the associations with bronchiectasis mortality. To explore potential lagged effects, we combined the distributed lag non-linear model (DLNM) with the conditional logistic regression model to account for potential lag effects and non-linear associations. Furthermore, according to some previous findings,16,30, 31, 32, 33 daily lags from the current day of death (lag 0 d) to up to 14 d prior (lag 14 d) were considered in this analysis. Specifically, we established a cross-basis function of temperature, in which the natural cubic splines with 3 df for temperature and 3 df for daily lags were used. Then, this cross-basis function was introduced into the conditional logistic regression model. Besides, we also introduced a natural cubic spline with 3 df for 14-d mean relative humidity and a binary variable for public holiday into the main model. The detailed model formulation for the analysis of temperature is shown below:
where t was the day of the study period; P (Yt = 1) was the probability of death occurring on day t; α0 was the intercept term; Temperaturet,l was the cross-basis matrix of temperature; l was the lag days; ns() meant the natural cubic spline for nonlinear variable; α1, α2, α3 corresponded to the coefficients for corresponding terms.
The odds ratios (ORs) and 95% CIs of bronchiectasis mortality were presented by comparing extremely low temperature (1st percentile of temperature distribution) or/and high temperature (99th percentile of temperature distribution) to referent temperature (the temperature corresponding to the lowest association from the exposure-response curve).
In addition, to identify potential effect modification, the stratified analyses were conducted by sex (male and female), age (<65 vs ≥65 years old), region, and season. The season was defined as cold (October to March) and warm (April to September)34, 35, 36; and the region was defined as north and south based on the geographic marker of the Qinling–Huaihe line.37,38 Furthermore, we examined between-stratum differences using the following formula:
where β1 and β2 were the coefficients of point estimates for two stratums, and SΕ1 and SΕ2 were the corresponding standard errors.
Besides, we also performed two sensitivity analyses to examine the robustness of the estimates. Firstly, we fitted co-pollutant models to control for potential confounding of co-pollutants. Secondly, we accordingly adjusted for the 14-d average concentrations of four air pollutants individually and jointly in the model of temperature analysis, to examine possible confounding by air pollutants.
In addition, some studies have explored and identified the interaction effects of pollutants and temperature on health.39, 40, 41, 42, 43, 44 Therefore, we also conducted an interaction analysis to clarify the potential interaction between air pollution and temperature on bronchiectasis mortality. Specifically, we utilized conditional logistic regression model to analyze the interaction effects. Considering the associations of pollutants are usually very acute, we selected the lag patterns consistent with foregoing pollutant analysis, including lag 0 d, lag 1 d, lag 2 d, lag 3 d, lag 01 d, lag 02 d, lag 03 d. The continuous variable temperature was converted to a dummy variable to facilitate interaction analysis. Accordingly, temperatures on corresponding lag days were classified as low temperature (temperature ≤25th percentile of temperature distribution of specific lag days), moderate temperature (between 25th percentile of temperature distribution and 75th percentile of temperature distribution), and high (temperature ≥75th percentile of temperature distribution). Then, the product term of air pollutants and temperature was introduced as an interaction term into the conditional logistic regression model. Moreover, a natural cubic spline with 3 df for mean relative humidity on the same lag days of air pollutants and a binary variable for public holiday were included to adjust for potential confounding.
All analyses were performed in R (Version 4.2.1) with the “survival” package for fitting the conditional logistic regression model, and the “dlnm” package for fitting DLNM. All statistical tests were two-sided, and the p-value < 0.05 was considered statistically significant.
Ethics approval
Informed consent was not applicable to our study, because the data used in this study was derived from National Death Registration and Reporting Information System which regulated by the authoritative government agency, and is anonymous. This study protocol was approved by the Institutional Review Board in School of Public Health, Fudan University (IRB#2021-04-0889).
Role of funders
Funders had no role in study design, data collection, data analyses, interpretation, and writing of report.
Results
Descriptive statistics
A total of 19,320 bronchiectasis death cases were included in this study, of whom 56.2% were males, 75.4% were aged over 65 years, and 92.3% were of the Han nationality. Other detailed demographic characteristics were shown in Table 1. The mean concentrations of PM2.5, PM2.5–10, NO2, and O3 on the day of bronchiectasis death were 46.0 μg/m3, 28.2 μg/m3, 33.7 μg/m3, and 81.8 μg/m3, respectively. The mean temperature and humidity on the day of bronchiectasis death were 16.1 °C (range: −13.4 to 31.9 °C) and 72.5% (range: 23.0%–99.0%), respectively (Table 2). Table S1 showed the spearman correlation coefficients among environmental variables, and the weak to moderate correlations were observed between air pollutants and meteorological factors, with the correlation coefficients ranging from 0.01 to 0.56.
Table 1.
Descriptive statistics of bronchiectasis death cases in Chinese mainland from 2013 to 2019.
| Characteristics | N (%) |
|---|---|
| Case | 19,320 |
| Control | 65,474 |
| Among all cases | |
| Sex | |
| Male | 10,859 (56.2) |
| Female | 8461 (43.8) |
| Age at death | |
| <65 | 4746 (24.6) |
| ≥65 | 14,574 (75.4) |
| Ethnicity | |
| Han | 17,825 (92.3) |
| Minority | 1495 (7.7) |
| Region | |
| North | 5457 (28.2) |
| South | 13,863 (71.8) |
| Season | |
| Warm | 8895 (46.0) |
| Cold | 10,425 (54.0) |
Note: The region was defined as north and south based on the geographic marker of the Qinling–Huaihe line.
The season was divided into warm (April to September) and cold (October to March).
Table 2.
Descriptive statistics for the average levels of air pollutants and meteorological variables on the present day of bronchiectasis death.
| Mean | SD | Min | P25 | P50 | P75 | Max | |
|---|---|---|---|---|---|---|---|
| Air pollutants (μg/m3) | |||||||
| PM2.5 | 46.0 | 32.9 | 6.7 | 22.9 | 36.8 | 58.9 | 202.9 |
| PM2.5–10 | 28.2 | 21.4 | 0.0 | 13.7 | 22.6 | 36.8 | 133.7 |
| NO2 | 33.7 | 19.4 | 4.2 | 18.7 | 29.9 | 45.0 | 101.7 |
| O3 | 81.8 | 43.8 | 6.4 | 48.8 | 75.9 | 109.4 | 217.9 |
| Meteorological variables | |||||||
| Temperature (ºC) | 16.1 | 9.7 | −13.4 | 9.0 | 17.5 | 24.2 | 31.9 |
| Relative humidity (%) | 72.5 | 16.3 | 23.0 | 63.0 | 75.0 | 85.0 | 99.0 |
Abbreviations: SD, standard deviation; P25, the 25th percentile of air pollutants and temperature; P50, the 50th percentile of air pollutants and temperature; P75, the 75th percentile of air pollutants and temperature; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; PM2.5–10, particulate matter with an aerodynamic diameter between 2.5 and 10 μm; NO2, nitrogen dioxide; O3, ozone.
