Abstract
The yeast SWR1 complex catalyses the exchange of histone H2A–H2B dimers in nucleosomes, with Htz1–H2B dimers1–3. Here we used single-molecule analysis to demonstrate two-step double exchange of the two H2A–H2B dimers in a canonical yeast nucleosome with Htz1–H2B dimers, and showed that double exchange can be processive without release of the nucleosome from the SWR1 complex. Further analysis showed that bound nucleosomes flip between two states, with each presenting a different face, and hence histone dimer, to SWR1. The bound dwell time is longer when an H2A–H2B dimer is presented for exchange than when presented with an Htz1–H2B dimer. A hexasome intermediate in the reaction is bound to the SWR1 complex in a single orientation with the ‘empty’ site presented for dimer insertion. Cryo-electron microscopy analysis revealed different populations of complexes showing nucleosomes caught ‘flipping’ between different conformations without release, each placing a different dimer into position for exchange, with the Swc2 subunit having a key role in this process. Together, the data reveal a processive mechanism for double dimer exchange that explains how SWR1 can ‘proofread’ the dimer identities within nucleosomes.
Subject terms: Single-molecule biophysics, Cryoelectron microscopy, Enzyme mechanisms
Nucleosome flipping mediates a proofreading and processive mechanism of histone exchange driven by the chromatin remodeller SWR1.
Main
A canonical nucleosome contains two copies each of the four histones H2A, H2B, H3 and H4 around which approximately 147 bp of DNA are wrapped4. However, additional variants have been discovered for each of these histones and, when present, that have special roles in cells, such as at centromeres, and in processes including transcription and DNA repair5. Most of these variants are laid down into chromatin during replication, but an exception in yeast is the H2A histone variant Htz1 (H2A.Z in higher eukaryotes). Htz1 is specifically incorporated into nucleosomes by the SWR1 complex1–3. In humans, there are two large multi-subunit complexes that incorporate H2A.Z into nucleosomes, SRCAP6 and TIP60 (ref. 7), the latter complex also being able to acetylate histones, as well as other proteins, as a part of DNA damage signalling7. In addition to being signals of DNA damage, nucleosomes that contain Htz1 (or H2A.Z) also have a role in transcriptional regulation8.
SWR1 is a 14-subunit complex that is a member of the INO80 remodeller family9. Cryo-electron microscopy (cryo-EM) structures of INO80 and SWR1 complexes bound to nucleosomes have been reported10–12. Despite significant similarity between the complexes in terms of subunits and sequence homology, the two complexes engage with nucleosomes in a very different manner13. The ATPase domains of the INO80 subunit engage at superhelical location 6 (SHL6), whereas those of SWR1 are located at SHL2. Both complexes unwrap significant sections of DNA from the nucleosome, but this is stabilized by the motor domains of INO80 (refs. 10,11) and the Arp6–Swc6 subunits in SWR1 (ref. 12). These differences may relate to the differing activities of the two complexes because SWR1, unlike INO80, lacks the ability to slide nucleosomes14, although ATP-dependent DNA translocation within the context of the nucleosome wrap is required for activity14. Instead, SWR1 catalyses the ATP-dependent exchange of H2A–H2B dimers with those comprising Htz1–H2B1–3. The exchange takes place in a stepwise manner with both dimers being exchanged15. For canonical nucleosomes, SWR1 shows specificity for exchange of H2A–H2B dimers with Htz1–H2B and will not catalyse the reverse exchange under any conditions so far identified15,16. How this remarkable specificity is achieved is unknown, although sequence differences between the α2 helix of H2A and Htz1 probably contribute to this14. Acetylation of K56 on H3 in nucleosomes appears to reduce the specificity of histone exchange by interfering with Swc2 function17.
Single-molecule studies have begun to reveal aspects of the complex process of histone exchange. In the initial complex, when nucleosomes first bind to SWR1, the DNA wrap becomes dynamic with small, but rapid, unwrapping events in addition to the significant unwrapping by Arp6–Swc6 subunits12. However, to progress towards dimer exchange, a larger unwrapping occurs18–20, presumably to fully expose the dimer, although the nature and full extent of this unwrapping remain unclear, as well as which subunits contribute to this process. It is also unclear whether dimer exchange is a processive process, with both dimers exchanged in a nucleosome after a single SWR1-binding event, or is distributive with nucleosome release between dimer exchanges12,18,20. If histone exchange is processive, this would suggest a higher propensity for double-exchanged dimer nucleosomes than for single exchanges, although the significance of double-exchanged versus single-exchanged nucleosomes is also unknown.
Single-molecule histone exchange by SWR1
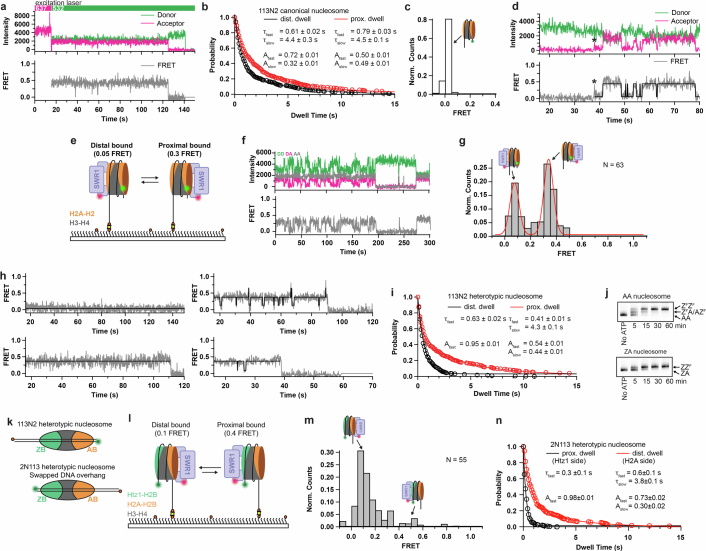
We have previously developed a fluorescence resonance energy transfer (FRET)-based assay to monitor histone exchange by SWR1 in bulk phase by monitoring loss of FRET when labelled H2A–H2B dimers are exchanged for unlabelled Htz1–H2B dimers16. We have adapted this methodology for single-molecule analysis by changing the dimer labelling so that a gain of FRET was observed when an unlabelled H2A–H2B dimer is exchanged for a labelled Htz1–H2B dimer (Fig. 1a). This makes interpretation of the data less ambiguous, because in a loss of FRET assay, it can be hard to distinguish between histone exchange and dye photobleaching19. This assay allows us to monitor two histone dimer exchanges on surface-immobilized nucleosomes as consecutive step increases in FRET (Fig. 1b and Extended Data Fig. 1). A histogram of the average FRET value between the first and second exchange reveals two possible intermediate states at approximately 0.6 and approximately 0.4 FRET (Fig. 1c), which corresponds to donor-proximal and donor-distal exchange, respectively. Control experiments in the presence of non-hydrolysable ATPγS analogue revealed no exchange (Extended Data Fig. 1). A dwell time analysis of the intermediate state (Fig. 1d) shows that the time between the first and the second exchange is τ2 = 246 ± 25 s, which is independent of the initial exchange (proximal or distal) and consistent with slow histone exchange observed in other single-molecule studies18,19 and in ensemble-averaged measurements2,15,16.
Fig. 1. Double-exchange events can be observed by smFRET.
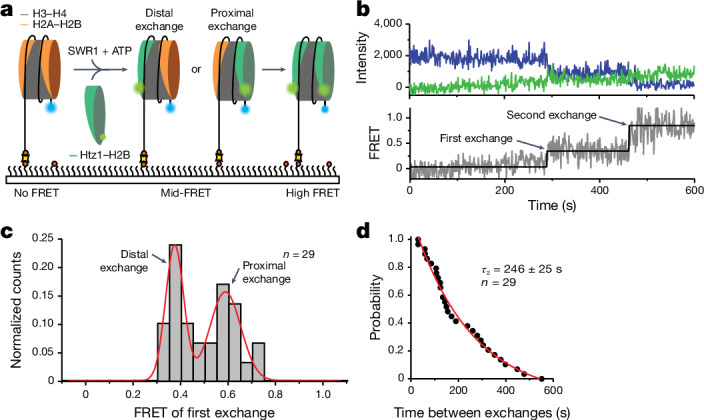
a, Schematic of the assay. Nucleosomes (113N2.AF488) labelled with AF488 (blue) on the short 2-bp overhang are surface immobilized on a PEGylated microscope slide. SWR1, ATP and AF555–Htz1–H2B dimers (green) are flowed in to start the exchange reaction. Histone exchange is detected as a FRET increase between AF488 and AF555. b, Intensity trajectory (top) and corresponding FRET trajectory (bottom) for a single nucleosome showing a stepwise gain in FRET signal following each dimer exchange. c, Idealized FRET histogram of the first-exchange event shows two approximately equal populations of approximately 0.4 and approximately 0.6 FRET corresponding to either dye-distal or dye-proximal exchange. d, Dwell time distribution between the first and second exchanges yields a second-exchange time τ2 = 246 ± 25 s. Reported errors are the error of the fit.
