Abstract
Aneurysms in the V1 segment of the extracranial vertebral artery are extremely rare. Furthermore, half of the cases are giant aneurysms larger than 25 mm. This study reports a case of unruptured giant V1 aneurysm of the right vertebral artery that was successfully treated with endovascular coil embolization. An 85-year-old woman experienced dizziness sporadically. Magnetic resonance imaging demonstrated a mass in the right subclavian region. Radiological examinations showed an aneurysm originating from the V1 segment of the right vertebral artery. The aneurysm was successfully embolized with platinum coils, with preserved patency of the vertebral artery. Although aneurysms in the V1 portion of the vertebral artery are extremely rare, there is a possibility of thrombosis or rupture. Endovascular treatment such as coil embolization may be effective for a V1 vertebral artery aneurysm.
Keywords: aneurysm, V1, vertebral artery, giant, coil embolization
Introduction
Extracranial vertebral artery (VA) aneurysms are rare, with a reported incidence of 1% among VA aneurysms and 0.5% among all aneurysms.1) Furthermore, giant aneurysms of the extracranial VA exceeding 25 mm in size are very rare.2) The VA has four segments, and fusiform or saccular aneurysms sometimes develops in the intracranial V4 portion of VA. V1-3 segments of VA are located outside the cranium. Among the V1-3 segments, aneurysms are the most frequently observed in the V3 segment, followed by V1.1) To the best of our knowledge, only 10 cases of aneurysms in V1 of VA have been reported, including our case.3) The etiology, characteristics, natural course, and management of aneurysms at this location have not been fully clarified. This study thus represents a case with an unruptured giant V1 aneurysm of VA that was successfully treated by coil embolization. Furthermore, the etiology, clinical characteristics, and treatment of V1 aneurysms along with a literature review are discussed.
Case Presentation
An 85-year-old woman with hypertension and hyperlipidemia occasionally experienced dizziness for more than 10 years and visited a local hospital. Magnetic resonance imaging (MRI) showed an abnormal mass lesion in the right subclavian region. An aneurysm or tumor was initially suspected (Fig. 1). MR angiography (MRA) demonstrated a patent right VA and subclavian artery. However, the origin of the lesion was unclear. The patient was referred to our department for further examination. Repeated MRI and computed tomographic angiography (CTA) demonstrated an aneurysmal lesion. The signal intensity of the lesion was not the same for neighboring arteries due to slow blood flow in the giant aneurysm. However, the origin of the aneurysm was unclear due to its large size (Fig. 2a, b, c). MRA and CTA showed no other abnormalities, such as an aneurysm in the thoracic or abdominal aorta. Angiography showed that the giant aneurysm originated in the V1 portion of the right VA. The diameter of the aneurysm was approximately 30 mm, and its neck was 7.6 mm. The diameter of the parent VA was 2.5-3.5 mm, and the proximal VA was tortuous (Fig. 2d, e). An aneurysm at this location has the potential to rupture or demonstrate thrombus formation as a natural course. Furthermore, the patient urged to receive treatment. Therefore, endovascular coil embolization of the aneurysm with or without stent placement was planned.
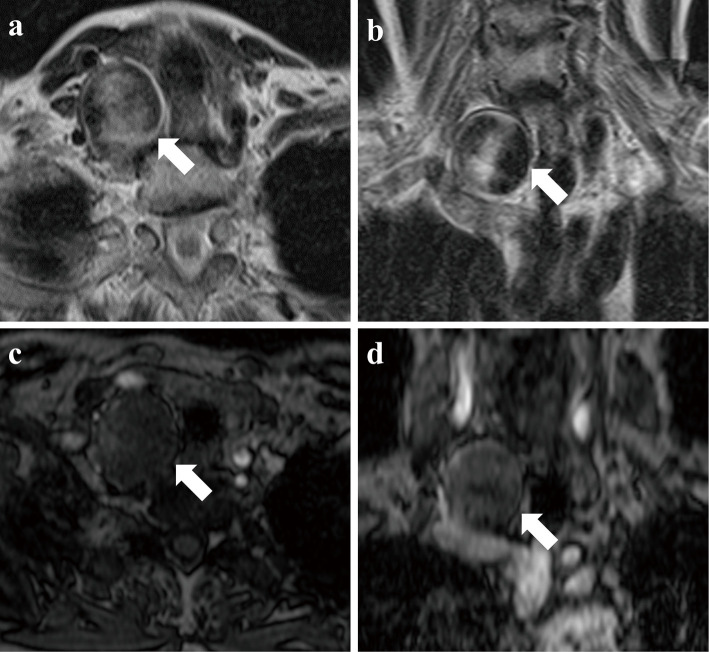
Fig. 1.