Associations between short-term exposure to air pollution and bronchiectasis mortality
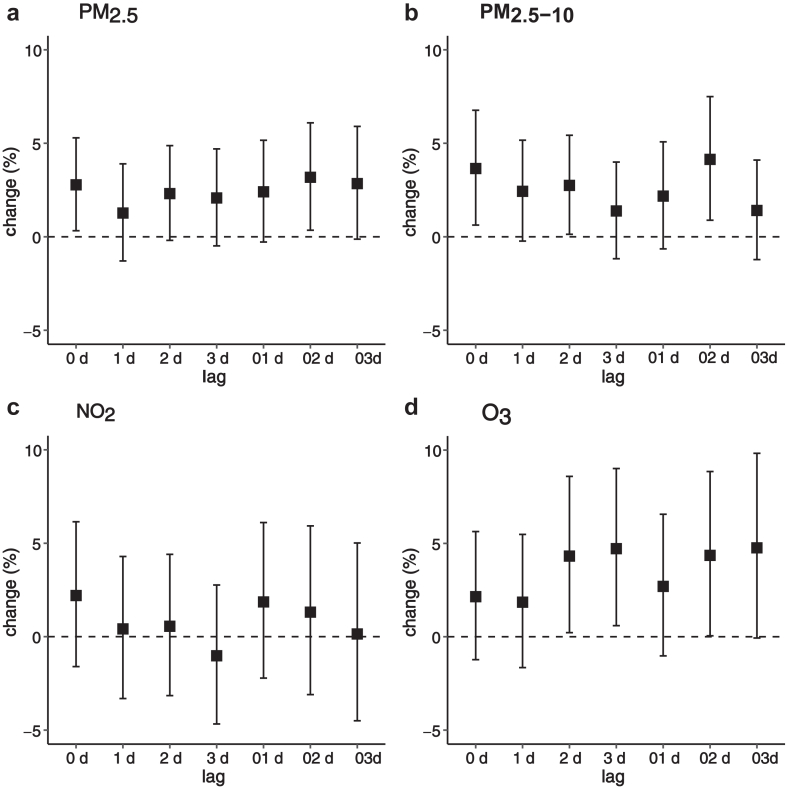
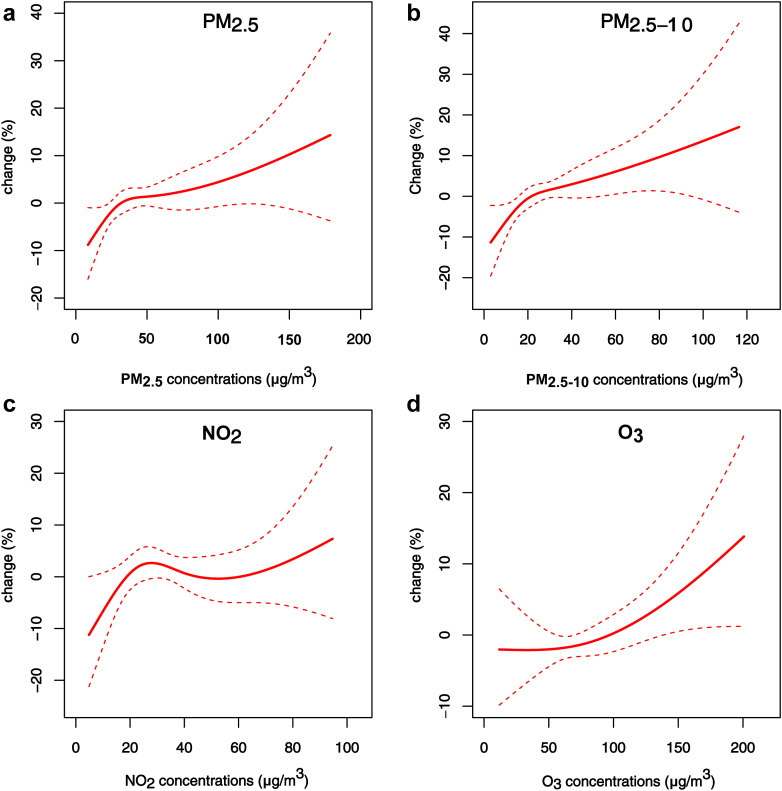
As illustrated in Fig. 1, transient exposure to PM2.5, PM2.5–10, and O3 was associated with higher odds of bronchiectasis mortality, while no association was found for NO2. Specifically, the associations of PM2.5 occurred at lag 0 and lag 02 days, the associations of PM2.5–10 occurred at lag 0, lag 2, and lag 02 days, and the associations of O3 appeared at lag 2, lag 3, and lag 02 days. Overall, PM2.5, PM2.5–10, and O3 exposures at lag 02 d generally had greater relationships of bronchiectasis mortality. Therefore, we primarily presented the results using a lag of 02 days in subsequent analyses. Among the four pollutants, O3 showed the strongest relationship with bronchiectasis mortality, followed by PM2.5–10 and PM2.5. Specifically, an IQR increase in the concentration of PM2.5 (32.8 μg/m3), PM2.5–10 (21.2 μg/m3), and O3 (56.1 μg/m3) was associated with increases of 3.18% (95% CI: 0.35%, 6.10%), 4.14% (95% CI: 0.89%, 7.50%), and 4.36% (95% CI: 0.05%, 8.85%) in the odds of bronchiectasis mortality at lag 02 d, respectively (Table 3). Moreover, the corresponding ORs of PM2.5, PM2.5–10, and O3 were 1.03 (95% CI: 1.004, 1.06), 1.04 (95% CI: 1.01, 1.08), 1.04 (95% CI: 1.04, 1.09), respectively. The exposure-response relationship curves showed higher associations of bronchiectasis mortality with higher concentrations of air pollutants, and were approximately linear without any apparent thresholds (Fig. 2).
Fig. 1.