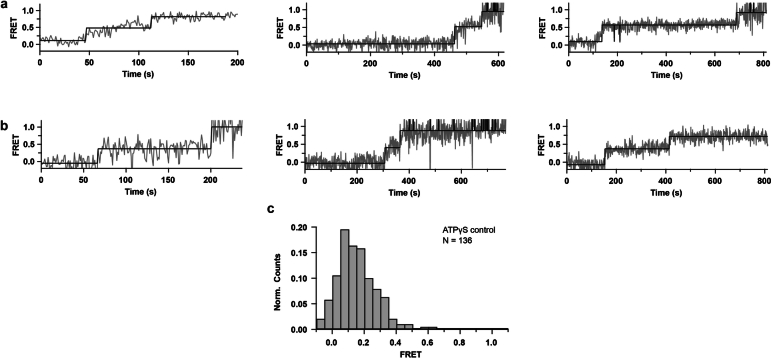
Extended Data Fig. 1. Additional examples of smFRET trajectories relating to the experiment described in Fig. 1 of the main text.
a, Three examples of double exchange events where the first exchange is on the dye proximal side. b, Three examples of double exchange events where the first exchange is on the dye distal side. c, Idealized FRET histogram of an exchange reaction carried out in the presence of ATPγS. No stepwise FRET increases like the examples shown in (a) and (b) are observed. Most molecules exhibit static low FRET.
Double exchange can be processive
The lifetime of SWR1–nucleosome complexes has been shown to be long (several tens of minutes18), although is reduced in the presence of ATP18,19. We have also determined lifetimes of SWR1–nucleosome complexes to be on the order of tens of minutes (Extended Data Fig. 2). Such long lifetimes, longer than that required for a single histone exchange (2–3 min (refs. 18,19)), raise the possibility of a processive mechanism for double histone exchange, as hinted at previously19. However, structural studies12 have strongly suggested that dimer exchange takes place in the position facing the enzyme complex, which has the DNA wrap partially unwound by the Arp6–Swc6 subunits. For double dimer exchange to be processive, different mechanisms for exchange would need to occur for each dimer, or a mechanism must exist to rotate the bound nucleosome in situ. An alternative, and seemingly more plausible, possibility is a distributive mechanism that allows the singly exchanged nucleosome to dissociate and then rebind to SWR1 with the appropriate face oriented for dimer exchange. However, it is important to note that any processive enzyme reactions that are observed need to be explained by a different mechanism.
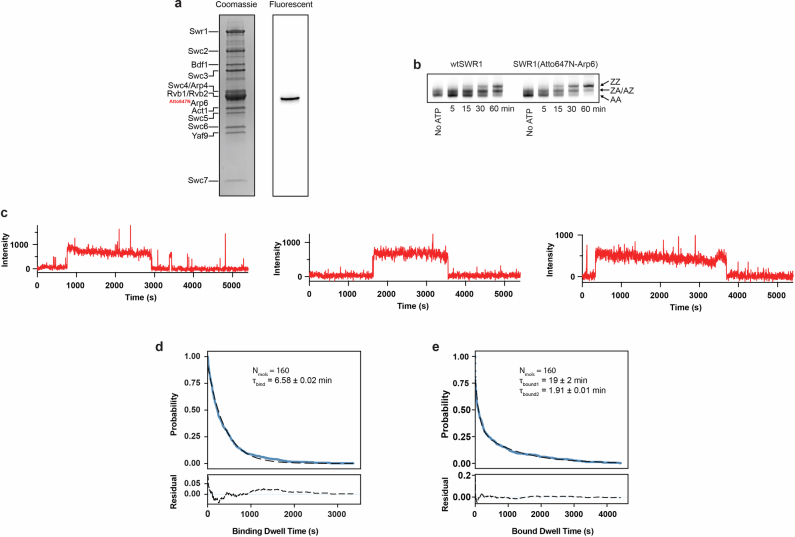
Extended Data Fig. 2. Fluorescently labelled SWR1 and measuring nucleosome bound lifetime.
a, SWR1 was specifically labelled with Atto647N on the N-terminus of the Arp6 subunit. Coomassie stained gel of the purified complex shows the presence of all expected SWR1 subunits (left). The same gel imaged for fluorescence shows that only the Arp6 subunit has been fluorescently labelled (right). Representative gel of three independent preparations. For gel source data, see Supplementary Fig. 1. b, Bulk activity assay using the insertion of a FLAG tagged Htz1–H2B dimer as a readout for exchange. Exchange activity of the labelled SWR1 complex is retained. Representative gel of two independent experiments using enzyme from separate purifications. For gel source data, see Supplementary Fig. 1. c, Three example single molecule intensity trajectories of SWR1(647N) colocalization to surface immobilized nucleosomes. d, Dwell time plot of SWR1(647N) binding times. Data is shown fit to a single exponential decay (with residuals below). On average SWR1 takes 6.58 ± 0.02 min to bind under our experimental conditions. e, Dwell time plot of the lifetime of SWR1(647N) bound to a nucleosome. Data is shown fit to a double exponential decay (with residuals below). Two types of bound complex are present, one stably bound (lifetime 19 ± 2 min) and one more transiently bound (lifetime 1.91 ± 0.01 min). We tentatively assign the transiently bound species to SWR1(647N) interacting with the extranucleosomal DNA, and the stably bound species to SWR1(647N) engaging properly with the nucleosome. Reported errors are the error of the fit.
To evaluate these alternatives directly, we expanded our single-molecule FRET (smFRET) assay to three colours with an additional dye (Atto647N) on SWR1 (see Methods) to colocalize enzyme binding and dissociation dynamics (Fig. 2a). The labelled SWR1 did not affect enzyme activity in bulk (Extended Data Fig. 2). Using alternating laser excitation, we can selectively follow histone exchange as stepwise FRET increases, while monitoring SWR1 binding by fluorescence intensity. The resulting single-molecule trajectories showed molecules that undergo single (43%; Fig. 2b) and double (57%; Fig. 2d) exchanges during a SWR1-binding event. In these trajectories, SWR1 binding precedes the first histone exchange by 36 ± 2 s (Fig. 2c), whereas the second exchange is approximately sixfold slower, taking 227 ± 11 s (Fig. 2e), consistent with the value measured in Fig. 1d. Ultimately, SWR1 dissociates or photobleaches (Fig. 2b,d).
Fig. 2. SWR1 processively exchanges H2A–H2B for Htz1–H2B.
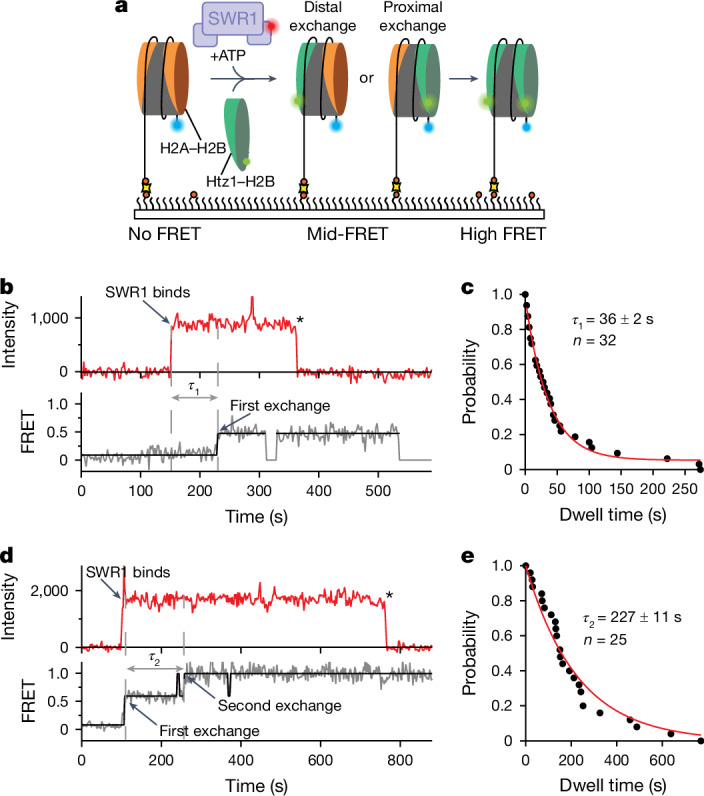
a, Three-colour smFRET assay with surface-immobilized AF488–nucleosome (blue), AF555–Htz1–H2B dimers (green) and SWR1(647N) (red). SWR1 binding is monitored by red fluorescence; histone exchange is detected as a FRET increase between AF488 and AF555. b, Example trace showing SWR1 binding (red; top) followed by a single-exchange event (bottom) after the exchange time (τ1). Asterisk indicates photobleaching or dissociation. c, Dwell time distribution between SWR1 binding and the first exchange (for both single and double exchanges) yields a τ1 = 36 ± 2 s. d, Example trace showing SWR1 binding (red; top) followed by two processive exchange events (bottom) with a second-exchange time (τ2). Asterisk indicates photobleaching or dissociation. e, Dwell time distribution between the first and second exchanges yields a second-exchange time τ2 = 227 ± 11 s. Reported errors are the error of the fit.