T2-weighted axial (a) and coronal (b) MR images showing an abnormal mass lesion (arrow) in the right subclavian region. The original axial (c) and coronal (d) images of MRA. The intensity of the lesion (arrow) is low compared with the surrounding arteries.
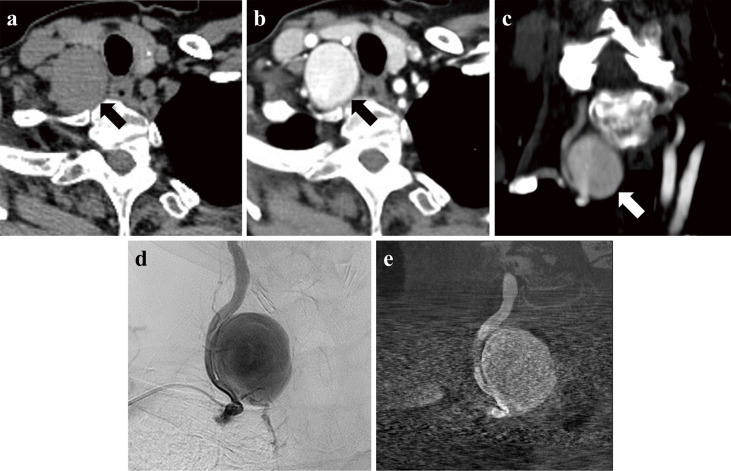
Fig. 2.
Axial images of plain computed tomography demonstrating a mass lesion (arrow) (a). Axial (b) and coronal (c) images of a computed tomographic angiogram demonstrating a contrast-enhanced aneurysmal lesion (arrow). (d) Pre-embolization angiography showing the giant aneurysm originating from the V1 portion of the right VA. (e) Vaso CT. The diameter of the aneurysm is about 30 mm, and its neck is 7.6 mm.
Transoral administration of 100 mg of aspirin and 3.75 mg of prasugrel hydrochloride was started 7 days prior to endovascular treatment. The procedures were performed under general anesthesia. A 4F sheath was introduced via the right radial artery. Next, the 4F sheath was changed to a 6F guiding sheath with balloon (FUBUKI, ASAHI INTECC, Japan) and was advanced to the right subclavian artery. A microcatheter (HeadwayPlus21 2-2.5Fr, TERUMO, Japan) was advanced to the cervical portion of the right VA distal to the aneurysm with the aid of a microguidewire (SynchroSELECT, Stryker, USA) in the case of stent insertion. Then, another microcatheter for coil delivery (1.7Fr SL-10, Stryker, USA) was advanced to the aneurysm, and the tip of microcatheter was positioned on the distal side of the aneurysm cavity (Fig. 3a). Initially, a platinum coil (22 × 500 mm, AXIUM PRIME FC3D coil, Medtronic, Ireland) was inserted into the aneurysm as a frame (Fig. 3b). Next, platinum coils were added, and a total of 30 coils were inserted into the aneurysm. Angiography showed almost complete occlusion of the aneurysm cavity with low-level blood flow in the aneurysm neck. The parent VA was confirmed to be patent, although it showed mild stenosis (Fig. 3c, d). Therefore, no stent was added. Ultrasound sonography performed on the day after embolization showed mild blood flow between the coil cast and aneurysm wall near the aneurysm neck. Postoperative MRI on the 2nd and CTA on the 7th day showed obliteration of the aneurysm dome and patency of the parent VA. Her postoperative course was uneventful, and she was discharged on the 8th day without complications. MRI/A after 3 months demonstrated obliteration of the aneurysm dome and patency of the parent VA (Supplementary Figure 1). Transoral administration of prasugrel hydrochloride was ceased 4 months after endovascular treatment. The administration of aspirin will be continued at least 1 year after the treatment. To date, the patient has experienced no symptoms, including dizziness.