Percent changes in the odds of bronchiectasis mortality associated with an interquartile range increase in concentrations of air pollutants in different lags. Note: lag 0 d refers to exposure at the concurrent day of death, and lag 01 d refers to the average exposure at lag 0 d and lag 1 d. Abbreviations: (a) PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; (b) PM2.5–10, particulate matter with an aerodynamic diameter between 2.5 and 10 μm; (c) NO2, nitrogen dioxide; (d) O3, ozone.
Table 3.
Percent changes in the odds of bronchiectasis mortality associated with an interquartile range (IQR) increase in air pollutant concentrations.
| Subgroups | PM2.5 | IQR | p value | PM2.5–10 | IQR | p value | NO2 | IQR | p value | O3 | IQR | p value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 3.18 (0.35, 6.10) | 32.8 | 4.14 (0.89, 7.50) | 21.2 | 1.31 (−3.10, 5.93) | 25.1 | 4.36 (0.05, 8.85) | 56.1 | ||||
| Sex | 0.51 | 0.43 | 0.85 | 0.21 | ||||||||
| Male | 3.90 (0.13, 7.82) | 32.7 | 5.38 (1.00, 9.94) | 21.6 | 1.50 (−4.43, 7.80) | 25.2 | 6.74 (0.89, 12.92) | 55.9 | ||||
| Female | 2.20 (−2.04, 6.63) | 33.0 | 2.82 (−2.00, 7.89) | 21.7 | 0.85 (−5.58, 7.70) | 24.8 | 0.68 (−5.53, 7.30) | 56.5 | ||||
| Age at death | 0.27 | 0.30 | 0.58 | 0.30 | ||||||||
| <65 | −0.03 (−5.98, 6.29) | 33.8 | 0.47 (−5.69, 7.03) | 22.6 | 3.23 (−5.70, 13.00) | 24.1 | 0.20 (−8.16, 9.33) | 55.9 | ||||
| ≥65 | 3.97 (0.77, 7.28) | 32.6 | 4.26 (0.76, 7.89) | 21.4 | 0.67 (−4.34, 5.94) | 25.3 | 5.74 (0.77, 10.95) | 56.2 | ||||
| Region | 0.99 | 0.60 | 0.5 | 0.49 | ||||||||
| North | 3.42 (0.11, 6.84) | 45.6 | 5.84 (0.23, 11.76) | 31.2 | 3.47 (−4.15, 11.69) | 25.6 | 6.00 (0.39, 11.92) | 64.1 | ||||
| South | 4.45 (−0.68, 9.85) | 43.5 | 2.96 (−0.76, 6.82) | 17.2 | 0.36 (−4.84, 5.85) | 23.8 | 4.17 (−0.53, 9.09) | 53.2 | ||||
| Season | 0.34 | 0.93 | 0.53 | 0.17 | ||||||||
| Warm | 3.88 (−0.59, 8.55) | 21.0 | 2.18 (−1.69, 6.20) | 12.9 | 4.39 (−2.78, 12.09) | 20.0 | 4.78 (−0.48, 10.32) | 54.5 | ||||
| Cold | 3.42 (0.10, 6.84) | 45.1 | 5.19 (0.16, 10.47) | 16.6 | 2.69 (−2.90, 8.61) | 27.3 | 5.17 (0.79, 9.74) | 43.0 |
Note: Abbreviations were shown in the footnote of Table 2. Air pollutant concentrations were averaged from lag 0 d to 2 d.
Fig. 2.
Exposure-response curves for the associations between air pollutants and bronchiectasis mortality at lag 02 d. The associations are presented as percent change in the odds of bronchiectasis mortality per unit change of 3-d average air pollutant concentrations (μg/m3) from the median concentration. The solid red lines represent the point estimates, and the intervals between dashed red lines represent 95% confidence intervals (CIs). Abbreviations were shown in the note of Fig. 1.
In stratified analyses, the relationships of PM2.5, PM2.5–10, and O3 with bronchiectasis mortality were stronger among males and the elderly. Additionally, exposure to air pollutants had stronger associations among people resided in the north and in the cold season (Table 3). However, these differences between stratums were not statistically significant (Table 3). In sensitivity analysis, the estimates did not appreciably change when adjusting for co-pollutants in two-pollutant models (Figure S1).
Associations between short-term exposure to ambient temperature and bronchiectasis mortality
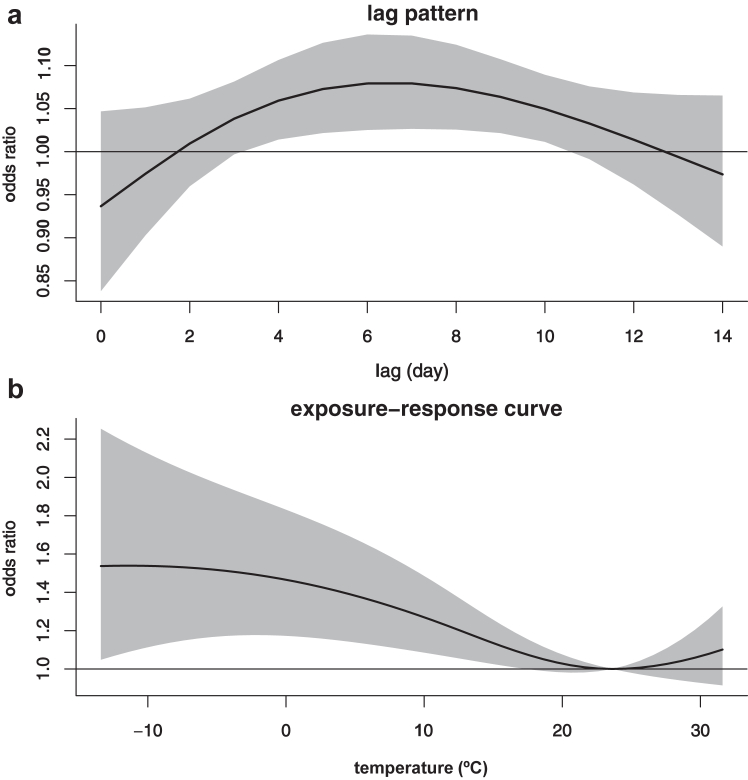
As shown in Fig. 3a, transient exposure to air temperature was associated with bronchiectasis mortality. This association occurred at around 3 days after exposure, thereafter gradually increased, and peaked at approximately lag 6 d. Afterward, this relationship gradually attenuated, and became approximately insignificant at lag 11 day. The exposure-response relationship curve revealed an inverse J-shaped relationship between air temperature and bronchiectasis mortality. It demonstrated that the relationship of high temperature was not significant, whereas low temperature increased the mortality of bronchiectasis, with the association monotonously increasing as temperature decreased (Fig. 3b). Compared to the referent temperature (23.6 °C), the cumulative odds ratio (OR) of bronchiectasis mortality associated with extremely low temperature (−13.4 °C) was 1.54 (95% CI: 1.05, 2.25) over lags 0–14 days (Fig. 3b; Table S2). Nevertheless, there was no significant association between high temperature and bronchiectasis mortality, and the cumulative OR of bronchiectasis mortality associated with extremely high temperature (31.9 °C) was 1.11 (95% CI: 0.91, 1.35). Additionally, given the almost linear relationship of low temperature with bronchiectasis mortality, we also estimated that a 10 °C decrease in temperature was associated with a 12% (OR: 1.12, 95% CI: 1.03, 1.22) increase in the odds of bronchiectasis mortality (Table S2).