These data demonstrate that SWR1 can exchange two histone dimers in a single-binding event, strongly supporting a processive exchange mechanism. A small fraction (approximately 10%) of distributive exchanges is observed, but this is expected for processive enzymes, as all processive enzymes are expected to exhibit a fraction of distributive events depending on experimental conditions. In some trajectories, we cannot observe the presence of SWR1, probably due to Atto647N photobleaching or incomplete labelling (Extended Data Fig. 3).
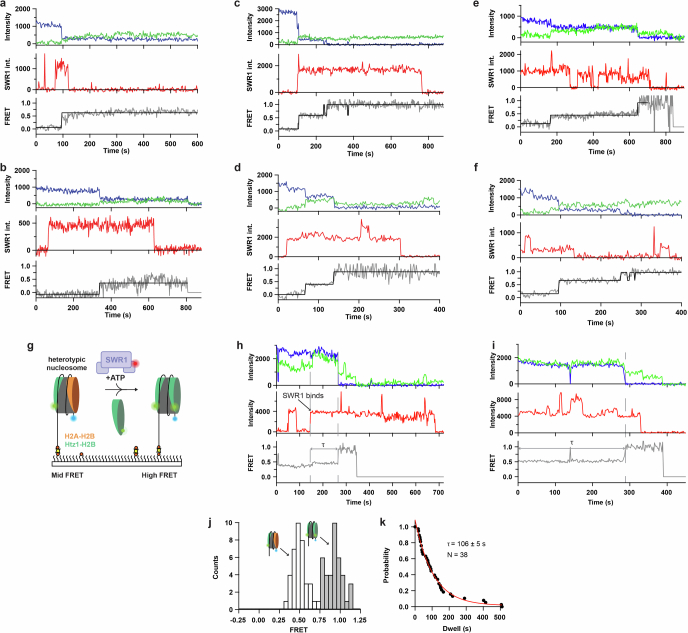
Extended Data Fig. 3. Additional examples of trajectories relating to the experiment described in Fig. 2 of the main text, and exchange of a heterotypic nucleosome.
a, & b, Single exchange events, where the exchange event is preceded by SWR1 binding. c, & d, Processive double exchange events. A single SWR1 binding event is followed by two consecutive exchange events. (Data in (c) is from Fig. 2d of the main text, replotted here to additionally show the donor and acceptor trajectories.) e, Distributive double exchange event. Following the first exchange SWR1 dissociates. The second exchange is preceded by a SWR1 binding event. f, Ambiguous double exchange example. SWR1 either dissociates or photobleaches between the first and second exchange events. g, Schematic of three-color smFRET assay using a heterotypic nucleosome as the substrate. Schematic is colored similarly to Fig. 2a of the main text. h, & i, Example trajectories showing SWR1 binding and histone exchange of a heterotypic nucleosome substrate. j, Histogram showing the FRET before (white bars) and after (grey bars) exchange, for a heterotypic nucleosome. k, Distribution of the time between SWR1 binding and histone exchange yields an exchange time of 106 ± 5 s for a heterotypic nucleosome. Reported errors are the error of the fit.
Nucleosomes flip between two bound states
Having recapitulated and monitored the complete double-exchange reaction at the single-molecule level, and established that this can be processive, we then sought to delve more deeply into the different steps of the reaction pathway. We set out to answer two questions: first, how SWR1 determines which dimer to exchange so that H2A–H2B dimers are always replaced with Htz1–H2B dimers and never the reverse; and second, how consecutive exchange reactions are carried out processively without release of the nucleosome.
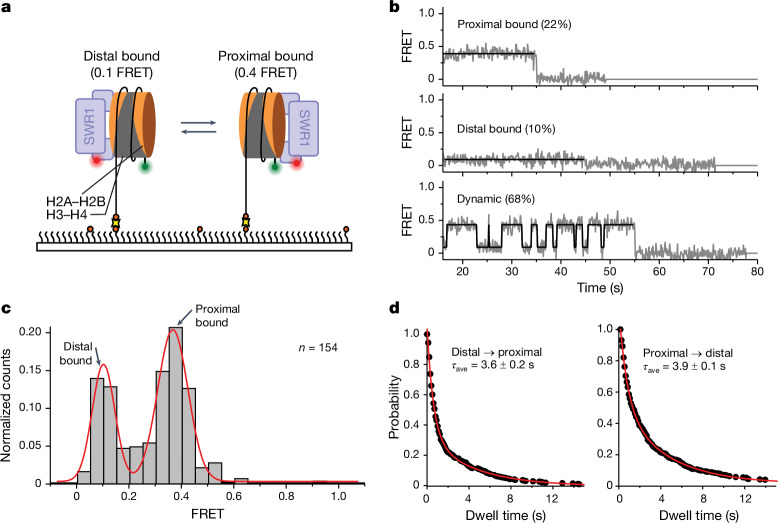
To answer the first question, we labelled the nucleosome (on the short DNA overhang) with a FRET donor and SWR1 complex with a FRET acceptor (Fig. 3a) to monitor nucleosome dynamics when bound to the complex. The resulting single-molecule FRET trajectories revealed two conformations for bound nucleosomes with different FRET efficiencies (approximately 0.1 and approximately 0.4; Fig. 3b and Extended Data Fig. 4a–d). The cryo-EM structure of the SWR1–nucleosome complex12 was used to evaluate the nature of the complexes, and the simplest interpretation is that binding of the nucleosome is in two pseudo-symmetric conformations, with each conformation presenting a different dimer to the surface of the SWR1 complex (Fig. 3a). These two bound states place the two dyes either close or further apart, termed dye-proximal and dye-distal conformations, respectively. Most molecules (68%; n = 154) showed that nucleosomes can flip between the distal and proximal states, although a small proportion remained in either the distal (10%) or proximal (22%) states (Fig. 3b). To rule out the possibility that the observed dynamic FRET stems from movement of DNA, we relocated the donor to H2A (Extended Data Fig. 4e–g), and observed similar dynamic FRET transitions that showed the same slight preference for the dye-proximal dimer. Alternative explanations for flipping, such as DNA unwrapping or SWR1 diffusing along the DNA overhang, were ruled out because unwrapping12 and diffusion21 require ATP binding, whereas flipping is not dependent on ATP.
Fig. 3. SWR1 flips between each face of a nucleosome.
a, Schematic of the assay. Nucleosomes (113N2.Cy3) labelled with Cy3 on the short 2-bp overhang are surface immobilized on a PEGylated microscope slide. SWR1, labelled with Atto647N on the N terminus of the Arp6 subunit (SWR1(647N)) is flowed in and allowed to bind to the nucleosome. Interactions between the nucleosome and SWR1(647N) are monitored via smFRET between the donor (green circle) and acceptor (red circle). b, Examples of typical smFRET (grey) and idealized (black) traces. Some molecules display a static FRET of either 0.4 or 0.1, whereas other molecules dynamically flip between these two FRET states. c, Idealized FRET histogram shows two major populations of SWR1(647N) bound to a nucleosome: a low-FRET (0.1) population corresponding to SWR1(647N) bound to the dye-distal side of the nucleosome, and a mid-FRET (approximately 0.4) population corresponding to SWR1(647N) bound to the dye-proximal side of the nucleosome. d, Dwell time plots for the distal-to-proximal (left) and proximal-to-distal (right) transition. The average dwell times (τave) for SWR1 bound in the distal and proximal orientations are approximately equal. Reported errors are the error of the fit.
