Abstract
Docetaxel is metabolized by cytochrome P450 3A4 (CYP3A4), and is transported by organic anion transporting peptides (OATPs) and ABCB1, and its blood concentration is known to affect the risk of some docetaxel-related adverse drug reactions (ADRs). Thus, the concomitant use of docetaxel with drugs that inhibit or induce these transporters or CYP3A4 requires careful attention. A 58-year-old woman was receiving clarithromycin (400 mg twice daily), rifampicin (450 mg once daily) and ethambutol (500 mg once daily) for nontuberculous mycobacteriosis. The patient was diagnosed as having stage IV HER2-positive breast cancer, which was treated with a regimen of trastuzumab (8 mg/kg), pertuzumab (first dose: 840 mg; second dose onward: 420 mg) and docetaxel (75 mg/m2) every 3 weeks. To predict the risk of serious drug interactions with rifampicin and clarithromycin, the blood concentration of docetaxel was analyzed after administration of the first course. The docetaxel levels at 22 and 159 h after administration were 36.1 and 6.5 ng/ml, respectively, which were higher than previously reported data. In addition, the calculated elimination half-life of 55.7 h was ~3 times longer than previously reported data. Although the docetaxel level was high, the same dosage was used in subsequent courses because no serious ADRs were observed during the first course of therapy. After 4 months of chemotherapy, the patient received complete remission. In conclusion, concomitant use of rifampicin and clarithromycin may increase the blood concentration of docetaxel.
Keywords: docetaxel, rifampicin, clarithromycin, drug interaction, nontuberculous mycobacteriosis, therapeutic drug monitoring
Introduction
Docetaxel is a cytotoxic anticancer drug that promotes and stabilizes microtubule polymerization and inhibits cell division. It is used to treat many types of cancer, including breast, non-small cell lung, esophageal, and ovarian cancers (1). Docetaxel is taken up into liver cells by organic anion transporting peptides (OATP) 1B1 and OATP1B3 and is metabolized and inactivated mainly by cytochrome P450 3A4 (CYP3A4) (1). Docetaxel and its metabolites in liver cells are excreted into the bile mainly by ABCB1 and ABCC2(1). Therefore, concomitant use of drugs that affect the activity of these transporters or CYP3A4 may pose a significant risk of affecting the therapeutic effect of docetaxel.
Here, we report a case of breast cancer that was diagnosed and treated with docetaxel during rifampicin and clarithromycin treatment for nontuberculous mycobacteriosis (NTM). The recommended treatment for NTM is a combination of two or three antimicrobial agents selected according to the species and the susceptibility of the organism (2). The patient in this case required rifampicin and clarithromycin combination therapy for NTM and docetaxel, a key drug for breast cancer. Rifampicin is a CYP3A4 inducer and OATP inhibitor (3-6), and clarithromycin is a CYP3A4 inhibitor and ABCB1 inhibitor (3,7,8). Drug interactions involving rifampicin and clarithromycin were expected to cause unusual pharmacokinetics of docetaxel, which could lead to unexpected adverse drug reactions (ADRs) or decreased effectiveness of treatment.
We measured the blood concentration of docetaxel to ensure the efficacy and safety of docetaxel administration, and to investigate the effects of rifampicin and clarithromycin combination therapy on the pharmacokinetics of docetaxel.
Case report
A 58-year-old woman presented to Gunma University Hospital (Maebashi, Japan) with a mass in the left breast in 2016. She was 168 cm tall and weighed 52 kg at presentation, and her only complaint was awareness of the left breast mass. Based on needle biopsy findings, the patient was diagnosed with advanced cancer of the left breast (stage IV, scirrhous carcinoma; estrogen receptor: negative, progesterone receptor: negative, HER2: 3+). Laboratory values for renal and hepatic function at admission were all within normal limits: total bilirubin, 0.9 mg/dl; alanine transaminase, 19 U/l; and serum creatinine: 0.71 mg/dl. The patient had NTM and had been taking clarithromycin tablets 200 mg twice daily, rifampicin capsules 450 mg once daily, and ethambutol tablets 500 mg once daily for 1 year, as well as famotidine tablets 10 mg twice daily and loxoprofen tablets 60 mg for pain.