Fig. 3.
Lag pattern (a) and exposure-response curve (b) for the association between ambient temperature and bronchiectasis mortality. Note: Lag pattern was presented for odds ratios by comparing mortality at extremely low temperature (−13.4 °C) with the referent temperature (23.6 °C). The black solid lines are the average odds ratios of bronchiectasis mortality and the gray areas are the 95% confidence intervals.
Stratified analyses show that the associations of low temperature were somewhat stronger for females (OR: 1.90, 95% CI: 1.04, 3.74) than males (OR: 1.43, 95% CI: 0.86, 2.40). Moreover, low temperature was associated with higher odds of bronchiectasis mortality in the southern region (OR: 1.39, 95% CI: 1.03, 1.89), in the cold season (OR: 1.90, 95% CI: 1.19, 3.04) and among the elderly (OR: 1.69, 95% CI: 1.08, 2.65), but not in the northern region (OR: 1.42, 95% CI: 0.79, 2.55), in the warm season (OR: 1.17, 95% CI: 0.79, 1.73) and among younger patients (OR: 1.81, 95% CI: 0.83, 3.96) (Table 4). However, similarly, the differences of two-stratum were not statistically significant. The result of sensitivity analysis showed that the association of low temperature with bronchiectasis mortality was not notably changed after adjusting for air pollution (Table S2).
Table 4.
Odds ratios (and 95% confidence intervals) of bronchiectasis mortality associated with specific temperatures, cumulated over lag 0 to 14 d.
| Subgroups | Odds ratioa | Extremely low temperature (P1) | Referent temperature | Odds ratiob | p value |
|---|---|---|---|---|---|
| Sex | 0.45 | ||||
| Male | 1.43 (0.86, 2.40) | −13.1 | 24.9 | 1.10 (0.98, 1.23) | |
| Female | 1.90 (1.04, 3.47) | −13.7 | 31.3 | 1.15 (1.01, 1.31) | |
| Age at death | 0.84 | ||||
| <65 | 1.81 (0.83, 3.96) | −15.1 | 20.9 | 1.07 (0.90, 1.28) | |
| ≥65 | 1.69 (1.08, 2.65) | −12.6 | 31.4 | 1.13 (1.03, 1.25) | |
| Region | 0.93 | ||||
| North | 1.42 (0.79, 2.55) | −19.0 | 19.0 | 1.12 (0.98, 1.27) | |
| South | 1.39 (1.03, 1.89) | 0.0 | 24.0 | 1.13 (1.02, 1.26) | |
| Season | 0.17 | ||||
| Warm | 1.17 (0.79, 1.73) | 6.1 | 26.1 | 1.00 (0.86, 1.17) | |
| Cold | 1.90 (1.19, 3.04) | −16.3 | 26.7 | 1.17 (1.06, 1.30) |
Odd ratios of bronchiectasis mortality comparing extremely low temperature (P1) to referent temperatures.
Odd ratios of bronchiectasis mortality per 10 °C decrease in daily temperature.
For the interaction analysis, we did not find any interaction effect between four air pollutants and temperature on bronchiectasis mortality at different lag days. Detailed results are provided in Table S3.
Discussion
We conducted a nationwide time-stratified case-crossover study exploring the relationships of short-term exposure to air pollutants and non-optimum temperature with bronchiectasis mortality. Using the national death dataset, we observed that short-term exposure to PM2.5, PM2.5–10, O3 and low temperature were associated with higher odds of bronchiectasis mortality at short lag periods. The exposure-response relationships of air pollutants and low temperature with bronchiectasis mortality were almost linear. Additionally, the stratified analyses revealed that the associations of air pollution and temperature varied by subpopulation and region.
Currently, there is a lack of studies exploring the association between short-term exposure pollutants and bronchiectasis mortality, with only a few studies identifying the relationships of air pollutants with bronchiectasis hospitalizations and the frequency of medical visits.14,15,19,20,45, 46, 47, 48 Most of these studies have found that short-term exposure to air pollutants was associated with bronchiectasis exacerbation. For example, Chalmers et al. conducted a case-crossover study among patients with bronchiectasis from a specialist bronchiectasis clinic at Ninewells Hospital in Dundee, and found that PM10 and NO2 could potentially exacerbate bronchiectasis condition (e.g., acute antibiotics prescription or hospital admissions).15 Wang et al. performed a time-series study in a province, and found that the RR of the hospitalization for bronchiectasis was 1.06 (95% CI: 1.01, 1.11) for PM10 at lag 06 d, 1.07 (95% CI: 1.02, 1.12) for PM2.5 at lag 06 d, 1.06 (95% CI: 1.03, 1.08) for NO2 at lag 0 d per IQR in air pollutant concentrations.47 However, the designs of these studies were typically simple, and they were often conducted in a single region with limited sample sizes, resulting in restricted generalizability of conclusions. Additionally, former studies have not discussed the association between PM2.5–10 exposure and bronchiectasis, posing a challenge to understanding the associations of acute air pollution exposure with bronchiectasis. Our study addressed several limitations of previous research and observed that short-term exposure to PM2.5, PM2.5–10, and O3 was associated with bronchiectasis mortality, whereas no significant relationship of NO2 was found. This finding filled the gap related to PM2.5–10, reinforced the conclusions on PM2.5 and O3, and found the contradictory conclusion on NO2. Besides, we also found that the association of PM2.5–10 was stronger than that of PM2.5. This may be because that PM2.5–10 primarily deposits in the nasal cavity and upper respiratory tract, while PM2.5 can penetrate deeper into the lower respiratory tract and translocate from the alveoli into the bloodstream, affecting multiple systems throughout the body.49 Therefore, it is commonly believed that PM2.5 is more hazardous to health than PM2.5–10.50,51 In the healthy population, approximately 90% of PM2.5–10 is expelled from the body via muco-ciliary clearance within 24 h of inhalation.52 However, up to 89.8% of patients with bronchiectasis have primary or secondary motile ciliary disorder.53 This pathological condition may affect the efficiency of mucus cilia in removing PM2.5–10, leading to increased mucus retention in patients with bronchiectasis. Consequently, the association of PM2.5–10 with bronchiectasis was found to be more significant.
