Abstract
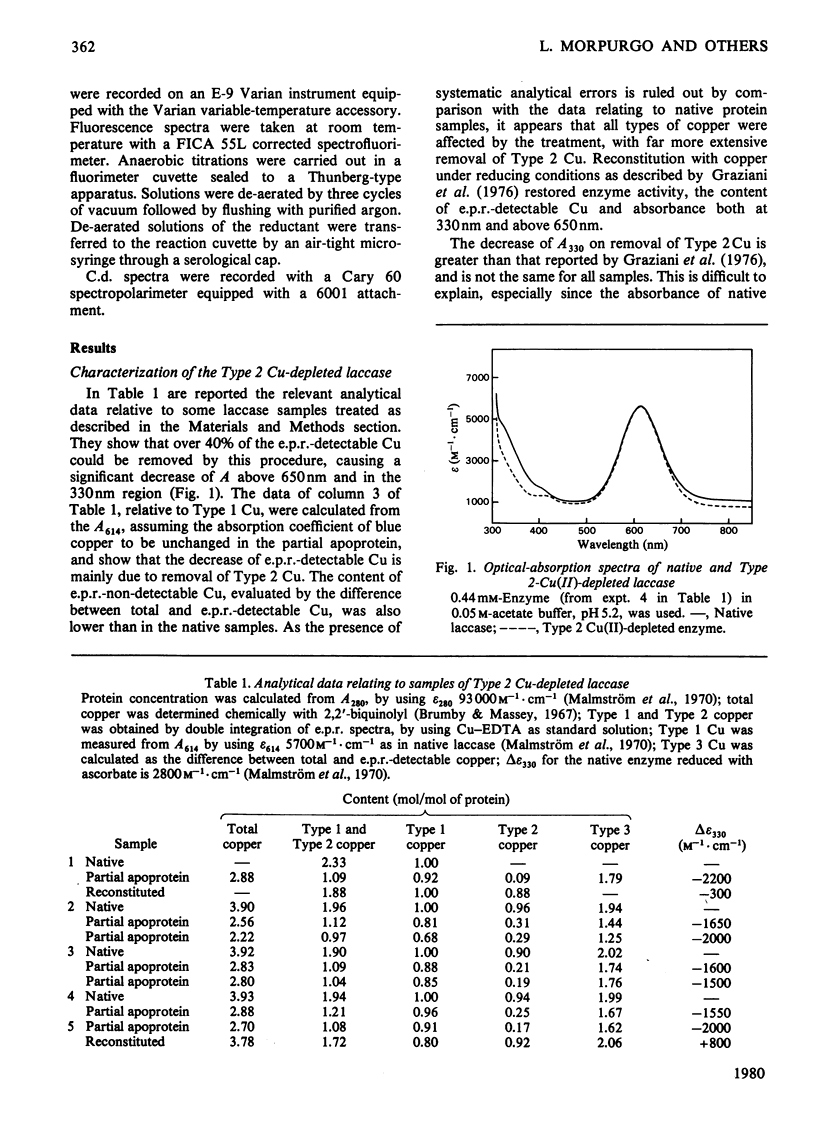
1. Spectroscopic and functional properties of Japanese-lacquer-tree (Rhus vernicifera) laccase were re-investigated, with special emphasis on the relationships between the different types of copper centres (Types 1, 2, and 3). 2. On removal of the Type 2 Cu(II), a decrease of absorbance occurred in the wavelength region above 650 nm (delta epsilon 750 = 300 M-1 . cm-1) and around 330 nm (delta episom 330 up to 2200 M-1 . cm-1). 3. Reductive titrations with ascorbic acid or ferrocyanide showed that the electron-accepting capacity of the partial apoprotein is one electron-equivalent lower than that of the native protein, i.e. the protein two-electron acceptor is present in the oxidized state in spite of absorbance loss at 330 nm. 4. The 330 nm chromophore apparently depends on the presence of both the Type 2 and the Type 3 copper in the oxidized state. 5. This finding may have implications in the relative location of Type 2 and 3 copper centres and on the redox behaviour of laccase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R., Brändén R., Deinum J., Malmström B. G., Reinhammar B., Vänngård T. A paramagnetic intermediate in the reduction of oxygen by reduced laccase. FEBS Lett. 1976 Jan 15;61(2):115–119. doi: 10.1016/0014-5793(76)81016-2. [DOI] [PubMed] [Google Scholar]
- Andréasson L. E., Malmström B. G., Strömberg C., Vänngård T. The kinetics of the anaerobic reduction of fungal laccase B. Eur J Biochem. 1973 May 2;34(3):434–439. doi: 10.1111/j.1432-1033.1973.tb02776.x. [DOI] [PubMed] [Google Scholar]
- Avigliano L., Desideri A., Urbanelli S., Mondovì B., Marchesini A. Removal of non-blue copper from ascorbate oxidase. FEBS Lett. 1979 Apr 15;100(2):318–320. doi: 10.1016/0014-5793(79)80360-9. [DOI] [PubMed] [Google Scholar]
- Avigliano L., Rotilio G., Urbanelli S., Mondovi B., Agrò A. F. Anaerobic reaction of ascorbate oxidase with ascorbate. Arch Biochem Biophys. 1978 Jan 30;185(2):419–422. doi: 10.1016/0003-9861(78)90184-4. [DOI] [PubMed] [Google Scholar]
- Bossa F., Rotilio G., Fasella P., Malmström B. G. An optical rotatory dispersion vestigation of fungal laccase. Eur J Biochem. 1969 Sep;10(2):395–398. doi: 10.1111/j.1432-1033.1969.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Brändén R., Malmström B. G., Vänngård T. The effect of fluoride on the spectral and catalytic properties of the three copper-containing oxidases. Eur J Biochem. 1973 Jul 2;36(1):195–200. doi: 10.1111/j.1432-1033.1973.tb02901.x. [DOI] [PubMed] [Google Scholar]
- Brändén R., Reinhammar B. EPR studies on the anaerobic reduction of fungal laccase. Evidence for participation of type 2 copper in the reduction mechanism. Biochim Biophys Acta. 1975 Oct 20;405(2):236–242. doi: 10.1016/0005-2795(75)90090-2. [DOI] [PubMed] [Google Scholar]
- Deinum J., Vänngård T. The stoichiometry of the paramagnetic copper and the oxidation-reduction potentials of type I copper in human ceruloplasmin. Biochim Biophys Acta. 1973 Jun 15;310(2):321–330. doi: 10.1016/0005-2795(73)90112-8. [DOI] [PubMed] [Google Scholar]
- Falk K. E., Reinhammar B. Visible and near-infrared circular dichroism of some blue copper proteins. Biochim Biophys Acta. 1972 Nov 28;285(1):84–90. doi: 10.1016/0005-2795(72)90182-1. [DOI] [PubMed] [Google Scholar]
- Farver O., Goldberg M., Lancet D., Pecht I. Oxidative titrations of Rhus vernicifera laccase and its specific interaction with hydrogen peroxide. Biochem Biophys Res Commun. 1976 Nov 22;73(2):494–500. doi: 10.1016/0006-291x(76)90734-8. [DOI] [PubMed] [Google Scholar]
