Highlights
-
•
The diagnosis relies on cerebrospinal fluid tests, which include molecular polymerase chain reaction.
-
•
It is important to search for additional sites of tuberculosis.
-
•
The lack of pleocytosis in the cerebrospinal fluid does not rule out this diagnosis.
Keywords: Meningitis, Mycobacterium tuberculosis, Absence of pleocytosis, CSF
Abstract
We report a case of tuberculous meningitis without pleocytosis of the cerebrospinal fluid (CSF) in a 27-year-old patient admitted for a meningeal syndrome with signs of basilar involvement and an infectious syndrome associated with a hacking cough with whitish sputum and night sweats, evolving for 15 days before her admission, in a context of weight loss of 2 kg, asthenia, and anorexia. Cytobacteriological and chemical analysis of the CSF revealed less than 3 cells/mm3 white blood cells, high protein levels of 2.54 g/l, and low glucose levels of 0.08 g/l. Molecular polymerase chain reaction testing of the CSF isolated Mycobacterium tuberculosis DNA. Cerebral magnetic resource imaging revealed multiple intra-axial lesions above and below the tentorial level. The hematologic analysis showed a white blood cell count of 8800/mm3 with lymphopenia of 1360/mm3, platelets 453,000/mm3, and C-reactive protein 17.4 mg/l. HIV-1 and -2 serology, anti-DNA, and anti-nuclear antibodies were negative; serum protein electrophoresis did not reveal polyclonal hypergammaglobulinemia. The lack of CSF pleocytosis in tuberculous meningitis should not rule out this diagnosis in immunocompetent patients.
Introduction
The standard white blood cell count in cerebrospinal fluid (CSF) from adults is normally between 0 and 5 × 106 cells/l as reported by Tunkel [1]. Erdem et al. [2] stated that the presence of CSF pleocytosis is crucial in confirming a diagnosis of meningitis. Therefore, the absence of CSF pleocytosis can pose a diagnostic challenge for clinicians, especially in cases of suspected meningitis, such as tuberculous meningitis in immunocompetent patients. We report a case of tuberculous meningitis in a patient with no history of immunosuppression, where CSF pleocytosis was absent.
Case presentation
Patient EI, 27 years old, with no known medical history, was admitted due to severe headache, vomiting, cough with whitish sputum, and night sweats. The patient also had a fever of 39°C, 2-kg weight loss, weakness, and loss of appetite persisting for 15 days before admission. During neurologic examination, signs of meningeal stiffness, convergent strabismus, Kernig's sign, and Brudzinski's sign were observed.
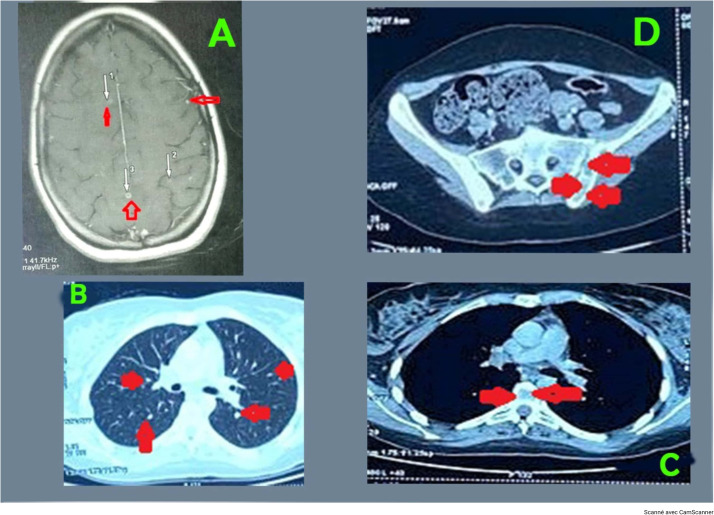
The brain magnetic resource imaging (MRI) revealed multiple intra-axial supratentorial and subtentorial infra centimetric lesions (Figure 1a). The CSF obtained through a lumbar puncture was clear. The analysis of the CSF showed a low white blood cell count (less than 3 cells/mm3), high albumin levels (2.54 g/l), and low glucose levels (0.08 g/l), consistent with a diagnosis of acellular meningitis. In addition, Mycobacterium tuberculosis DNA without rifampicin resistance was detected in the CSF using molecular polymerase chain reaction, confirming the diagnosis of tuberculous meningitis.
Figure 1.
(a) The brain magnetic resource imaging revealed multiple intra-axial supratentorial infra-centimetric lesions; (b) an injected chest computed tomography scan showed symmetrical micronodular opacities in both lungs resembling millet seeds; (c) a computed tomography scan of the thorax (without contrast medium) revealed osteolytic lesions of the vertebral body of D11 surrounded by an osteocondensing border graded Lodwick 1A; (d) osteolytic lesions of the sacroiliac joint in the abdominopelvic region (abdominopelvic computed tomography scan).