Additionally, previous studies have found varied lag patterns in the associations between air pollutants and bronchiectasis. Few study observed the strongest associations occurred at lag 01 d,28 while others found the strongest associations could appear at lag 1 d,14 lag 0 d,15,48 lag 4 d,54 lag 04 d47 or lag 06 d.47 Currently, there is no consistent conclusion on the sensitive exposure windows, which may be due to the differences in study populations and pollutant levels in different regions, resulting in varying timings of the effects. Our study observed the relationships of short-term exposure to air pollution with bronchiectasis mortality primarily occurring within 2 days after exposure, with the strongest associations at lag 02 d. Besides, the exposure-response relationship curves depicted that the relationships of PM2.5, PM2.5–10, and O3 with bronchiectasis mortality were approximately linear without apparent threshold, with the relationships increasing as higher levels of air pollutants. Consequently, there might be a need for a re-evaluation of suitable safety thresholds of air pollutants for patients with bronchiectasis. Given the limited research evidence on bronchiectasis mortality, our conclusions require further validation in broader study areas with a larger sample size.
To date, studies discussing the relationships of short-term exposure to non-optimal temperature with bronchiectasis exacerbation are very limited and inconclusive.19 Only a retrospective observational study reported that the higher percent changes of hospital admission due to bronchiectasis exacerbation associated with decreasing temperature.19 Our study filled this gap and revealed that low temperature was associated with a higher odds of bronchiectasis mortality. Furthermore, the exposure-response relationship was nearly linear, with the relationship progressively increasing as temperature decreased. From a physiological mechanism perspective, our findings are highly reasonable and reliable. Low temperature can facilitate the increased transmission of viruses and bacteria,10 leading to airway constriction,55 impaired gas exchange,56 ciliary dyskinesia,57 and immune defense dysfunction,58 all of which may be likely to aggravate bronchiectasis condition. Additionally, patients with bronchiectasis may have some comorbid respiratory diseases, such as asthma or COPD.2 Low temperature may trigger the exacerbations of these comorbidities,7,8 thereby adding an extra burden to the respiratory system and potentially associated with a higher odds of bronchiectasis mortality. It is noteworthy that bronchiectasis is a clinical syndrome with diverse etiology. For instance, a study in Germany screened 1000 patients with bronchiectasis, identifying the five most common causes as idiopathic (36%), post-infection (21%), COPD (15%), asthma (11%), and primary ciliary dyskinesia (9%).59 In our study, we focused on cases where bronchiectasis was registered as the primary cause of death and excluding cases which the primary cause was registered as asthma or COPD. This selection criterion was established to effectively eliminate the potential interference of COPD, asthma, and other complications, thereby ensuring the validity and independence of our research conclusions.
In the stratified analysis of air pollutants, we observed stronger associations in the northern region, which may be due to the varied levels of air pollutants. Data from the Chinese Ministry of Ecology and Environment indicates that ambient pollution is a more severe problem in northern China.60 This issue may be attributed to the prevalence of thermal power generation and coal-fired heating in the north. Our stratified analysis of temperature showed that the OR of bronchiectasis mortality due to low temperature was stronger in the southern region, which could be attributed to the lack of centralized heating during the winter. Additionally, both the analyses of temperature and air pollutants found stronger relationships during the cold season. During winter, lower temperature can exacerbate respiratory conditions, making patients with bronchiectasis more susceptible to air pollution. Furthermore, the immune system may be weaker in colder temperature, further increasing opportunity to respiratory infections and aggravating the influence of pollutants. In terms of demographic characteristics, males were more vulnerable to air pollutants, while females were more sensitive to low temperature. This vulnerability may be due to the physiological differences in lung structure between sex. Males usually have larger airways, leading to a greater airway surface area exposed to air pollutants.61 Additionally, females generally have smaller airways, making them more prone to bronchoconstriction and spasms in cold environments, thereby worsening the bronchiectasis symptoms. Furthermore, there are differences in physiological thermoregulation between sex, with females being more susceptible to cold air temperature, potentially increasing the negative impacts on the respiratory system. We also observed that both air pollution and cold temperature are likely to have greater influence on the elderly, which may be attributed to age-related declines in lung function, lung elasticity, and airway clearance,62 as well as diminished thermoregulatory and immune function in old people.63
Our research holds significant clinical and public health implications for the development of effective prevention and treatment strategies. For healthcare providers, our findings provide a scientific basis for personalized treatment. For example, adjusting medication dosages or implementing preventive measures during periods of heavy air pollution or temperature drops could be beneficial for the management of patients with bronchiectasis. Additionally, our study also highlights the need for healthcare workers to enhance the monitoring and early warning for high-risk populations to promptly adjust treatment plans and prevent acute exacerbations. For policy makers and implementers, this study suggests the importance of strengthening air pollution monitoring and control, establishing health-related temperature warning systems, and providing air purifiers and warm clothes for low-income populations. Furthermore, media and public health education campaigns should widely disseminate information about the relationships of air pollution and extreme temperature with respiratory system to raise public awareness. At the individual level, the public should stay informed about air quality and weather forecasts, minimize outdoor activities during severe pollution days or cold weather, and wear protective masks appropriately. They can also use air purifiers, maintain indoor warm during cold weather, and wear suitable warm clothing.
This study presents several significant strengths. First, this is a nationwide and population-based study to explore the relationships of short-term exposure to air pollutants and non-optimal temperature with bronchiectasis mortality. We provided the comprehensive study regions and population, thereby enriching the evidence and enlarging the representativeness. Second, we utilized an individual-level, time-stratified, case-crossover study design to control for a wide range of potential confounders that did not vary in a short period, such as age, sex, health status, thus enhancing the robustness of our findings. Additionally, we used high spatiotemporal resolution satellite models to estimate the residential levels of air pollution and temperature, enhancing the accuracy and precision of exposure assessment. We ensured the representativeness based on the national death registry of China, reduced the exposure measurement errors using satellite-based models of high resolutions and enhanced the causal inference by virtue of the individual-level case crossover design.