Extended Data Fig. 4. Additional data and controls relating to the experiments in Figs. 3 and 4 of the main text.
a, Example fluorescence intensity trajectory (top) and corresponding FRET trajectory (bottom) resulting from SWR1(647N) bound to a surface immobilized nucleosome. The excitation scheme used is illustrated with the magenta and green bars (top). After locating SWR1(647N) bound nucleosomes with red excitation, FRET between the nucleosome and SWR1(647N) is monitored using green excitation. Single step photobleaching of the acceptor and donor indicate a single FRET pair. b, Dwell time plot of the dwell times in the proximal or distal bound configurations for a nucleosome containing two H2A–H2B dimers (data from Fig. 3d, replotted here to show additional details of the fit). Dwell time is fit to a double exponential decay. The lifetimes in the fast (τfast) and slow (τslow) phases are indicated, along with the corresponding amplitudes (Afast, Aslow). The lifetimes are approximately equal regardless of SWR1 orientation. c, FRET histogram of nucleosome (donor) only control displaying zero FRET in the absence of any SWR1(647N) (acceptor). d, Example fluorescence intensity trajectory (top) and corresponding FRET trajectory (bottom) showing SWR1(647N) binding to a surface immobilized nucleosome (indicated by *), and subsequently flipping between dye-distal and dye-proximal orientations. SWR1(647N) binding results in a small but detectable non-zero FRET. (Note: such a trajectory would not be included in subsequent analysis as it does not satisfy the criterion of having SWR1(647N) bound at the start of data acquisition, but is shown here to illustrate detection of SWR1(647N) binding and flipping.) e, Schematic of the assay where the donor fluorophore is placed on one of the H2A histones: Nucleosomes (113N2) labelled with Cy3B on the linker-distal H2A are surface immobilized. SWR1(647N) is flowed in and allowed to bind the nucleosomes. SWR1(647N)–nucleosome interactions are monitored via FRET. Repositioning the FRET donor from the short DNA overhang (as used throughout the rest of this work) to the linker-distal H2A results in lower FRET efficiencies. To identify these true low-FRET values we employed alternating laser excitation throughout the entire acquisition. f, Trajectory of a dynamic SWR1(647N) bound nucleosome showing donor emission upon donor excitation (DD, green trace, top); acceptor emission upon donor excitation (DA, magenta trace, top); acceptor emission upon acceptor excitation (AA, gray trace, top). DD and DA are used for calculating apparent FRET efficiency (gray trace, bottom). g, Idealized FRET histogram shows two major populations of SWR1(647N)–bound nucleosomes. We observe similar ratios of the two states regardless of FRET donor position (c.f. Fig. 3). h, Additional smFRET traces from the experiment described in Fig. 4b. i, Dwell time plot of the dwell times in the proximal or distal bound configurations for a heterotypic nucleosome containing one Htz1–H2B and one H2A–H2B dimer (data from Fig. 4d, replotted here to show additional details of the fit). While the time spent on the distal side (i.e., the side containing Htz1) is well described by a single exponential decay, the proximal (H2A containing side) is best fit to a double exponential decay. Compare with (b). The lifetimes in the fast (τfast) and slow (τslow) phases are indicated, along with the corresponding amplitudes (Afast, Aslow). j, Bulk assay showing the exchange of a canonical H2A–H2B nucleosome (AA) compared to a heterotypic nucleosome containing one Htz1–H2B and one H2A–H2B dimer (ZA). The insertion of a FLAG tagged Htz1–H2B dimer is used as a readout for exchange. The AA nucleosome undergoes two consecutive rounds of exchange (indicated by the appearance of a double band shift). However, the ZA nucleosome can only be exchanged once (single band shift) indicating that SWR1 does not remove Htz1–H2B dimers from a nucleosome. Representative gel of two independent experiments using enzyme from separate purifications. For gel source data, see Supplementary Fig. 1. k, Cartoons of 113N2 heterotypic nucleosome (top) and 2N113 swapped DNA overhang heterotypic nucleosome (bottom). The position of the Cy3 fluorophore (green circle) and biotin (orange circle) are shown. Swapping the DNA overhang orientation with respect to the 601 positioning sequence results in the Htz1–H2B variant histone either being adjacent or opposite to the long DNA overhang. l, Swapped DNA overhang heterotypic nucleosomes (Cy3.2N113) containing one Htz1–H2B dimer (green) and one canonical H2A–H2B dimer (orange) Cy3-labeled on the 2 bp overhang are surface immobilized. SWR1647N is flowed in and allowed to bind to the nucleosome. SWR1(647N)–nucleosome interactions are monitored via FRET. m, Idealized FRET histogram shows a main population at low (0.1) FRET (c.f. Fig. 4c). n, Dwell time plots for the distal to proximal (red) and proximal to distal (black) transition for a 2N113 heterotypic nucleosome. Binding to the H2A–H2B face of the nucleosome is more stable than binding to the Htz1–H2B face, irrespective of the location of the long DNA overhang.
A time-binned FRET histogram of all the trajectories (Fig. 3c) showed that each state is sampled with approximately equal probability with a slight preference for the dye-proximal state. A dwell time analysis of the dynamic molecules showed that the nucleosome flips with approximately equal average time (3–4 s; Fig. 3d) from either the proximal or the distal face of the nucleosome. This observation is consistent with previous data from our group (and that in Fig. 1) that show an approximately equal propensity for exchange of each of the dimers in a yeast nucleosome in the first step12. The dwell time analysis further revealed biphasic exponential kinetics with a slow (4–5 s) and a fast (0.6–0.8 s) population, indicating the presence of flipping intermediates that cannot be distinguished by FRET alone (Extended Data Fig. 4b). A possible explanation for the biphasic kinetics is that SWR1 engages the nucleosome in either a more (slow) or less (fast) stable conformation. The corrected exponential amplitudes showed that the molecules spend approximately 80% of the time in the slow (more engaged) configuration.
SWR1 senses heterotypic nucleosome dimer
Although each dimer in a canonical yeast nucleosome containing two H2A–H2B dimers has, in principle, an equal likelihood to be exchanged, the second-exchange reaction is exclusively of the remaining H2A-containing dimer15. Furthermore, a nucleosome in which both H2A–H2B dimers have been exchanged for Htz1–H2B cannot undergo SWR1-catalysed replacement by Htz1, and even futile cycling, in which one Htz1–H2B dimer is exchanged for another, does not seem to occur15. These observations indicate that the SWR1 complex has a mechanism to distinguish between Htz1 and H2A within a nucleosome.
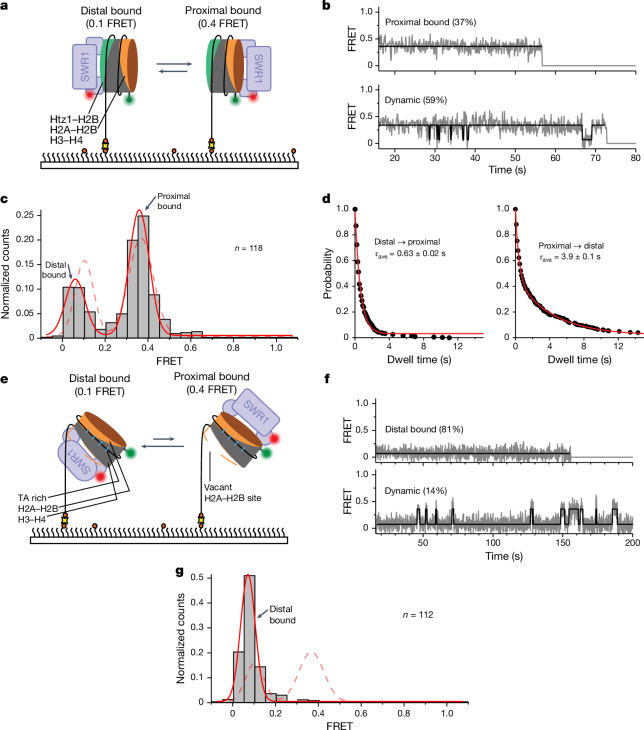
Having determined that the bound nucleosome flips between conformations and that each presents a different nucleosome face, and hence dimer, to the SWR1 complex, we then sought to test whether this mechanism allowed SWR1 to probe the identity of the dimer with which it was presented. We prepared nucleosomes with a single copy each of H2A and Htz1 (Fig. 4a) and then repeated the experiments described above to monitor the flipping process. The data show that these ‘heterotypic’ nucleosomes bind to SWR1 in a similar manner to the canonical nucleosomes and are able to flip between both distal and proximal orientations (Fig. 4b and Extended Data Fig. 4h). However, in contrast to canonical nucleosomes, the static trajectories were almost exclusively in the proximal orientation that presents the H2A–H2B dimer to SWR1 (Fig. 4b). Furthermore, dwell time analysis for each orientation revealed clear kinetic differences between the two states (Fig. 4d and Extended Data Fig. 4i). The side containing the H2A–H2B dimer exhibits almost identical kinetics to the canonical nucleosome (complare with Fig. 3d). By contrast, the side containing the Htz1–H2B dimer exhibits only a single fast exponential decay (0.63 ± 0.02 s), comparable with the fast component of the canonical dimer face. This is also reflected in the time-binned FRET histogram (Fig. 4c), which shows a clear preference for the proximal (H2A–H2B) face.