The patient underwent morphologic and microbiologic examinations for tuberculosis in other parts of the body. A thorax, abdomen, and pelvis computed tomography scan revealed symmetrical micronodular opacities in both lungs resembling millet seeds (Figure 1b). In addition, osteolytic lesions were observed at the 11th dorsal vertebra (Figure 1c) and the sacroiliac joint (Figure 1d).
GeneXpert (molecular testing) of the sputum confirmed the presence of M. tuberculosis DNA without rifampicin resistance, indicating pulmonary tuberculosis.
Biological analyses were conducted: hemoglobin 11.3 g/dl, white blood cells 8800/mm3, neutrophils 6860/mm3, lymphopenia 1360/mm3, platelets 453,000/mm3, C-reactive protein 17.4 mg/l, lactate dehydrogenase 390 IU/l, creatinine 5.4 mg/l, estimated glomerular filtration rate 60 ml/min, and urea 0.10 g/l.
The patient underwent an immunologic assessment to rule out immunosuppression, specifically testing for HIV-1 and -2 serologies, anti-DNA (less than 80), and anti-nuclear antibodies (less than 10), all of which yielded negative results. In addition, serum protein electrophoresis showed no signs of polyclonal hypergammaglobulinemia (16%), which could indicate an autoimmune disease. Furthermore, an hemoglobin A1c level of 5.6% ruled out diabetes mellitus.
Antibacillary chemotherapy, which includes isoniazid, rifampicin, ethambutol, and pyrazinamide, is combined with methylprednisolone corticosteroid therapy at a bolus dose of 500 mg, adjuvant treatment, and a low-salt diet.
The CSF was examined during a follow-up lumbar puncture on the 8th day of antibacterial treatment. The results showed acellular meningitis with low white blood cell count, high protein, and low glucose levels. After 10 days of treatment, the patient showed good progress and was discharged.
Discussion
Denkinger et al. [3] and Khanna et al. [4] described the diverse clinical manifestations of tuberculous meningitis. These include headache, vomiting, meningeal signs, focal deficits, loss of vision, paralysis of the cranial nerves (with the sixth cranial nerve most often affected, leading to convergent strabismus), and signs of intracranial hypertension.
Karandanis et al. [5] suggested that cytologic, bacteriologic, and chemical examination of CSF is crucial for an early diagnosis of tuberculous meningitis, characterized by lymphocytic pleocytosis, high protein levels, and low glucose levels.
Hakim et al. [6] found that meningitis without pleocytosis is quite common in severely immunocompromised patients with HIV with clusters of differentiation 4 counts below 50 cells/mm3 and confirmed tuberculous meningitis. Scriven et al. [7] observed acellular meningitis in patients with HIV with cryptococcal meningitis. Thao et al. [8] explained the absence of pleocytosis in tuberculous meningitis by the level of immunosuppression affecting the intracerebral inflammatory process. This pathophysiology is also reported in cryptococcal meningitis [7].
Acellular meningitis is rare in bacterial infections but more common in viral infections in individuals with healthy immune systems [2].
In a multicenter study by Erdem et al. [9] in less endemic countries, tuberculous meningitis was associated with an absence of pleocytosis observed in non-immunodeficient individuals. Based on the literature review, the lack of CSF pleocytosis in cases of tuberculous meningitis is rarely reported in Morocco and Africa. The most reliable way to confirm the presence of M. tuberculosis in CSF is through microbiological analysis [6].
Ozates et al. [10] demonstrated that the molecular polymerase chain reaction test of CSF is an effective method for diagnosing tuberculous meningitis. This molecular test detects the genetic material of M. tuberculosis and determines resistance to rifampin, with a sensitivity of 81% and a specificity of 98% [10].
Garg et al. [11] showed that brain computed tomography and MRI are essential for diagnosing tuberculosis due to their high sensitivity.
Subedi et al. [12] described the MRI images characteristic of tuberculous meningitis, such as the presence of nodules above and below the tentorial region, surrounded by areas of T2 and fluid-attenuated inversion recovery hypersignal associated with perilesional edema, as non-specific. However, similar images may be seen in other diseases in immunocompromised patients, such as cryptococcal meningitis and viral encephalitis [12]. Marcus et al. [13] also identified supratentorial and subtentorial lesions in their study of patients with HIV admitted for cerebral toxoplasmosis. Cerebral tuberculous miliary, often associated with tuberculous meningitis, presents as tiny, hyperintense T2 hypersignal foci [4].
Houston et al. [14] discovered that disseminated and miliary tuberculosis occur less frequently in immunocompetent individuals than seropositive patients with a low clusters of differentiation 4 count. In addition, de Vuyst et al. [15] observed that sacroiliitis localization of tuberculosis is rare in immunocompetent individuals, accounting for only 10% of osteoarticular tuberculous cases.