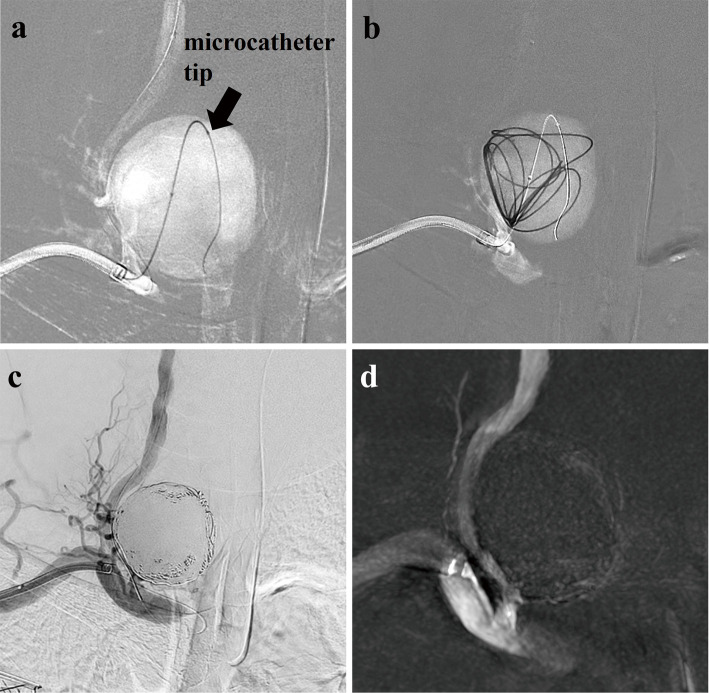
Fig. 3.
Fluoroscopic X-ray images showing that the tip of the microcatheter (arrow) is positioned on the distal side of the aneurysm cavity, (a) and the first coil is inserted into the aneurysm as a frame (b). Postembolization angiography (c) and Vaso CT (d) showing almost complete occlusion of the aneurysm cavity with low-level blood flow in the aneurysm neck.
Discussion
Aneurysms in the V1 segment of VA are very rare. To the best of our knowledge, only 10 cases were reported between 1996 and 2023, including the present case (Table 1). In addition, four other cases have been reported, but without detailed information.4) Among the 10 cases, at least six involved aneurysms with large diameters exceeding 25 mm. Aneurysms at this location tend toy to develop to a large size. This may be due to characteristics of the vascular structure or softness of the surrounding tissues.
Table 1.