A triweekly regimen of docetaxel, trastuzumab, and pertuzumab was initiated on an outpatient basis to treat the breast cancer. The first course consisted of docetaxel (75 mg/m2), trastuzumab (8 mg/kg), and pertuzumab (840 mg) on day 1. From the second course, the pertuzumab dose was reduced to 420 mg according to the planned regimen. For the first 3 days of each course, 8 mg of dexamethasone was administered intravenously for prophylaxis against nausea. After consultation with the respiratory physician and breast surgeon, it was decided that clarithromycin tablets, rifampicin capsules, and ethambutol tablets for the treatment of NTM would be continued during treatment with anticancer agents. Because the effects of drug interactions involving clarithromycin and rifampicin on the pharmacokinetics of docetaxel are not known, pegfilgrastim 3.6 mg was administered on day 2 of the first course to account for the risk of neutropenia. The patient contracted influenza 7 days after the first dose of docetaxel, so a 7-day rest period was added after the end of the course. After the second course, anticancer agents and pegfilgrastim 3.6 mg were administered as scheduled. Neutropenia was not observed during the entire treatment course, and there were no signs of infection, except for the aforementioned infection with influenza virus and NTM. There were no grade 3 or higher ADRs according to the Common Terminology Criteria for Adverse Events (Table I) (9), and the cancer disappeared on imaging after the fourth courses, indicating a complete response. The patient was then switched to maintenance therapy with trastuzumab and pertuzumab. After 5 years of maintenance therapy, there were no signs of recurrence.
Table I.
CTCAE grade of ADRs at the start of each course.
| ADR (CTCAE grade) | ||||
|---|---|---|---|---|
| Course | Neutropeniaa | Alopecia | Dysgeusia | Dysesthesia |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 2 | 1 | 0 |
| 3 | 0 | 2 | 1 | 0 |
| 4 | 0 | 2 | 1 | 1 |
| 5 | 0 | 2 | 0 | 1 |
| 6 | 0 | 2 | 0 | 1 |
| 7 | 0 | 2 | 0 | 1 |
aSeverity under pegfilgrastim 3.6 mg administration. ADRs, adverse drug reactions; CTCAE, Common Terminology Criteria for Adverse Events (9).
Blood samples were taken 22 h after the first course of docetaxel administration, and blood levels of docetaxel, rifampicin, and clarithromycin were measured by ultra high-performance liquid chromatography-tandem mass spectrometry (ACQUITY UPLC-Xevo TQ MS). The blood concentrations of docetaxel at 22 and 159 h after its administration (i.e., in the elimination phase) were 36.1 and 6.5 ng/ml (Fig. 1), respectively, which are higher than previously reported (10). The half-life of docetaxel was calculated to be 55.4 h based on the blood concentration values at 22 and 159 h using the following formula:
Figure 1.
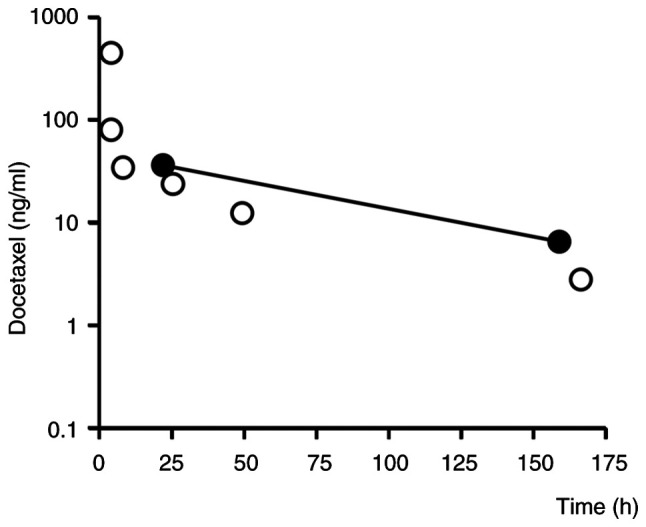
Blood concentration of docetaxel. The open circles are previously reported mean blood concentration data for patients who received the same dose of docetaxel (75 mg/m2) as the patient in this case (10). The closed circles are the values measured in this case.