Conclusion
The absence of an increased number of white blood cells in the CSF during tuberculous meningitis should not rule out this diagnosis in an immunocompetent patient who exhibits symptoms of meningitis and has clinical, biological, and radiologic indications of tuberculosis. Further research is necessary to comprehend why tuberculous meningitis may occur without an elevated white blood cell count in immunocompetent patients.
Declarations of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-p or not-for-profit sectors.
Ethical approval statement
The patient was informed of the study's purpose, and her written consent was obtained before the study began. She requested that her name be used as an acronym.
Author contributions
Kamena Mwana Yile Hassan contributed to the conception and writing of the clinical case.
Kamena Mwana Yile and Khadija Rida contributed to interpreting the MRI images.
Kamena Mwana Yile Hassan, Fatima Ihbibane and El Filali Kamal Marhoum contributed to interpreting the MRI images and corrected the manuscript.
References
- 1.Tunkel AR. In: Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Bennett JE, Dolin R, Blaser MJ, editors. Elsevier; Philadelphia: 2015. Approach to the patient with central nervous system infection; pp. 1091–1096. [Google Scholar]
- 2.Erdem H, Ozturk-Engin D, Cag Y, Senbayrak S, Inan A, Kazak E, et al. Central nervous system infections in the absence of cerebrospinal fluid pleocytosis. Int J Infect Dis. 2017;65:107–109. doi: 10.1016/j.ijid.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: A systematic review and meta-analysis. Eur Respir J. 2014;44:435–446. doi: 10.1183/09031936.00007814. [DOI] [PubMed] [Google Scholar]
- 4.Khanna SR, Kralovic SM, Prakash R. Tuberculous meningitis in an immunocompetent host: a case report. Am J Case Rep. 2016;17:977–981. doi: 10.12659/ajcr.900762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karandanis D, Shulman JA. Recent survey of infectious meningitis in adults: review of laboratory findings in bacterial, tuberculous, and aseptic meningitis. South Med J. 1976;69:449–457. doi: 10.1097/00007611-197604000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Hakim JG, Gangaidzo IT, Heyderman RS, Mielke J, Mushangi E, Taziwa A, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000;14:1401–1407. doi: 10.1097/00002030-200007070-00013. [DOI] [PubMed] [Google Scholar]
- 7.Scriven JE, Rhein J, Hullsiek KH, Von Hohenberg M, Linder G, Rolfes MA, et al. Early ART after cryptococcal meningitis is associated with cerebrospinal fluid pleocytosis and macrophage activation in a multisite randomized trial. J Infect Dis. 2015;212:769–778. doi: 10.1093/infdis/jiv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thao LTP, Heemskerk AD, Geskus RB, Mai NTH, Ha DTM, Chau TTH, et al. Prognostic models for 9-month mortality in tuberculous meningitis. Clin Infect Dis. 2017;66:523–532. doi: 10.1093/cid/cix849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdem H, Ozturk-Engin D, Tireli H, et al. Hamsi scoring in the prediction of unfavorable outcomes from tuberculous meningitis: results of the Haydarpasa-II study. J Neurol. 2015;262:890–898. doi: 10.1007/s00415-015-7651-5. [DOI] [PubMed] [Google Scholar]
- 10.Ozates M, Kemaloglu S, Gurkan F, Ozkan U, Hoşoglu S, Simşek MM. CT of the brain in tuberculous meningitis, a review of 289 patients. Acta Radiol. 2000;41:13–17. doi: 10.1034/j.1600-0455.2000.041001013.x. [DOI] [PubMed] [Google Scholar]
- 11.Garg RK, Malhotra HS, Jain A. Neuroimaging in tuberculous meningitis. Neurol India. 2016;64:219–227. doi: 10.4103/0028-3886.177608. [DOI] [PubMed] [Google Scholar]
- 12.Subedi RC, Acharya S, Adhikari A, Banmala S, Shiwakoti TK, Karki P, et al. Disseminated tuberculosis in an immunocompetent woman from the Himalayan region of Nepal: a case report. Clin Case Rep. 2023;11:e7754. doi: 10.1002/ccr3.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus C, Feizi P, Hogg J, Summerfield H, Castellani R, Sriwastava S, et al. Imaging in differentiating cerebral toxoplasmosis and primary CNS lymphoma with special focus on FDG PET/CT. AJR Am J Roentgenol. 2021;216:157–164. doi: 10.2214/AJR.19.22629. [DOI] [PubMed] [Google Scholar]
- 14.Houston A, Macallan DC. Extrapulmonary tuberculosis. Medicine. 2014;42:18–22. doi: 10.1016/j.mpmed.2013.10.008. [DOI] [Google Scholar]
- 15.Vuyst de D, Vanhoenacker F, Gielen J, Bernaerts A, Schepper de AM. Imaging features of musculoskeletal tuberculosis. Eur Radiol. 2003;13:1809–1819. doi: 10.1007/s00330-002-1609-6. [DOI] [PubMed] [Google Scholar]