Some limitations of this study should be noted. Firstly, although we used a time-stratified case-crossover study design to control for confounders that remain constant over time, it is challenging to entirely eliminate the confounding caused by factors that can vary temporarily. The residual confounding is inevitable. However, we believe that the influence of such temporary fluctuation tended to be negligible given the large sample size and nationwide coverage. Secondly, due to the lack of detailed information on bronchiectasis subtypes, we were not able to analyze the associations of short-term exposure to environmental factors with the mortality of specific bronchiectasis subtypes, which limited our ability to gain a broader and deeper understanding. Thirdly, this national death registration system did not collect more detailed and relevant information on disease exacerbation or medical history of patients. However, it is unlikely that medication use would confound the associations, because it is generally randomized and not influenced by some factors such as ambient air pollution and temperature. Furthermore, given the large sample size, study region, the time-stratified case-crossover design, and rigorous diagnosis of causes of death, we believe that the findings from this study are reliable and convincing. Fourthly, given that current discussion mainly focuses on the relationships of short-term exposure to pollutants and temperature with bronchiectasis, more cohort studies are needed to further elucidate causal relationships in the future. Lastly, the diagnosis of bronchiectasis faces the challenges in Chinese rural areas due to limited medical resources, especially the lack of chest CT scans; and consequently, some bronchiectasis deaths may be ignored in this nationwide study due to the limitations in data sources and poor healthcare conditions, especially in rural areas.
In conclusion, our nationwide study provides compelling evidence that short-term exposure to air pollution and low temperature were associated with bronchiectasis mortality. Our conclusions underscore the need for tailored public health strategies to reduce air pollution levels and adapt to non-optimum temperature, thereby alleviating the disease burden of bronchiectasis and related mortality. Additionally, these findings also emphasize the importance of adapting healthcare practices and enhancing patient awareness in line with local climate and air quality conditions.
Contributors
R.C., M.Z., and J-F.X. designed and conceptualized the project, reviewed the manuscript for significant intellectual content, and approved the final version. R.C., M.Z., and J-F.X. are all senior authors and contributed equally to the correspondence work. S.H., X.X., and J-F.X. contributed to the data analysis and interpretation and reviewed the manuscript for significant intellectual content. P.Y. collected the death data. X.M. and H.K. collected the exposure data. J.X. provided guidance and feedback on the overall work. S.H. and X.X. drafted the manuscript. R.C. and J-F.X. finalized the manuscript. All authors contributed to the manuscript preparation. R.C., M.Z., and J-F.X. verified the underlying data. All authors have read and approved the final manuscript.
Data sharing statement
The death data that support the findings of this study are obtained from the National Centre for Chronic Non-communicable Disease Control and Prevention, Chinese Centre for Disease Control and Prevention, and the license for the use of the data was restricted only to the current study, so the death data are not publicly available. Mortality data are, however, available from the authors upon reasonable request and with permission of the National Centre for Chronic Non-communicable Disease Control and Prevention. The environmental data could be available upon reasonable request.
Declaration of interests
All authors have declared that no conflict of interest exists.
Acknowledgements
We appreciate the contributions of all participants in this study. We also thank all staff involved in the data collection, processing and construction of the database. This work was supported by the National Natural Science Foundation of China (81925001; 82330070); the Innovation Program of Shanghai Municipal Education Commission (202101070007-E00097); Program of Shanghai Municipal Science and Technology Commission (21DZ2201800); Program of Shanghai Shenkang Development Center (SHDC12023110); Innovative research team of high-level local universities in Shanghai.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105465.
Contributor Information
Renjie Chen, Email: chenrenjie@fudan.edu.cn.
Maigeng Zhou, Email: zhoumaigeng@ncncd.chinacdc.cn.
Jin-Fu Xu, Email: jfxucn@163.com.
Appendix ASupplementary data
References
- 1.Xu J.F., Gao Y.H., Song Y.L., et al. Research advances and clinical management of bronchiectasis: Chinese perspective. ERJ Open Res. 2022;8 doi: 10.1183/23120541.00017-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flume P.A., Chalmers J.D., Olivier K.N. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet. 2018;392:880–890. doi: 10.1016/S0140-6736(18)31767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y.H., Zheng H.Z., Lu H.W., et al. Quality-of-life bronchiectasis respiratory symptom scale predicts the risk of exacerbations in adults with bronchiectasis: a prospective observational study. Ann Am Thorac Soc. 2024;21:393–401. doi: 10.1513/AnnalsATS.202302-133OC. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers J.D., Chang A.B., Chotirmall S.H., et al. Bronchiectasis. Nat Rev Dis Prim. 2018;4:45. doi: 10.1038/s41572-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 5.Quint J.K., Millett E.R., Joshi M., et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J. 2016;47:186–193. doi: 10.1183/13993003.01033-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sin D.D., Doiron D., Agusti A., et al. Air pollution and COPD: GOLD 2023 committee report. Eur Respir J. 2023;61 doi: 10.1183/13993003.02469-2022. [DOI] [PubMed] [Google Scholar]
- 7.Hansel N.N., McCormack M.C., Kim V. The effects of air pollution and temperature on COPD. COPD. 2016;13:372–379. doi: 10.3109/15412555.2015.1089846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirabelli M.C., Vaidyanathan A., Flanders W.D., et al. Outdoor PM2.5, ambient air temperature, and asthma symptoms in the past 14 Days among adults with active asthma. Environ Health Perspect. 2016;124:1882–1890. doi: 10.1289/EHP92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Li D., Ma Y., et al. Long-term exposure to ambient air pollutants and increased risk of pneumonia in the UK Biobank. Chest. 2023;164:39–52. doi: 10.1016/j.chest.2023.02.018. [DOI] [PubMed] [Google Scholar]