Summary of reported cases with V1 aneurysms
| Case no. | Author (year) | Age (years)/Sex | Side | Dominancy | Location (segment) | Configuration | Size (mm) | Thrombosis | Symptoms | Ruptured or Unruptured | Endovascular Treatment | Followup Period | Outcome | Etiology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hiramatsu et al. (2007)[12] | 67/M | Left | Left | V1 | Saccular | 20×20 | NR | Dizziness | Unruptured | BTO→Trapping | 6 weeks | GR | NF-1 |
| 2 | Shang et al. (2013)[13] | 26/M | Right | NR | V1 | Saccular | 40 | NR | Arm and chest pain, arm paresthesias, pulsatile nontender mass | Unruptured | PAO (proximal VA) Covered stent (subclavian artery) |
1 month | GR | Unclear |
| 3 | Kollmann et al. (2016)[6] | 48/F | Left | NR | V1 | Saccular | 11×10 | NR | Massive hematoma of neck | Ruptured | Embolization (Coil and Onyx) | 1 year | GR | NR |
| 4 | Kiyohira et al. (2017)[7] | 59/F | Left | NR | V1 | Saccular | 42×38×48 | Yes (Partially) | Swelling | Unruptured | BTO→PAO (proximal VA) | 2 years | GR | NR |
| 5 | Strambo et al. (2017)[9] | 53/M | Left | NR | V1 | Fusiform | 40 | Yes (Eccentric) | Dysarthria, right side paresthesias, left limb ataxia | Unruptured | PAO (proximal VA)→Ligation of distal left VA (6 years after PAO) | 6 years (After PAO) 2 years (After ligation) |
NR | NF-1 |
| 6 | Zhang et al. (2018)[10] | 57/F | Left | Codominant | V1 | Fusiform | 18×15.4 | NR | No symptoms | Unruptured | Aneurysm resection, vascular graft reconstruction | 6 months | GR | NR |
| 7 | Fuga et al. (2021)[3] | 68/F | Left | Right | V1-V2 | Saccular | 47×58×47 | Yes (Partially) | Swelling and neck pain | Unruptured | Coil embolization | 1 year | GR | Idiopathic |
| 8 | Pataki et al. (2022)[1] | 74/M | Right | Right | V1 | Fusiform | 55×57 | Yes (Partially) | No symptoms | Unruptured | Stent grafts | 3 years | GR | Idiopathic |
| 9 | Topaloğlu et al. (2023)[14] | 35/F | Right | NR | V1 | Saccular | 75×65 | NR | Neck pain | Unruptured | Antiplatelet and immunosuppressive therapy | NR | NR | Behçet’s disease |
| 10 | Present case | 85/F | Right | Left | V1 | Saccular | 30 | No | Dizziness | Unruptured | Coil embolization | 3 months | GR | Idiopathic |
BTO: balloon test occlusion, F: female, GR: good recovery, M: male, NF-1: neurofibromatosis type 1, NR: not reported, PAO: parent artery occlusion, VA: vertebral artery
Extracranial VA aneurysms can be caused by trauma, vasculitis, atherosclerosis, dissection, or occur due to idiopathic reasons. They can also be induced by hereditary diseases such as Behçet's disease, Ehler-Danlos syndrome, and neurofibromatosis type 1. Among them, trauma is the most common cause of extracranial VA aneurysms.3,5) In cases of V1 aneurysms, reported definite etiologies were neurofibromatosis type 1 and Behçet's disease. Therefore, etiologies in most cases were unclear. Our patient had only hypertension and hyperlipidemia, with no history of collagen disease or congenital abnormalities. Therefore, the etiology was considered to be atherosclerosis.
Regarding symptoms, V1 aneurysms sometimes reportedly caused dizziness, paresthesia, ataxia, dysarthria, swelling, and pain of the arm, neck, and chest.3) Our patient occasionally felt dizziness for 10 years. If blood flow in VA decreases due to the aneurysm, dizziness may occur. However, the symptom was not persistent in our patient. Therefore, it was considered unlikely that the symptom of dizziness was caused by the V1 aneurysm. During the 3-month followup following embolization, the patient did not complain of any symptoms, including dizziness.
Among the 10 reported cases of V1 aneurysms, one case showed aneurysmal rupture and initially presented with massive hematoma in the neck.6) In the case of a ruptured V1 aneurysm, a massive hematoma may form in the neck, and airway obstruction may occur.7) The incidence of rupture of large intracranial aneurysms greater than 25 mm in diameter is about 25% within 1 year after diagnosis, about 63% after 2 years, and about 75% after 3 years.8) Although an aneurysm in V1 of VA is an unusual location, and only 1 in 10 cases rupture, a large aneurysm in V1 is associated with the possibility of rupture. In contrast, there is a possibility of thrombus formation in a large aneurysm due to stagnant blood flow. Thrombosed aneurysms not only increase the risk of rupture but also that of ischemic stroke. Four out of the 10 reported cases showed thrombus formation in the aneurysms (Table 1). Three lesions were partially thrombosed aneurysms and showed no ischemic symptom.1,3,7) One lesion was eccentrically thrombosed.9) The patient developed cerebellar infarction and showed neurological symptoms at both initial onset and recurrence. Cerebellar infarction was caused by embolic material from the thrombus in the aneurysm.