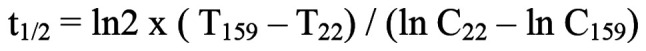 |
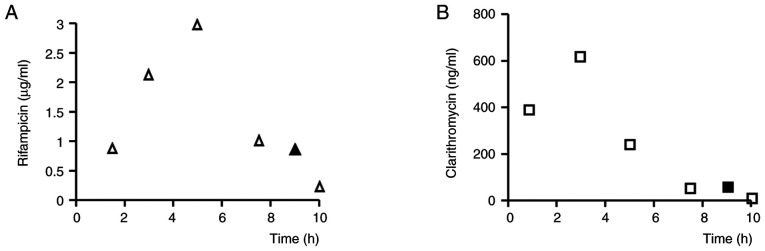
where T22 and T159 are the times at 22 and 159 h after administration and C22 and C159 are the corresponding docetaxel concentrations. The blood levels of rifampicin and clarithromycin were 0.75 µg/ml and 62 ng/ml, respectively, at 22 h after docetaxel administration, which was 9 h after oral administration of rifampicin and clarithromycin (Fig. 2).
Figure 2.
Blood concentrations of (A) rifampicin and (B) clarithromycin. (A) Open triangles are average data previously reported in patients receiving rifampicin (22). The closed triangle is the measured value in the present case. (B) Open squares are average data previously reported in patients receiving clarithromycin (22). The closed square is the measured value in the present case.
Discussion
Although the prevalence of NTM is low at approximately 6 per 100,000, the number of affected patients is reported to be increasing worldwide (11,12), and there is a possibility of that patients receiving rifampicin and clarithromycin will require administration of anticancer drugs as in the present case. Therefore, it is necessary to accumulate more information regarding the concomitant use of rifampicin and clarithromycin.
The measured blood concentration of docetaxel following administration of rifampicin and clarithromycin in our patient was clearly higher than that in typical patients who receive the same dose of docetaxel. The elimination half-life of docetaxel was also clearly longer and was calculated from blood concentrations at two time points to be 55.7 h, about three times longer than previously reported values (10). The distribution volume of docetaxel was 74 l/m2 (13), and the patient's body surface area was calculated to be 1.58 m2 using the Du Bois equation. Assuming that elimination follows a linear one-compartment model, the clearance of docetaxel was calculated from C22 and C159 using the following formula, and was found to be 1.45 l/h.
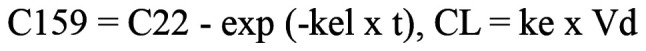 |
The docetaxel clearance in patients (n=40) who received docetaxel 75 mg/m2, as this patient did, was 30.0±14.2 l/h (mean ± SD) (10). The calculated clearance in this patient was more than 2 SD lower than the mean value in previously reported patients, and was considered to be significantly lower than the clearance in the general patient population.