- 10.He Q., Liu Y., Yin P., et al. Differentiating the impacts of ambient temperature on pneumonia mortality of various infectious causes: a nationwide, individual-level, case-crossover study. EBioMedicine. 2023;98 doi: 10.1016/j.ebiom.2023.104854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X., Zhang Q., Du X., et al. Respiratory effects of traffic-related air pollution: a randomized, crossover analysis of lung function, airway metabolome, and biomarkers of airway injury. Environ Health Perspect. 2023;131 doi: 10.1289/EHP11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kayalar Ö., Rajabi H., Konyalilar N., et al. Impact of particulate air pollution on airway injury and epithelial plasticity; underlying mechanisms. Front Immunol. 2024;15 doi: 10.3389/fimmu.2024.1324552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghapour M., Ubags N.D., Bruder D., et al. Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur Respir Rev. 2022;31 doi: 10.1183/16000617.0112-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Olive I., Stojanovic Z., Radua J., et al. Effect of air pollution on exacerbations of bronchiectasis in Badalona, Spain, 2008-2016. Respiration. 2018;96:111–116. doi: 10.1159/000488646. [DOI] [PubMed] [Google Scholar]
- 15.Goeminne P.C., Cox B., Finch S., et al. The impact of acute air pollution fluctuations on bronchiectasis pulmonary exacerbation: a case-crossover analysis. Eur Respir J. 2018;52 doi: 10.1183/13993003.02557-2017. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y., Huang Z., Wang S., et al. Morbidity burden of respiratory diseases attributable to ambient temperature: a case study in a subtropical city in China. Environ Health. 2019;18:89. doi: 10.1186/s12940-019-0529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donaldson G.C., Seemungal T., Jeffries D.J., et al. Effect of temperature on lung function and symptoms in chronic obstructive pulmonary disease. Eur Respir J. 1999;13:844–849. doi: 10.1034/j.1399-3003.1999.13d25.x. [DOI] [PubMed] [Google Scholar]
- 18.Borsi S.H., Khodadadi N., Khanjani N., et al. Physiological equivalent temperature (PET) index and respiratory hospital admissions in Ahvaz, southwest of Iran. Environ Sci Pollut Res Int. 2021;28:51888–51896. doi: 10.1007/s11356-021-14345-z. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Olive I., Radua J., Sanchez-Berenguer D., et al. Association between environmental factors and hospitalisations for bronchiectasis in Badalona, Barcelona, Spain (2007-2015) Med Clin (Barc) 2018;150:257–261. doi: 10.1016/j.medcli.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Lee H., Kim S.H., Lee S.-K., et al. Impact of air pollution on healthcare utilization in patients with bronchiectasis. Front Med (Lausanne) 2023;10 doi: 10.3389/fmed.2023.1233516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villeneuve P.J., Huynh D., Lavigne E., et al. Daily changes in ambient air pollution concentrations and temperature and suicide mortality in Canada: findings from a national time-stratified case-crossover study. Environ Res. 2023;223 doi: 10.1016/j.envres.2023.115477. [DOI] [PubMed] [Google Scholar]
- 22.Casas L., Cox B., Nemery B., et al. High temperatures trigger suicide mortality in Brussels, Belgium: a case-crossover study (2002-2011) Environ Res. 2022;207 doi: 10.1016/j.envres.2021.112159. [DOI] [PubMed] [Google Scholar]
- 23.Chen J., Gao Y., Jiang Y., et al. Low ambient temperature and temperature drop between neighbouring days and acute aortic dissection: a case-crossover study. Eur Heart J. 2022;43:228–235. doi: 10.1093/eurheartj/ehab803. [DOI] [PubMed] [Google Scholar]
- 24.Li X., Wang P., Wang W., et al. Mortality burden due to ambient nitrogen dioxide pollution in China: application of high-resolution models. Environ Int. 2023;176 doi: 10.1016/j.envint.2023.107967. [DOI] [PubMed] [Google Scholar]
- 25.Meng X., Wang W., Shi S., et al. Evaluating the spatiotemporal ozone characteristics with high-resolution predictions in mainland China, 2013-2019. Environ Pollut. 2022;299 doi: 10.1016/j.envpol.2022.118865. [DOI] [PubMed] [Google Scholar]
- 26.Shi S., Wang W., Li X., et al. Optimizing modeling windows to better capture the long-term variation of PM(2.5) concentrations in China during 2005-2019. Sci Total Environ. 2023;854 doi: 10.1016/j.scitotenv.2022.158624. [DOI] [PubMed] [Google Scholar]
- 27.Meng X., Liu C., Zhang L., et al. Estimating PM2.5 concentrations in Northeastern China with full spatiotemporal coverage, 2005-2016. Remote Sens Environ. 2021;253 doi: 10.1016/j.rse.2020.112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Liang L., Zhang S., et al. Short-term ambient particulate matter pollution of different sizes and respiratory hospital admission in the Beibu Gulf area of Southern China. Atmos Environ. 2023;294 [Google Scholar]
- 29.Zhai S., Zeng J., Zhang Y., et al. Combined health effects of PM2.5 components on respiratory mortality in short-term exposure using BKMR: a case study in Sichuan, China. Sci Total Environ. 2023;897 doi: 10.1016/j.scitotenv.2023.165365. [DOI] [PubMed] [Google Scholar]
- 30.Agache I., Canelo-Aybar C., Annesi-Maesano I., et al. The impact of outdoor pollution and extreme temperatures on asthma-related outcomes: a systematic review for the EAACI guidelines on environmental science for allergic diseases and asthma. Allergy. 2024;79:1725–1760. doi: 10.1111/all.16041. [DOI] [PubMed] [Google Scholar]
- 31.Lam H.C.-Y., Li A.M., Chan E.Y.-Y., et al. The short-term association between asthma hospitalisations, ambient temperature, other meteorological factors and air pollutants in Hong Kong: a time-series study. Thorax. 2016;71:1097–1109. doi: 10.1136/thoraxjnl-2015-208054. [DOI] [PubMed] [Google Scholar]
- 32.Li M., Zhou M., Yang J., et al. Temperature, temperature extremes, and cause-specific respiratory mortality in China: a multi-city time series analysis. Air Qual Atmos Health. 2019;12:539–548. [Google Scholar]
- 33.Xue X., Hu J., Peng L., et al. Low ambient temperature might trigger the symptom onset of pulmonary embolism: a nationwide case-crossover study at hourly level in China. Sci Total Environ. 2022;853 doi: 10.1016/j.scitotenv.2022.158524. [DOI] [PubMed] [Google Scholar]
- 34.Xue X., Hu J., Xiang D., et al. Hourly air pollution exposure and the onset of symptomatic arrhythmia: an individual-level case-crossover study in 322 Chinese cities. CMAJ. 2023;195:E601–E611. doi: 10.1503/cmaj.220929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y., Chen R., Peng W., et al. Hourly ultrafine particle exposure and acute myocardial infarction onset: an individual-level case-crossover study in Shanghai, China, 2015-2020. Environ Sci Technol. 2023;57:1701–1711. doi: 10.1021/acs.est.2c06651. [DOI] [PubMed] [Google Scholar]
