Abstract
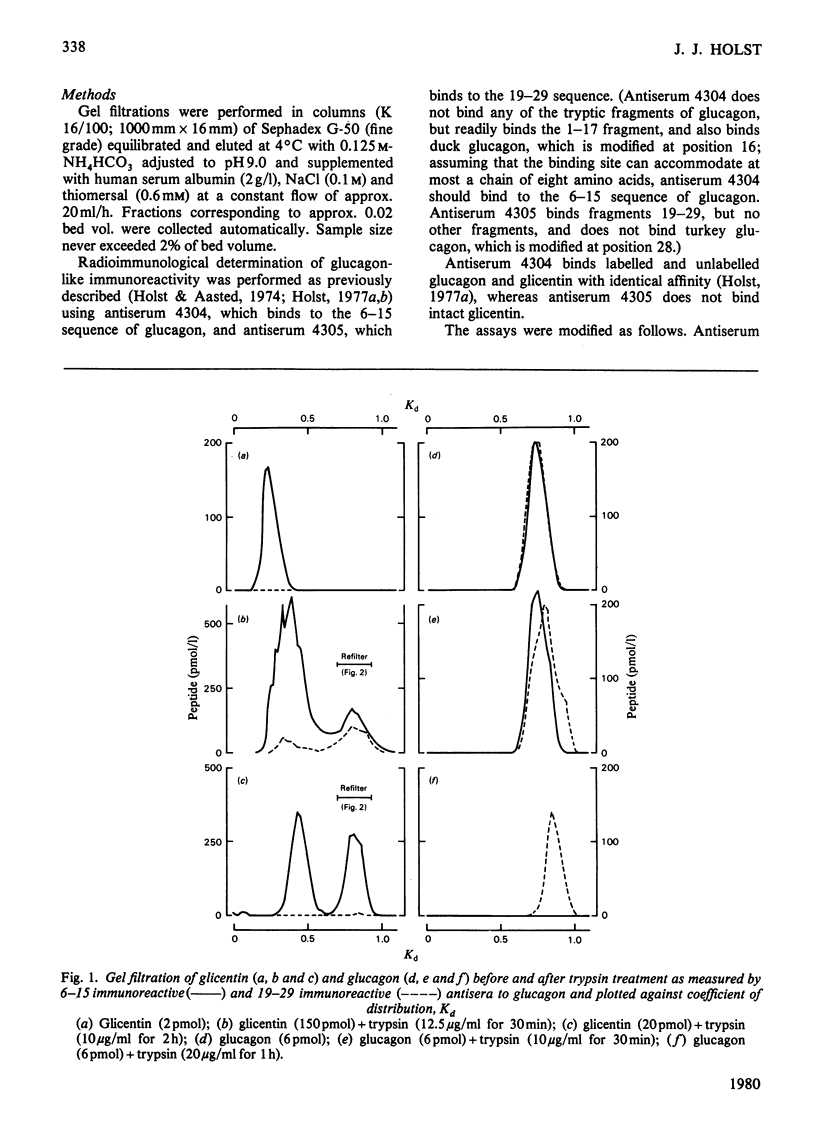
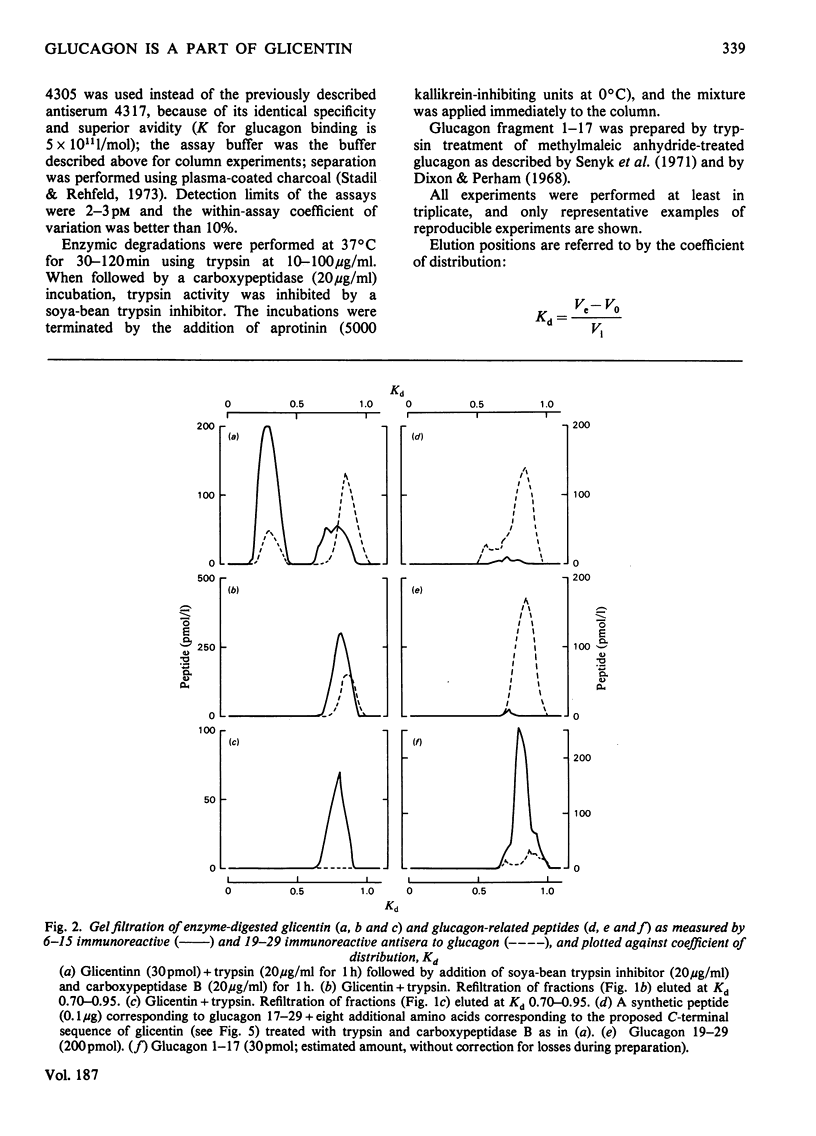
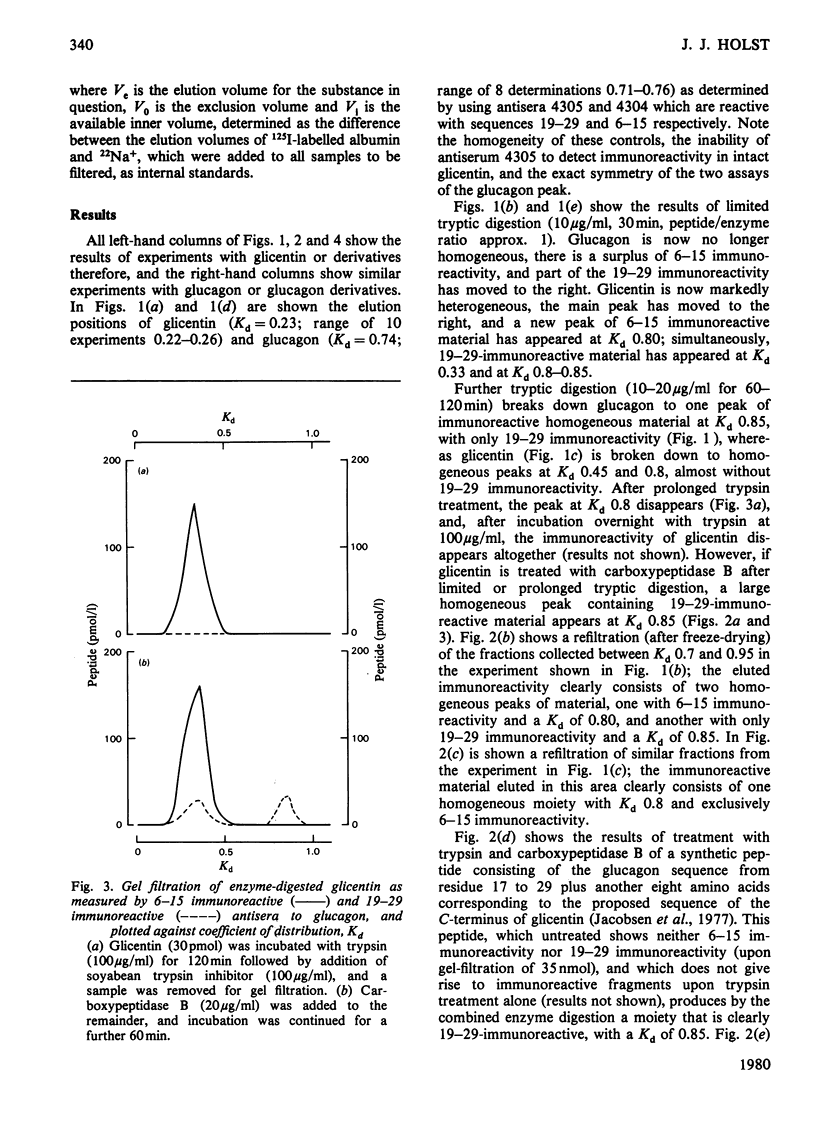
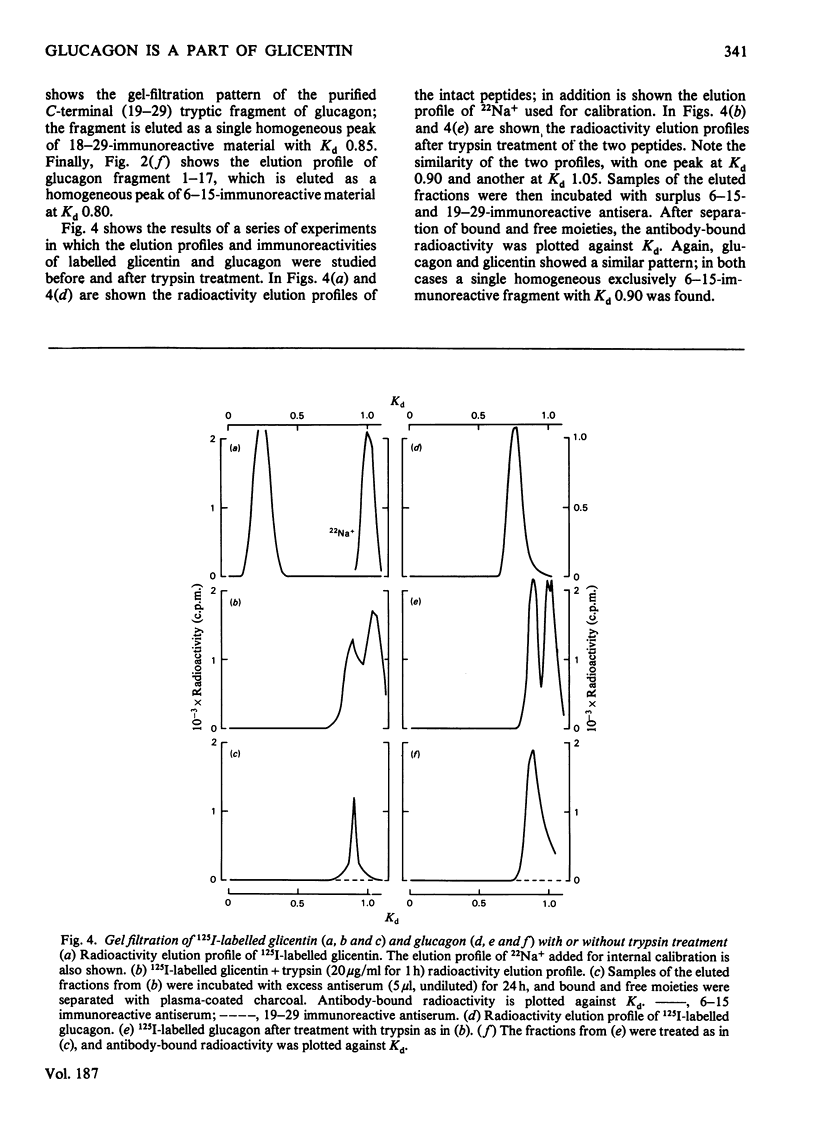
Glicentin (a highly purified 100-amino acid peptide with glucagon-like immunoreactivity from porcine gut) was subjected to limited digestion with trypsin and carboxypeptidase B, and the resulting peptides were studied by gel filtration and region-specific glucagon radioimmunoassays. Similar digests of glucagon and purified fragments of glucagon were studied in parallel. Glicentin gave rise to peptides that corresponded closely to the 1-17 and 19-29 fragments of glucagon. Also, 125I-labelled glicentin and 125I-labelled glucagon gave rise to identical fragments after trypsin treatment. On the basis of this and other evidence [Jacobsen, Demandt, Moody & Sundby (1977) Biochim. Biophys. Acta 493, 452-459] it is concluded that glicentin contains the entire glucagon sequence at residues number 64-92 and thus fulfills one of the requirements for being a 'proglucagon'.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dixon H. B., Perham R. N. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968 Sep;109(2):312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. J., Aasted B. Production and evaluation of glucagon antibodies for radioimmunoassay. Acta Endocrinol (Copenh) 1974 Dec;77(4):715–726. doi: 10.1530/acta.0.0770715. [DOI] [PubMed] [Google Scholar]
- Holst J. J. Extraction, gel filtration pattern, and receptor binding of porcine gastrointestinal glucagon-like immunoreactivity. Diabetologia. 1977 Apr;13(2):159–169. doi: 10.1007/BF00745145. [DOI] [PubMed] [Google Scholar]
- Holst J. J. Extrapancreatic glucagons. Digestion. 1978;17(2):168–190. doi: 10.1159/000198107. [DOI] [PubMed] [Google Scholar]
- Jacobsen H., Demandt A., Moody A. J., Sundby F. Sequence analysis of porcine gut GLI-1. Biochim Biophys Acta. 1977 Aug 23;493(2):452–459. doi: 10.1016/0005-2795(77)90201-x. [DOI] [PubMed] [Google Scholar]
- Jorgensen K. H., Larsen U. D. Purification of 125 I-glucagon by anion exchange chromatography. Horm Metab Res. 1972 May;4(3):223–224. doi: 10.1055/s-0028-1097092. [DOI] [PubMed] [Google Scholar]
- Ravazzola M., Siperstein A., Moody A. J., Sundby F., Jacobsen H., Orci L. Glicentin immunoreactive cells: their relationship to glucagon-producing cells. Endocrinology. 1979 Aug;105(2):499–508. doi: 10.1210/endo-105-2-499. [DOI] [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F. Determination of gastrin in serum. An evaluation of the reliability of a radioimmunoassay. Scand J Gastroenterol. 1973;8(2):101–112. [PubMed] [Google Scholar]
- Sundby F., Jacobsen H., Moody A. J. Purification and characterization of a protein from porcine gut with glucagon-like immunoreactivity. Horm Metab Res. 1976 Sep;8(5):366–371. doi: 10.1055/s-0028-1093615. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Isolation of a glucagon-containing peptide: primary structure of a possible fragment of proglucagon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2321–2325. doi: 10.1073/pnas.70.8.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Schenck H., Jeppsson J. O. Preparation of monoiodotyrosine-13-glucagon. Biochim Biophys Acta. 1977 Apr 25;491(2):503–508. doi: 10.1016/0005-2795(77)90294-x. [DOI] [PubMed] [Google Scholar]