More than 95% of the total metabolic clearance of docetaxel (22 l/h/m2) is via hepatic metabolism (13), so its metabolism may be affected by variations in hepatic clearance. Since the clearance reduction when docetaxel is combined with ketoconazole is reported to be 49% (14) and the CYP3A4 inhibitory activity of ketoconazole is considered to be 100%, the contribution of CYP3A4 to docetaxel metabolism is considered to be 49%. Akiyama et al (15) reported that the metabolism of midazolam, a substrate of CYP3A4, is enhanced in NTM patients receiving rifampicin and clarithromycin. CYP3A4 induction by rifampicin is due to transcriptional promotion via activation of the pregnane X receptor, and according to a report by Hisaka et al (16), it increases the clearance of midazolam about 8-fold. In contrast, CYP3A4 is inhibited by clarithromycin via an irreversible reaction that occurs when an intermediate metabolite of clarithromycin forms a complex with CYP3A4. This is called mechanism-based inhibition (MBI). Hisaka et al (16) reported that clarithromycin inhibits the clearance of midazolam to 20% of the original value. Since docetaxel and midazolam may differ in their reactivity to various enzymes, a definite answer cannot be given. However, based on the information about midazolam, it is possible to envision that the clearance increases about 8-fold due to the increase in the expression level of CYP3A4 caused by the action of rifampicin, but that the activity is inhibited to 20% of the original value due to MBI by clarithromycin. As a result, it is thought that the contribution of the increase in expression level by rifampicin is large, and the activity of CYP3A4 becomes about 1.6-fold larger. Further basic research is needed to examine this hypothesis. However, in our patient, factors other than CYP3A4 may have had a significant influence, since the increase in blood levels cannot be explained by CYP3A4-mediated drug-drug interactions alone.
OATP1B3 and ABCB1 are transporters that can affect the pharmacokinetics of docetaxel. Hu et al (17) reported a 2- to 3-fold increase in the area under the curve (AUC) of docetaxel by OATP1B knockout (KO) in an in vivo study using mice. Similarly, Iusuf et al (18) reported a 3-fold increase in the AUC of docetaxel in a study using OATP KO mice. Furthermore, clinical trials have found an increase in the AUC of docetaxel when sorafenib, an OATP1B1 inhibitor, was combined with docetaxel (19). When ritonavir, an inhibitor of both CYP3A4 and ABCB1, was concomitantly administered with docetaxel, the clearance of docetaxel decreased by more than 90% (20). These results suggest that metabolism by CYP3A4 and transport by OATP1B3 and ABCB1 have a major influence on docetaxel elimination. Like docetaxel, atorvastatin is a substrate for OATPs and CYP3A4, and concomitant use of rifampicin induces CYP3A4. Therefore, considering only the metabolism by CYP3A4, the AUC of atorvastatin would be expected to decrease by 80-90% when the two are combined (16). Clinically, however, the concomitant use of rifampicin has been reported to increase the AUC of atorvastatin in a dose-dependent manner, with the increase being as much as 10-fold (4,5). Moreover, although the effects of clarithromycin on the pharmacokinetics of docetaxel have not been reported, a clinical study evaluating the effect of clarithromycin on the pharmacokinetics of the ABCB1 substrates dabigatran and rivaroxaban found that clarithromycin increased the AUC of both drugs by approximately 2-fold (7). Therefore, a concern is that, in addition to inhibiting CYP3A4, clarithromycin may inhibit ABCB1 at clinical doses, potentially leading to a large decrease in docetaxel clearance.
Although the contributions of OATPs and ABCB1 to the hepatic clearance of docetaxel have not been determined, our findings suggest that the inhibitory effect of rifampicin on OATPs and that of clarithromycin on ABCB1 possibly reduced hepatic clearance.
Overall, CYP3A4 activity is likely to be elevated in patients concomitantly receiving rifampicin and clarithromycin. However, for docetaxel, which is transported by OATPs and ABCB1, the inhibition of both transporters by rifampicin and clarithromycin greatly reduces systemic clearance. In the same way that the metabolism of atorvastatin, a substrate of OATP and 3A4, is rate-limiting due to hepatic uptake by OATP (21), the rate-limiting factor for the metabolism of docetaxel is OATP rather than CYP, and OATP may have a greater influence on clearance. The blood levels of rifampicin and clarithromycin measured in our patient were similar to those reported for patients with NTM (22), indicating that her exposure to rifampicin and clarithromycin was typical NTM patients treated with these drugs.