- 36.Chen R., Jiang Y., Hu J., et al. Hourly air pollutants and acute coronary syndrome onset in 1.29 million patients. Circulation. 2022;145:1749–1760. doi: 10.1161/CIRCULATIONAHA.121.057179. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z., Dennell R., Huang W., et al. Hominin occupation of the Chinese Loess Plateau since about 2.1 million years ago. Nature. 2018;559:608–612. doi: 10.1038/s41586-018-0299-4. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z., Jiang Z., Li T., et al. Structural variants in the Chinese population and their impact on phenotypes, diseases and population adaptation. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aboubakri O., Shoraka H.R., Karamoozian A., et al. Seasonal impact of air particulate matter on morbidity: interaction effect assessment in a time-stratified case-crossover design. Hum Ecol Risk Assess. 2021;27:2328–2341. [Google Scholar]
- 40.Lee H., Myung W., Cheong H.-K., et al. Ambient air pollution exposure and risk of migraine: synergistic effect with high temperature. Environ Int. 2018;121:383–391. doi: 10.1016/j.envint.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Murtas R., Russo A.G. Effects of pollution, low temperature and influenza syndrome on the excess mortality risk in winter 2016-2017. BMC Public Health. 2019;19 doi: 10.1186/s12889-019-7788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ning Z., He S., Liao X., et al. Cold waves and fine particulate matter in high-altitude Chinese cities: assessing their interactive impact on outpatient visits for respiratory disease. BMC Public Health. 2024;24 doi: 10.1186/s12889-024-18896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ning Z., He S., Liu Q., et al. Effects of the interaction between cold spells and fine particulate matter on mortality risk in Xining: a case-crossover study at high altitude. Front Public Health. 2024;12 doi: 10.3389/fpubh.2024.1414945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu R., Huang S., Shi C., et al. Extreme temperature events, fine particulate matter, and myocardial infarction mortality. Circulation. 2023;148:312–323. doi: 10.1161/CIRCULATIONAHA.122.063504. [DOI] [PubMed] [Google Scholar]
- 45.Raji H., Riahi A., Borsi S.H., et al. Acute effects of air pollution on hospital admissions for asthma, COPD, and bronchiectasis in Ahvaz, Iran. Int J Chronic Obstr Pulm Dis. 2020;15:501–514. doi: 10.2147/COPD.S231317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li R., Jiang N., Liu Q., et al. Impact of air pollutants on outpatient visits for acute respiratory outcomes. Int J Environ Res Public Health. 2017;14:47. doi: 10.3390/ijerph14010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z., Zhou Y., Zhang Y., et al. Association of hospital admission for bronchiectasis with air pollution: a province-wide time-series study in southern China. Int J Hyg Environ Health. 2021;231 doi: 10.1016/j.ijheh.2020.113654. [DOI] [PubMed] [Google Scholar]
- 48.Zhuang J., Bai H., Sun J., et al. The association between fine particulate matter and acute lower respiratory infections in Yancheng City, China. Environ Sci Pollut Res Int. 2021;28:61723–61731. doi: 10.1007/s11356-021-15102-y. [DOI] [PubMed] [Google Scholar]
- 49.Wang T.H., Huang K.Y., Chen C.C., et al. PM2.5 promotes lung cancer progression through activation of the AhR-TMPRSS2-IL18 pathway. EMBO Mol Med. 2023;15 doi: 10.15252/emmm.202217014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou N., Jiang C., Chen Q., et al. Exposures to atmospheric PM10 and PM2.5-10 affect male semen quality: results of MARHCS study. Environ Sci Technol. 2018;52:1571–1581. doi: 10.1021/acs.est.7b05206. [DOI] [PubMed] [Google Scholar]
- 51.Ji Y., Cong S., Fan J., et al. Prevalence of nicotine dependence among smokers aged 40 years and older in China. Chin Med J Pulm Crit Care Med. 2024;2:119–131. doi: 10.1016/j.pccm.2024.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hofmann W., Asgharian B. The effect of lung structure on mucociliary clearance and particle retention in human and rat lungs. Toxicol Sci. 2003;73:448–456. doi: 10.1093/toxsci/kfg075. [DOI] [PubMed] [Google Scholar]
- 53.Zhang R.L., Pan C.X., Tang C.L., et al. Motile ciliary disorders of the nasal epithelium in adults with bronchiectasis. Chest. 2023;163:1038–1050. doi: 10.1016/j.chest.2022.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Szyszkowicz M., Kousha T., Castner J., et al. Air pollution and emergency department visits for respiratory diseases: a multi-city case crossover study. Environ Res. 2018;163:263–269. doi: 10.1016/j.envres.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 55.Rundell K.W., Anderson S.D., Sue-Chu M., et al. Air quality and temperature effects on exercise-induced bronchoconstriction. Compr Physiol. 2015;5:579–610. doi: 10.1002/cphy.c130013. [DOI] [PubMed] [Google Scholar]
- 56.Mortola J.P., Frappell P.B. Ventilatory responses to changes in temperature in mammals and other vertebrates. Annu Rev Physiol. 2000;62:847–874. doi: 10.1146/annurev.physiol.62.1.847. [DOI] [PubMed] [Google Scholar]
- 57.Reula A., Pitarch-Fabregat J., Milara J., et al. High-speed video microscopy for primary ciliary dyskinesia diagnosis: a study of ciliary motility variations with time and temperature. Diagnostics (Basel) 2021;11:1301. doi: 10.3390/diagnostics11071301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang D., Taha M.S., Nocera A.L., et al. Cold exposure impairs extracellular vesicle swarm-mediated nasal antiviral immunity. J Allergy Clin Immunol. 2023;151:509–525.e8. doi: 10.1016/j.jaci.2022.09.037. [DOI] [PubMed] [Google Scholar]
- 59.Ewen R., Pink I., Sutharsan S., et al. Primary ciliary dyskinesia in adult bronchiectasis - data from the German Bronchiectasis Registry PROGNOSIS. Chest. 2024;166:938–950. doi: 10.1016/j.chest.2024.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z., Xue T., Jin X. Effects of meteorological conditions and air pollution on COVID-19 transmission: evidence from 219 Chinese cities. Sci Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vidaillac C., Yong V.F.L., Jaggi T.K., et al. Gender differences in bronchiectasis: a real issue? Breathe (Sheff) 2018;14:108–121. doi: 10.1183/20734735.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandström T., Frew A.J., Svartengren M., et al. The need for a focus on air pollution research in the elderly. Eur Respir J. 2003;21:92S–95S. doi: 10.1183/09031936.03.00403503. [DOI] [PubMed] [Google Scholar]
- 63.Contarini M., Finch S., Chalmers J.D. Bronchiectasis: a case-based approach to investigation and management. Eur Respir Rev. 2018;27 doi: 10.1183/16000617.0016-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.