As mentioned, an aneurysm in V1 of VA is associated with the possibility of rupture or thrombus formation. Therefore, we decided to treat the V1 aneurysm in our patient. The treatment options for extracranial VA aneurysms include direct surgery and endovascular treatment. Among nine reported cases of V1 aneurysms, a case involving a ruptured aneurysm was treated by direct surgery, such as aneurysm resection and vascular graft reconstruction.10) Strambo et al.9) reported the case of a V1 aneurysm recanalized 6 years after endovascular coil embolization. In this case, the proximal VA was embolized by platinum coils to prevent thromboembolism from the thrombosed aneurysm as the initial treatment. The recurrent aneurysm was treated by ligation of the distal VA and excision of the coiled aneurysm.9) Neurosurgeons are not familiar with direct surgery involving the periclavicular region. In the case of direct surgery, collaboration with thoracic or vascular surgeons is necessary. We discussed the possible treatment of our patient with cardiovascular surgeons, and endovascular treatment was selected. Of nine reported cases, endovascular treatment alone was performed in seven. Regarding endovascular treatment, coil embolization with or without stent placement and proximal VA occlusion are additional options. If the vascular size and configuration are suitable, placement of a covered stent or flow diverter may be considered. In our case, the patient was treated by simple coil embolization. Although VA showed mild stenosis based on a protruded coil cast after coiling, blood flow was considered adequate. Therefore, no stent was placed. Due to the paucity of such cases, to the best of our knowledge, no clinical studies have made a comparison between simple coiling and stent-assisted coiling for extracranial aneurysms. In one clinical study of intracranial aneurysms, 62% (224/361) of patients were treated without, and 38% (137/361) were treated with a stent.11) According to the report, the incidence of total periprocedural serious adverse events was 4.5% with coil embolization alone and 6.6% with stent-assisted coil embolization. Likewise, the incidence of ischemic stroke was lower in cases with simple coil (2.2%) than stent-assisted coil embolization (8.8%).11) In our case, no stent was placed, and the treatment was appropriate for preventing postoperative ischemic stroke. Furthermore, no thrombus was observed on preoperative radiological examinations. Of nine reported cases, four had an intramural thrombus. If thrombus formation in an aneurysm is suspected, direct surgery, proximal VA occlusion including the aneurysm, or endovascular treatment with prevention of thromboembolism to the distal VA might be necessary. For endovascular treatment with preservation of VA, protection with a balloon or filter, or flow reversal, if possible, might be required. Gentle manipulation of coils or other materials is also mandatory.
Most giant intracranial aneurysms reopen partially following coil embolization because of coil compaction or migration.15) Furthermore, Dorfer et al.16) reported that the mechanism of aneurysm recurrence was coil compaction in seven cases in giant intracranial aneurysms. Of the seven cases, retreatment was done in the four cases by 2 months after coil embolization, one in 6 months, and two in 19 months.16) Therefore, although our case is an extracranial aneurysm, long-term followup is required.
Although neurosurgeons and neurologists pay little attention to the periclavicular region, there is a possibility that a rare extracranial aneurysm of VA may exist in this region. For a V1 aneurysm of VA, endovascular treatment might be one of the effective options.
In conclusion, although aneurisms in V1 of VA is extremely rare, it is associated with the possibility of rupture or thrombosis. Endovascular treatment such as coil embolization may be an effective option for unruptured giant V1 vertebral aneurysms.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of Interest Disclosure
There are no conflicts of interest to disclose.