A limitation of this study is that the metabolic capacity of docetaxel in this patient was not determined in the absence of rifampicin and clarithromycin, and the possibility that the patient's basal metabolic capacity is atypical cannot be ruled out. The attending physician determined that rifampicin and clarithromycin could not be discontinued as treatment for NTM, so all of this patient's docetaxel treatments were administered with the concomitant use of rifampicin and clarithromycin. Therefore, it is important to evaluate the effects of rifampicin and clarithromycin combination therapy on the pharmacokinetics of docetaxel in a larger number of similar cases, and basic research data are also needed in order to support pharmacokinetic studies. Furthermore, the mechanism of this drug-drug interaction could not be understood from the data in this case. Therefore, in the future, it is hoped that basic research will be conducted on the pharmacokinetics of docetaxel when used in combination with rifampicin and clarithromycin. Specifically, studies are needed to clarify the differences in the influence of docetaxel as a substrate for CYP3A4, OATP, and ABCB1 depending on the presence or absence of rifampicin and clarithromycin, and in vivo studies are needed to comprehensively evaluate these effects.
In conclusion, this case demonstrated that the elimination of docetaxel is likely to be delayed in cases with blood concentrations of rifampicin and clarithromycin commonly seen in the treatment of NTM. In cases of cancer treated with docetaxel where combination therapy with clarithromycin and rifampicin is also required, it is desirable to monitor the blood concentration of docetaxel, given that the blood concentration of docetaxel in individual patients may vary widely due to complex drug interactions.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
HY, TA, YI, SO, DN, KO, JH and KY participated in the design of the study and reviewed the results. JH, HY, TA, YI and SO made the diagnosis, and discussed this case and carried out the acquisition of data. HY, TA and DN analyzed serum concentrations. HY, TA, DN and KY were responsible for the calculation of pharmacokinetic parameters. HY, TA and KY drafted the manuscript. All authors helped to draft the manuscript, and read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent as obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nieuweboer AJ, de Morrée ES, de Graan AJ, Sparreboom A, de Wit R, Mathijssen RH. Inter-patient variability in docetaxel pharmacokinetics: A review. Cancer Treat Rev. 2015;41:605–613. doi: 10.1016/j.ctrv.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin Infect Dis. 2020;71:e1–e36. doi: 10.1183/13993003.00535-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9:310–322. doi: 10.2174/138920008784220664. [DOI] [PubMed] [Google Scholar]
- 4.Mori D, Kimoto E, Rago B, Kondo Y, King-Ahmad A, Ramanathan R, Wood LS, Johnson JG, Le VH, Vourvahis M, et al. Dose-Dependent Inhibition of OATP1B by Rifampicin in Healthy Volunteers: Comprehensive Evaluation of Candidate Biomarkers and OATP1B Probe Drugs. Clin Pharmacol Ther. 2020;107:1004–1013. doi: 10.1002/cpt.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda K. Organic anion transporting polypeptide (OATP)1B1 and OATP1B3 as important regulators of the pharmacokinetics of substrate drugs. Biol Pharm Bull. 2015;38:155–168. doi: 10.1248/bpb.b14-00767. [DOI] [PubMed] [Google Scholar]
- 6.Bolleddula J, Gopalakrishnan S, Hu P, Dong J, Venkatakrishnan K. Alternatives to rifampicin: A review and perspectives on the choice of strong CYP3A inducers for clinical drug-drug interaction studies. Clin Transl Sci. 2022;15:2075–2095. doi: 10.1111/cts.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouin-Thibault I, Delavenne X, Blanchard A, Siguret V, Salem JE, Narjoz C, Gaussem P, Beaune P, Funck-Brentano C, Azizi M, et al. Interindividual variability in dabigatran and rivaroxaban exposure: Contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J Thromb Haemost. 2017;15:273–283. doi: 10.1111/jth.13577. [DOI] [PubMed] [Google Scholar]