- Farver O., Goldberg M., Wherland S., Pecht I. Reductant-dependent electron distribution among redox sites of laccase. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5245–5249. doi: 10.1073/pnas.75.11.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee J. A., Briggs R. G. Studies on the reconstitution of bovine erythrocyte superoxide dismutase. V. Preparation and properties of derivatives in which both zinc and copper sites contain copper. Biochim Biophys Acta. 1975 Aug 19;400(2):439–450. doi: 10.1016/0005-2795(75)90200-7. [DOI] [PubMed] [Google Scholar]
- Fee J. A., DiCorleto P. E. Observations on the oxidation-reduction properties of bovine erythrocyte superoxide dismutase. Biochemistry. 1973 Nov 20;12(24):4893–4899. doi: 10.1021/bi00748a013. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Gaber B. P. Anion binding to bovine erythrocyte superoxide dismutase. Evidence for multiple binding sites with qualitatively different properties. J Biol Chem. 1972 Jan 10;247(1):60–65. [PubMed] [Google Scholar]
- Fee J. A., Phillips W. D. The behavior of holo- and apo-forms of bovine superoxide dismutase at low pH. Biochim Biophys Acta. 1975 Nov 18;412(1):26–38. doi: 10.1016/0005-2795(75)90336-0. [DOI] [PubMed] [Google Scholar]
- Goldberg M., Pecht I. Fluorescence enhancement of laccase induced by reduction of Cu(II) sites. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4684–4687. doi: 10.1073/pnas.71.12.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani M. T., Morpurgo L., Rotilio G., Mondovì B. Selective removal of type 2 copper from Rhus vernicifera laccase. FEBS Lett. 1976 Nov;70(1):87–90. doi: 10.1016/0014-5793(76)80732-6. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Mavelli I., Calabrese L., Agrò A. F., Rotilio G. A ferrocyanide charge-transfer complex of bovine superoxide dismutase. Relevance of the zinc imidazolate bond to the redox properties of the enzyme. Biochem Biophys Res Commun. 1976 May 17;70(2):607–614. doi: 10.1016/0006-291x(76)91091-3. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. R. Oxidation-reduction potentials of the electron acceptors in laccases and stellacyanin. Biochim Biophys Acta. 1972 Aug 17;275(2):245–259. doi: 10.1016/0005-2728(72)90045-x. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. R., Vänngård T. I. The electron-accepting sites in Rhus vernicifera laccase as studied by anaerobic oxidation-reduction titrations. Eur J Biochem. 1971 Feb;18(4):463–468. doi: 10.1111/j.1432-1033.1971.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Reinhammar B., Oda Y. Spectroscopic and catalytic properties of Rhus vernicifera laccase depleted in type 2 copper. J Inorg Biochem. 1979 Oct;11(2):115–127. doi: 10.1016/s0162-0134(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Agrò A. F., Calabrese L., Bossa F., Guerrieri P., Mondovì B. Studies of the metal sites of copper proteins. Ligands of copper in hemocuprein. Biochemistry. 1971 Feb 16;10(4):616–621. doi: 10.1021/bi00780a011. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Morpurgo L., Calabrese L., Mondovì B. On the mechanism of superoxide dismutase. Reaction of the bovine enzyme with hydrogen peroxide and ferrocyanide. Biochim Biophys Acta. 1973 Apr 12;302(2):229–235. doi: 10.1016/0005-2744(73)90151-4. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Dooley D. M., Wang R. H., Gray H. B., Credonio M., Mogno F., Romani G. L. Letter: Susceptibility studies of laccase and oxyhemocyanin using an ultrasensitive magnetometer. Antiferromagnetic behavior of the type 3 copper in Rhus laccase. J Am Chem Soc. 1976 Feb 18;98(4):1029–1031. doi: 10.1021/ja00420a035. [DOI] [PubMed] [Google Scholar]