Fig. 4. Histone composition regulates SWR1 flipping kinetics.
a, Cy3-labelled surface-immobilized heterotypic nucleosomes (113N2.Cy3) containing Htz1–H2B (green) and canonical H2A–H2B dimers (orange). SWR1(647N) is flowed in and nucleosome binding is monitored via smFRET between the donor (green circle) and acceptor (red circle). b, Characteristic smFRET (grey) and idealized (black) trajectories. Some molecules (37%; ntotal = 118) display static 0.4 FRET, whereas others (59%) flip dynamically between 0.4 and 0.1 FRET. c, Idealized FRET histogram showing two populations corresponding to SWR1(647N) bound to the dye-distal Htz1–H2B (0.1 FRET) or to the dye-proximal H2A–H2B (approximately 0.4 FRET). The dashed line indicates the canonical nucleosome distribution from Fig. 3c. A small (0.04) shift of the low-FRET population may indicate altered binding to the Htz1 side. d, Dwell time plots for the distal-to-proximal (left) and proximal-to-distal (right) transition for a heterotypic nucleosome. Average dwell time (τave) on the Htz1–H2B side (distal) is shorter than the H2A–H2B side (proximal). e, Surface-immobilized hexasomes (113H2.Cy3) lacking the dye-distal H2A–H2B dimer (dashed orange line). SWR1(647N) is flowed in and hexasome binding is monitored via smFRET. f, Characteristic smFRET (grey) and idealized (black) trajectories. Molecules display a low (0.1) FRET. A small number of molecules show infrequent transitions from 0.1 to 0.4 FRET. g, Idealized FRET histogram showing one major population of SWR1(647N) bound to a hexasome. Only the low-FRET (0.1) population corresponding to SWR1(647N) bound to the vacant (dye-distal) side of the hexasome is present. The dashed line indicates the canonical nucleosome distribution from Fig. 3c. A small (0.03) shift of the low-FRET population may indicate altered binding when SWR1 faces the empty side. Reported errors are the error of the fit.
To rule out the possibility that the observed kinetic differences stem from the asymmetric DNA overhangs, we prepared heterotypic nucleosomes with swapped DNA overhangs (Extended Data Fig. 4k–n). The data show that the observed biased flipping kinetics is maintained, confirming that the SWR1 selection against the Htz1–H2B side is based on histone content rather than on DNA overhang. These data show that SWR1 is able to distinguish between H2A and Htz1 within the context of the nucleosome, discriminating against Htz1 by rapidly flipping back to the canonical side. We thus propose that a form of kinetic proofreading22,23 places the appropriate face of the nucleosome into the position proficient for dimer removal and exchange, thus contributing to the exquisite selectivity of the enzyme for replacing H2A with Htz1 and not the reverse. However, the ratio of the two dwell times (Fig. 4d) only gives a selectivity of sixfold, which is less than the apparent selectivity reported for SWR1 (refs. 15,16), so although this kinetic proofreading contributes significantly to specificity, additional steps (such as the multiple ATP hydrolysis events during dimer exchange or selective binding of Htz1–H2B versus H2A–H2B dimers for insertion) probably increase this selectivity further.
The hexasome vacant site hinders flipping
A necessary intermediate in the histone dimer exchange reaction is a hexasome intermediate in which one H2A–H2B dimer has been removed but has not yet been replaced with an Htz1–H2B dimer. We next prepared hexasomes labelled in the same way as nucleosomes (Fig. 4e and Extended Data Fig. 5) to determine whether there was any effect on the kinetics and distribution of binding orientations. The single-molecule trajectories exhibit an almost complete loss of the proximal orientation with almost all hexasomes bound in the distal orientation, with the fraction of molecules flipping between the distal and the proximal sites decreasing to 14% (Fig. 4f). The distal orientation places the ‘empty dimer’ site against the SWR1 complex surface ready for insertion by an incoming Htz1–H2B dimer, consistent with structural data suggesting that this is the face of the nucleosome that undergoes histone exchange12. Although we did observe occasional flipping into the proximal conformation, this state is very short lived and reverts quickly to the distal configuration. A time-binned histogram of all trajectories confirms that the proximal orientation becomes almost undetectable (Fig. 4g). These results are consistent with SWR1 placing the nucleosome empty site in position ready to accept the incoming Htz1–H2B dimer. This change in the flipping dynamics suggests an active stabilization of the hexasome intermediate, produced on-enzyme, retaining the orientation that places the empty site in position to accept the incoming dimer. Indeed, our recent cryo-EM structure of the hexasome-bound SWR1 complex demonstrates that Swc5 has a role in complex stabilization24.
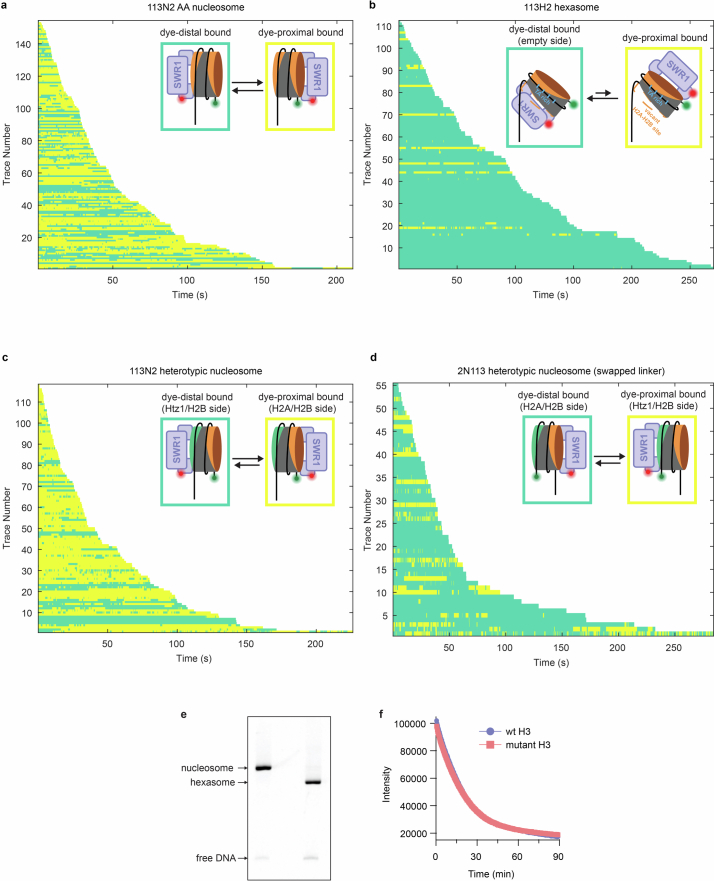
Extended Data Fig. 5. Rastergrams summarising the nucleosome flipping data for different nucleosomes/hexasome, and preparation of yeast hexasomes.
a-d, Each horizontal line represents a smFRET trajectory, ordered by photobleaching/dissociation time. Color indicates whether SWR1 is bound in the dye-distal (green) or dye-proximal (yellow) orientations. Thresholding (at 0.25 FRET) of the idealized FRET trajectories was used to determine the two states. Data is shown for: a, Canonical H2A–H2B 113N2 nucleosomes. b, Hexasomes 113H2 containing only one H2A–H2B dimer. c, Heterotypic nucleosomes 113N2 containing both H2A–H2B and Htz1–H2B histones. d, Swapped linker heterotypic nucleosomes 2N113 containing both H2A–H2B and Htz1–H2B histones. e, Native PAGE comparing a nucleosome and hexasome sample. Representative gel of two independent preparations. f, H3MPQ mutations required for formation of S. cerevisiae hexasomes and heterotypic nucleosomes (see Methods) have no effect on SWR1 exchange activity as measured by bulk FRET decrease.