- 8.Gessner A, König J, Fromm MF. Clinical aspects of transporter-mediated drug-drug interactions. Clin Pharmacol Ther. 2019;105:1386–1394. doi: 10.1002/cpt.1360. [DOI] [PubMed] [Google Scholar]
- 9. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf, 2017. [Google Scholar]
- 10.Baker SD, Li J, ten Tije AJ, Figg WD, Graveland W, Verweij J, Sparreboom A. Relationship of systemic exposure to unbound docetaxel and neutropenia. Clin Pharmacol Ther. 2005;77:43–53. doi: 10.1016/j.clpt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Sharma SK, Upadhyay V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J Med Res. 2020;152:185–226. doi: 10.4103/ijmr.IJMR_902_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namkoong H, Kurashima A, Morimoto K, Hoshino Y, Hasegawa N, Ato M, Mitarai S. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect Dis. 2016;22:1116–1117. doi: 10.3201/eid2206.151086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke SJ, Rivory LP. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet. 1999;36:99–114. doi: 10.2165/00003088-199936020-00002. [DOI] [PubMed] [Google Scholar]
- 14.Engels FK, Ten Tije AJ, Baker SD, Lee CK, Loos WJ, Vulto AG, Verweij J, Sparreboom A. Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther. 2004;75:448–454. doi: 10.1016/j.clpt.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Akiyama N, Inui N, Mori K, Nakamura Y, Hayakawa H, Tanaka S, Uchida S, Namiki N, Watanabe H, Suda T. Effect of rifampicin and clarithromycin on the CYP3A activity in patients with Mycobacterium avium complex. J Thorac Dis. 2019;11:3814–3821. doi: 10.21037/jtd.2019.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hisaka A, Ohno Y, Yamamoto T, Suzuki H. Prediction of pharmacokinetic drug-drug interaction caused by changes in cytochrome P450 activity using in vivo information. Pharmacol Ther. 2010;125:230–248. doi: 10.1016/j.pharmthera.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Hu S, Mathijssen RHJ, de Bruijn P, Baker SD, Sparreboom A. Inhibition of OATP1B1 by tyrosine kinase inhibitors: In vitro-in vivo correlations. Br J Cancer. 2017;117(e3) doi: 10.1038/bjc.2013.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iusuf D, Hendrikx JJ, van Esch A, van de Steeg E, Wagenaar E, Rosing H, Beijnen JH, Schinkel AH. Human OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and clearance of docetaxel. Int J Cancer. 2015;136:225–233. doi: 10.1002/ijc.28970. [DOI] [PubMed] [Google Scholar]
- 19.Awada A, Hendlisz A, Christensen O, Lathia CD, Bartholomeus S, Lebrun F, de Valeriola D, Brendel E, Radtke M, Delaunoit T, et al. Phase I trial to investigate the safety, pharmacokinetics and efficacy of sorafenib combined with docetaxel in patients with advanced refractory solid tumours. Eur J Cancer. 2012;48:465–474. doi: 10.1016/j.ejca.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Koolen SL, Oostendorp RL, Beijnen JH, Schellens JH, Huitema AD. Population pharmacokinetics of intravenously and orally administered docetaxel with or without co-administration of ritonavir in patients with advanced cancer. Br J Clin Pharmacol. 2010;69:465–474. doi: 10.1111/j.1365-2125.2010.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshikado T, Yoshida K, Kotani N, Nakada T, Asaumi R, Toshimoto K, Maeda K, Kusuhara H, Sugiyama Y. Quantitative analyses of hepatic OATP-mediated interactions between statins and inhibitors using PBPK modeling with a parameter optimization method. Clin Pharmacol Ther. 2016;100:513–523. doi: 10.1002/cpt.391. [DOI] [PubMed] [Google Scholar]
- 22.Alffenaar JW, Nienhuis WA, de Velde F, Zuur AT, Wessels AM, Almeida D, Grosset J, Adjei O, Uges DR, van der Werf TS. Pharmacokinetics of rifampin and clarithromycin in patients treated for Mycobacterium ulcerans infection. Antimicrob Agents Chemother. 2010;54:3878–3883. doi: 10.1128/AAC.00099-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.