Supplementary Material
Original axial images of MRI (a) and MRA (b). MRI/A conducted 3 months following embolization showing obliteration of the V1 aneurysm (arrow) and patency of the parent artery
References
- 1). Pataki Á, Nguyen DT, Nagy Z, Nardai S, Nemes B: Stent-graft treatment of a giant asymptomatic extracranial vertebral artery aneurysm: case report and literature review. Ann Vasc Surg 79: 442.e1-442.e6, 2022 [DOI] [PubMed] [Google Scholar]
- 2). Weir B, Disney L, Karrison T: Sizes of ruptured and unruptured aneurysms in relation to their sites and the ages of patients. J Neurosurg 96: 64-70, 2002 [DOI] [PubMed] [Google Scholar]
- 3). Fuga M, Tanaka T, Tachi R, et al. : Successful endovascular trapping for symptomatic thrombosed giant unruptured aneurysms of the V1 and V2 segments of the vertebral artery: case report and literature review. NMC Case Rep J 8: 681-690, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Morasch MD, Phade SV, Naughton P, Garcia-Toca M, Escobar G, Berguer R: Primary extracranial vertebral artery aneurysms. Ann Vasc Surg 27: 418-423, 2013 [DOI] [PubMed] [Google Scholar]
- 5). Muhammad S, Raj R, Numminen J, Niemelä M: Successful endovascular coil embolisation of a ruptured V1-segment vertebral artery dissecting aneurysm making a fistula with the adjacent vein. BMJ Case Rep 12: e229108, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Kollmann D, Kinstner C, Teleky K, et al. : Successful treatment of a ruptured extracranial vertebral artery aneurysm with onyx instillation. Ann Vasc Surg 36: 290.e7-290.e10, 2016 [DOI] [PubMed] [Google Scholar]
- 7). Kiyohira M, Ishihara H, Oku T, Kawano A, Oka F, Suzuki M: Successful endovascular treatment for thrombosed giant aneurysm of the V1 segment of the vertebral artery: a case report. Interv Neuroradiol 23: 628-631, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). UCAS Japan Investigators, Morita A, Kirino T, et al. : The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366: 2474-2482, 2012 [DOI] [PubMed] [Google Scholar]
- 9). Strambo D, Peruzzotti-Jametti L, Semerano A, et al. : Treatment challenges of a primary vertebral artery aneurysm causing recurrent ischemic strokes. Case Rep Neurol Med 2017: 2571630, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Zhang H, Zhao Y, Naha G, Hou C, Wang Z, Yang X: Successful management of extracranial vertebral artery aneurysm by artificial vessel reconstruction. World Neurosurg 116: 249-254, 2018 [DOI] [PubMed] [Google Scholar]
- 11). Hetts SW, Turk A, English JD, et al. : Stent-assisted coiling versus coiling alone in unruptured intracranial aneurysms in the matrix and platinum science trial: safety, efficacy, and mid-term outcomes. AJNR Am J Neuroradiol 35: 698-705, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Hiramatsu H, Negoro M, Hayakawa M, et al. : Extracranial vertebral artery aneurysm associated with neurofibromatosis type 1: a case report. Interv Neuroradiol 13: 90-93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Shang EK, Fairman RM, Foley PJ, Jackson BM: Endovascular treatment of a symptomatic extracranial vertebral artery aneurysm. J Vasc Surg 58: 1391-1393, 2013 [DOI] [PubMed] [Google Scholar]
- 14). Topaloğlu ÖF, Kılınçer A, Nayman A: Giant vertebral artery aneurysm in Behçet's disease: a rare case and review of the literature. Curr Med Imaging 19: 389-393, 2023 [DOI] [PubMed] [Google Scholar]
- 15). Sluzewski M, Menovsky T, van Rooij WJ, Wijnalda D: Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. Am J Neuroradiol 24: 257-262, 2003 [PMC free article] [PubMed] [Google Scholar]
- 16). Dorfer C, Gruber A, Standhardt H, Bavinzski G, Knosp E: Management of residual and recurrent aneurysms after initial endovascular treatment. Neurosurgery 70: 537-554, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Original axial images of MRI (a) and MRA (b). MRI/A conducted 3 months following embolization showing obliteration of the V1 aneurysm (arrow) and patency of the parent artery