Structural basis for nucleosome flipping
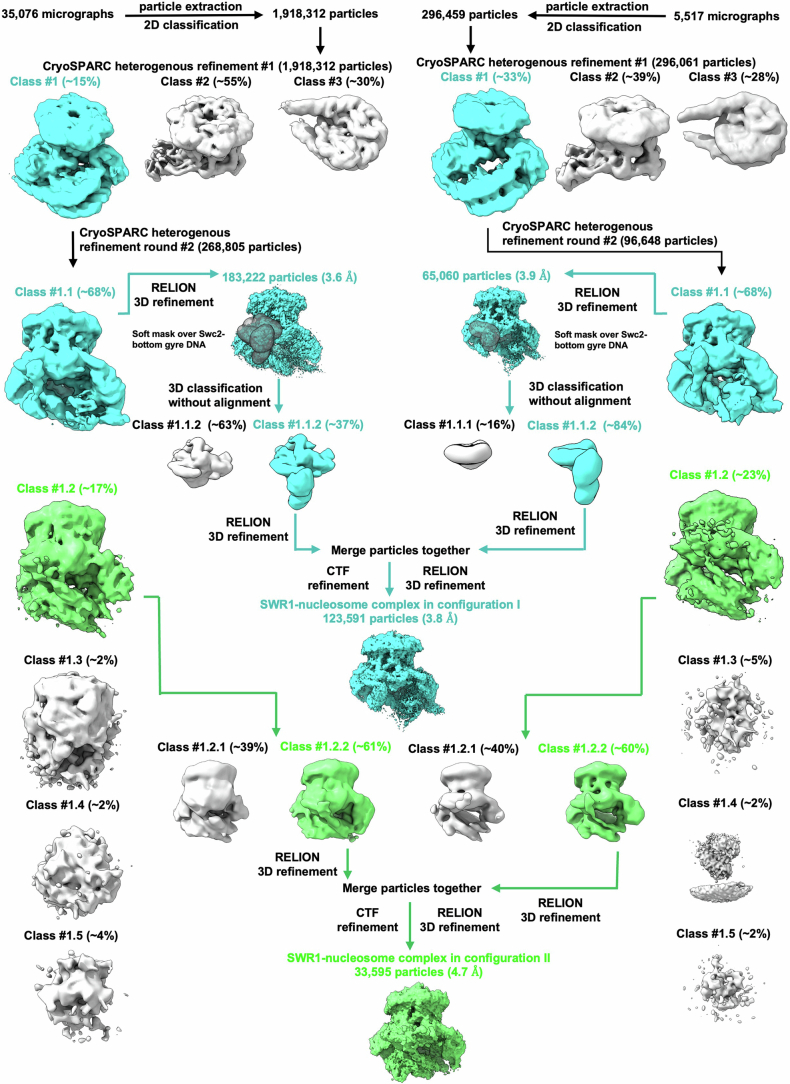
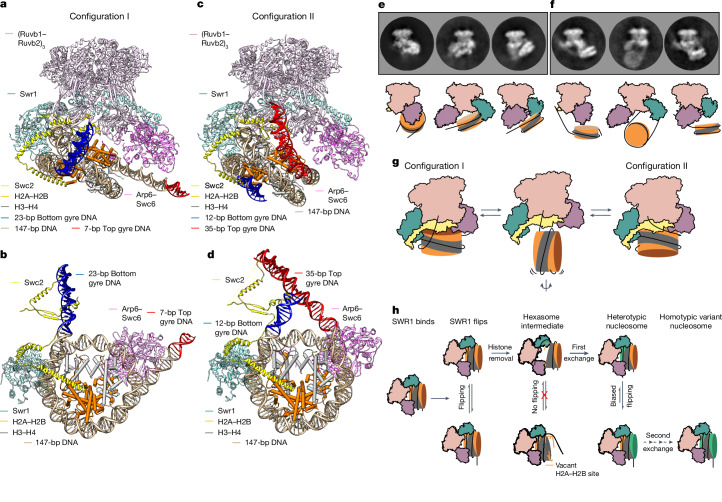
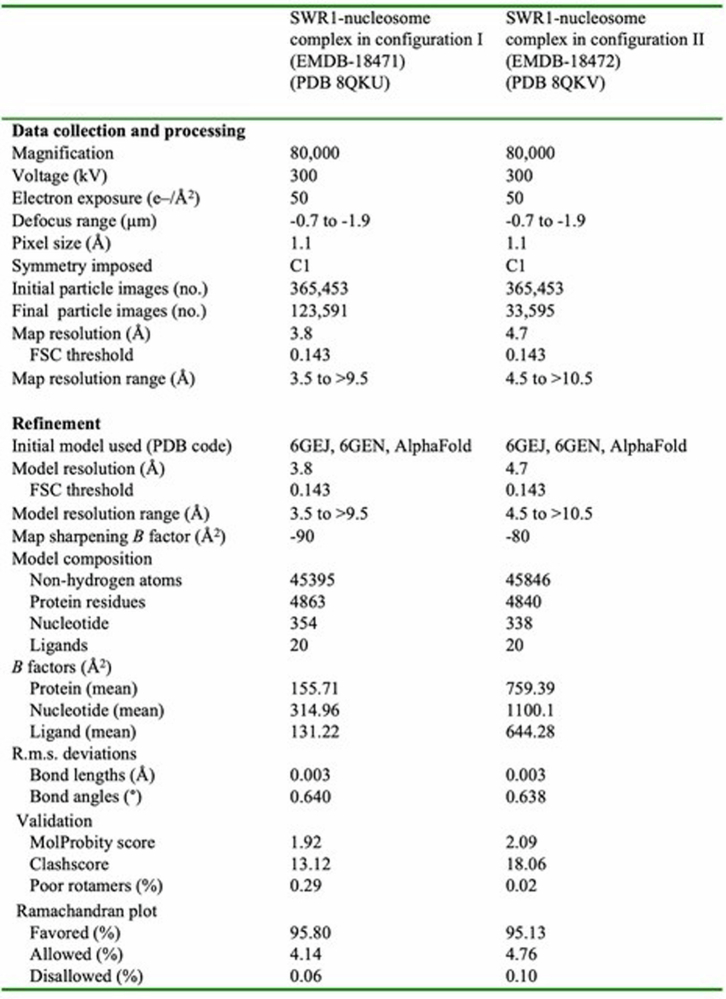
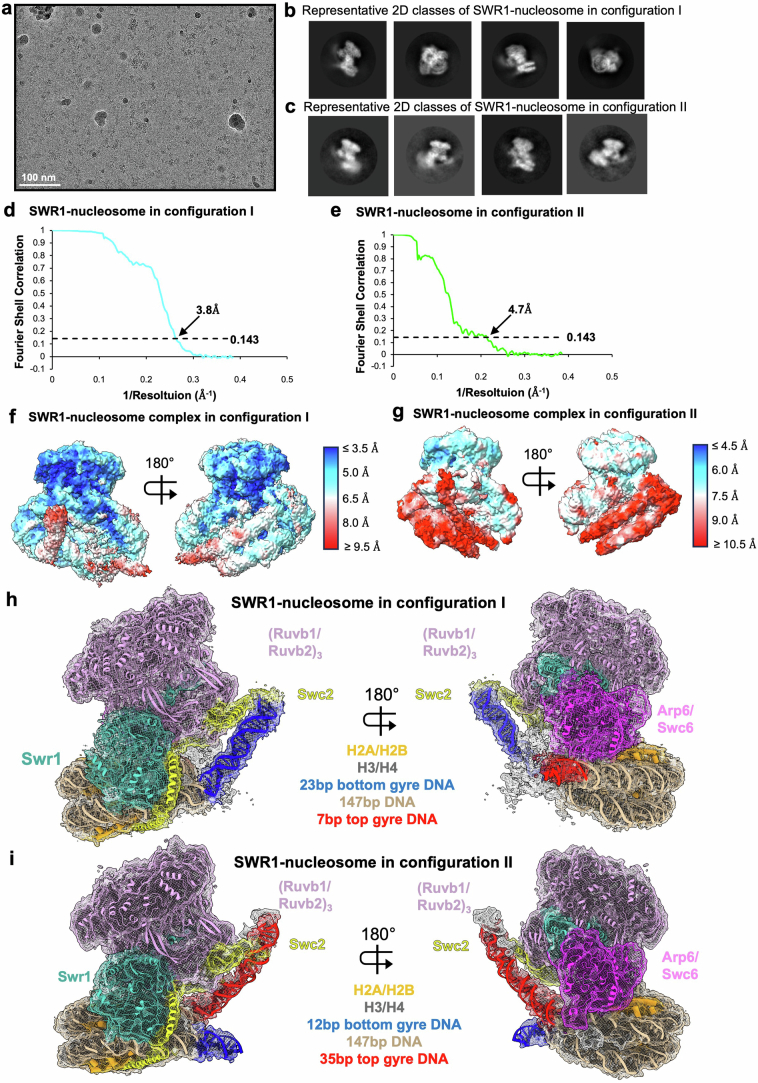
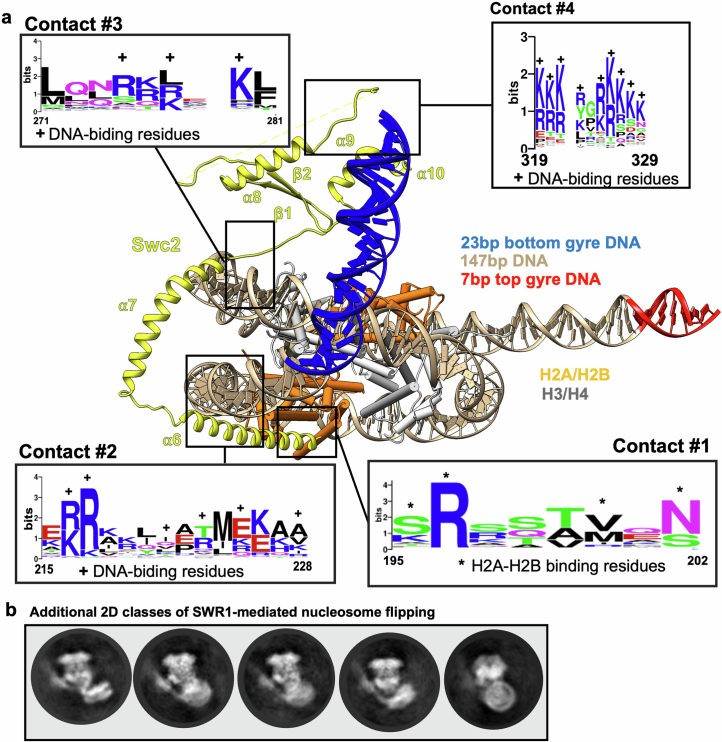
To gain a better understanding of the flipping mechanism, we used cryo-EM analysis to examine different states of SWR1 complexed with nucleosomes. Our previous cryo-EM structures have shown one major state for the complex but also revealed several minor states, the function of which was not evident at that time12. However, in light of the new single-molecule data above, we re-examined these less-populated structural states in an expanded dataset to see whether these provided information about how nucleosomes might flip between different conformations. Further analysis and processing focused on these minor classes, revealing additional details (Extended Data Fig. 6). Two classes were of particular interest and resulted in structures at 3.8 Å and 4.7 Å, respectively (Fig. 5, Extended Data Table 1, Extended Data Figs. 6 and 7 and Supplementary Videos 1 and 2). One of these classes (described briefly in our previous work12) was very similar to the major structure, but a longer section of overhang DNA is evident that emanates from the lower gyre of the nucleosome and binds across the surface of SWR1 (configuration I; Fig. 5a,b and Supplementary Video 1). The DNA extending from the upper gyre is unwrapped from the nucleosome and binds to Arp6–Swc6 in the same manner as that described for the main structure12. The second structure (configuration II; Fig. 5c,d and Supplementary Video 2) also showed a longer section of DNA overhang bound to SWR1, but this time, it was the DNA that extended from the upper gyre, which is released from Arp6–Swc6, that now binds across the same surface of SWR1 as the DNA from the lower gyre in configuration I. The DNA overhang from the lower gyre is released in this structure. Thus, the same DNA-binding surface on SWR1 binds different overhangs in each structure (Extended Data Figs. 7 and 8).
Extended Data Fig. 6. Schematic overview of the cryoEM processing.
Schemes for the 3.8 Å SWR1–nucleosome complex in configuration I (cyan) and the 4.7 Å SWR1–nucleosome complex in configuration II (green) datasets.
Fig. 5. Structural basis of SWR1-mediated nucleosome flipping.
a, The built-in coordinates of SWR1 in complex with a canonical nucleosome at 3.8 Å resolution in configuration I. Note that the DNA emanating from the lower gyre of the nucleosome (highlighted in blue) is bent up and binding across the surface of SWR1. b, A bottom view of the SWR1–nucleosome structure in configuration I. For clarity, only Swr1 (HD1 and HD2), Swc2 and the Arp6–Swc6 complex of SWR1 are shown. c, The built-in coordinates of SWR1 in complex with a canonical nucleosome at 4.7 Å resolution in configuration II. Note that the DNA emanating from the upper gyre of the nucleosome (highlighted in red) is binding across the surface of SWR1. d, A bottom view of the SWR1–nucleosome structure in configuration II. For clarity, only Swr1 (HD1 and HD2), Swc2 and the Arp6–Swc6 complex of SWR1 are shown. e, Three representative 2D class averages of SWR1–nucleosome in the canonical conformation. A cartoon representation of each 2D class is shown beneath. f, Three representative 2D class averages of SWR1-mediated nucleosome flipping with SWR1 orientated as in panel e. A cartoon representation of each 2D class is shown beneath. g, Cartoon summary of SWR1-mediated nucleosome flipping. h, Cartoon summary of kinetic proofreading and processivity of histone exchange by the SWR1 remodeller.
Extended Data Table 1.
Cryo-EM data collection, refinement and validation statistics
Extended Data Fig. 7. Cryo-EM analysis of the SWR1–nucleosome in configuration I (3.8 Å) and SWR1–nucleosome in configuration II (4.7 Å) volumes.
a, Representative micrograph out of 35,076 micrographs from the SWR1–nucleosome dataset. A scale bar is shown at the bottom left. b, Four representative 2D classes of SWR1–nucleosome complex in configuration I. c, Four representative 2D classes of SWR1–nucleosome complex in configuration II. d, gFSC curve of the SWR1–nucleosome in configuration I volume. e, gFSC curve of the SWR1–nucleosome configuration II volume. f, Local resolution of the SWR1–nucleosome complex in configuration I. g, Local resolution of SWR1–nucleosome in configuration II. h, Overview of the SWR1–nucleosome complex in configuration I at 3.8 Å. i, Overview of the SWR1–nucleosome complex in configuration II at 4.7 Å.
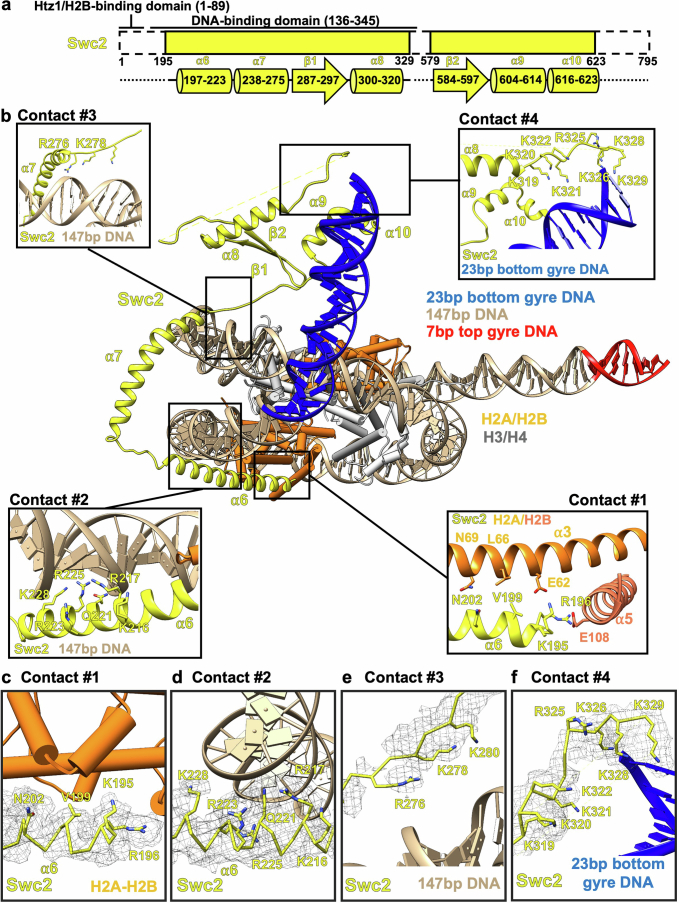
Extended Data Fig. 8. Details of the interaction between Swc2 and the nucleosome in the SWR1–nucleosome in configuration I.
a, Linearized cartoon of the Swc2 subunit, the built-in coordinates are represented in yellow. The Htz1–H2B binding domain (residues 1–89) and the DNA-binding domain (residues 136–345) are highlighted. b, The residues of Swc2 that binds the DNA or interact with the H2A–H2B histones are highlighted The interaction between Swc2 and the nucleosome in the SWR1–nucleosome complex in configuration I. For simplicity, only the built in coordinates of Swc2 and the nucleosome is shown. c, Representative density for Swc2 at contact 1 (contoured at 2σ) in the SWR1–nucleosome in configuration I complex. d, Representative density for Swc2 at contact 2 (contoured at 2.5σ) in the SWR1–nucleosome in configuration I complex. The side chains of Swc2 that interacts with nucleosomal DNA is shown. e, Representative density for Swc2 at contact #3 (contoured at 5.5σ). in the SWR1–nucleosome in configuration I complex. The side chains of Swc2 that interacts with the nucleosomal DNA is shown. f, Representative density for Swc2 at contact #4 (contoured at 3σ). in the SWR1–nucleosome in configuration I complex. The side chains of Swc2 that interacts with the bottom gyre nucleosomal DNA is shown.
The improved resolution, combined with the availability of an AlphaFold model for Swc2 (ref. 25) allows us to assign, locate and build regions of the Swc2 subunit that we were previously unable to assign confidently. Swc2 has an essential role in SWR1-mediated histone exchange12,26. The N-terminal region of Swc2 binds to Htz1–H2B dimers26,27; however, we are still unable to assign that part of Swc2 in our structure. The central portion of yeast Swc2 (residues 136–345) is a DNA-binding module that probably localizes the SWR1 complex towards the nucleosome-depleted region21,28 (Extended Data Fig. 8a). We can confidently build a portion of this DNA-binding region (residues 195–329) into the density (Extended Data Fig. 8b). Our structure indicates that there are three contacts between this region of Swc2 and the DNA (Extended Data Fig. 8b). Several positively charged residues in these regions are conserved across Swc2 subunits from different species (Extended Data Fig. 9a), consistent with a role in interacting with DNA. Two of these contact regions have been previously observed12, although specific residue contacts could not be unambiguously determined. The AlphaFold model of Swc2 now allows us to better define these regions, which both contact the DNA wrap of the nucleosome (Extended Data Fig. 8b). A third contact region, involving eight conserved basic residues (K319–K322, R325, K326, K328 and K329), are in a loop that sits across the surface of the SWR1 complex (Extended Data Figs. 8 and 9). This surface contacts the DNA overhang adjacent to the nucleosome wrap (Extended Data Fig. 8), but a different overhang in structures emanating from either the lower (configuration I) or the upper (configuration II) DNA gyre.
Extended Data Fig. 9. Residues that interact with the nucleosome are conserved between Swc2-like proteins, and additional 2D classes of SWR1-mediated nucleosome flipping.
a, The interaction between Swc2 and the nucleosome in the SWR1–nucleosome complex in configuration I. For simplicity, only the built-in coordinates of Swc2 and the nucleosome are shown. The four different contacts between Swc2 and the nucleosome are highlighted. An alignment of 116 Swc2-like proteins across various species was used to generate a sequence logo to display sequence conservation. The residues of Swc2 that bind the DNA or interact with the H2A–H2B histones are highlighted. b, Five additional intermediates of SWR1-mediated nucleosome flipping are visible after 2D classification.
These two states suggest a simple mechanism to allow flipping of nucleosome orientations between the proximal and distal states (Fig. 5g). By swapping which DNA overhang is bound to SWR1, and then releasing the nucleosome but without releasing the DNA overhang, the nucleosome can flip and rebind in the opposite orientation but remain tethered to SWR1 by the DNA contact with the Swc2 subunit (Supplementary Video 3). Owing to the symmetry of the histone octamer, we cannot distinguish whether the nucleosome orientation relative to SWR1 switches between configurations I and II. An alternative explanation could be that SWR1 remains bound to the same face of the nucleosome in both configurations, and only the DNA overhang interacting with Swc2 is swapped. However, the smFRET experiments in which the donor is located on H2A still exhibit the nucleosome flipping dynamics (Extended Data Fig. 4e–g), thereby ruling out this possibility. Consequently, we interpret that the two major states that we observed by cryo-EM represent intermediates on the flipping pathway. In principle, SWR1 could also use a single approximately 35-bp or longer DNA overhang while flipping the nucleosome (Fig. 5c), as shown in our single-molecule experiments (Fig. 3a). In the cell, however, the chromatin context (that is, the presence or absence of a neighbouring nucleosome) would dictate whether one or two overhangs can be used for flipping.
The single-molecule analysis presented above shows that although nucleosomes bound to SWR1 flip between configurations I and II, they only spend a very small amount of time flipping between these configurations. We would, therefore, expect to see very few complexes caught in this process in our cryo-EM dataset and these would probably be in various conformations with the nucleosome only interacting via the DNA overhangs that are so hard to define and average. Nonetheless, by careful classification of the particle dataset where bound nucleosome could not be visualized (Extended Data Fig. 6), we were able to identify several 2D classes in which the nucleosome could be observed in a flipped state where the nucleosome was disengaged from SWR1 but the flanking DNA remained bound. Three particularly well-defined examples are shown in Fig. 5 (compare Fig. 5e and 5f), but others were also visible (Extended Data Fig. 9b). We interpret these as direct visualization of nucleosomes frozen in the act of flipping between configurations I and II. The dynamic nature of the flipping, however, precluded a 3D analysis of intermediate flipped states.
Discussion
The ATP-dependent exchange of H2A–H2B dimers for those containing Htz1–H2B is a two-step process that replaces each dimer in turn15. Structural data12 have strongly suggested that the dimer to be exchanged makes close contacts with SWR1, and unwrapping of the DNA from around this dimer begins upon binding and then progresses in an ATP-dependent process18–20. However, both dimers in a nucleosome can be exchanged, implying dissociation of the nucleosome to allow it to rebind with the appropriate dimer exposed for exchange in a distributive mechanism. On the basis of ensemble-averaged experiments, we have previously suggested that histone exchange proceeds via a distributive mechanism12. However, this conclusion was based on the assumptions that both exchanges proceeded with comparable rates, and that SWR1 would be completely processive or distributive. The three-colour single-molecule exchange experiments (Fig. 2) showed that, under these conditions, the second exchange is much slower and that not all exchanges are processive, illustrating the need for such single-molecule experiments to unambiguously show processivity of the SWR1 complex. Why the second exchange is slower than the first one remains unclear. One possibility is that heterotypic nucleosomes have slower exchange rates. To test this possibility, we performed single-molecule exchange assays using a heterotypic nucleosome substrate (Extended Data Fig. 3). The resulting exchange time is approximately 100 s, still threefold slower than the first exchange (approximately 36 s), confirming that exchange is slower on heterotypic nucleosomes and in agreement with previous results20,29.
However, demonstration that dimer exchange is processive raises a conundrum. We questioned how SWR1 accesses both faces of a single nucleosome without dissociation or by utilizing different mechanisms for each exchange given the asymmetric manner of association with the SWR1 complex. The answer is by a partial release of the nucleosome, while retaining a hold on the flanking DNA, to allow it to flip 180° and then rebind with the opposite face towards the enzyme to allow the second-exchange event (Fig. 5g,h and Supplementary Video 3). This simple, yet elegant, mechanism also explains the exquisite specificity of dimer exchange by SWR1 through dynamic, kinetic proofreading that favours placing a nucleosome face containing H2A rather than Htz1 in the position for exchange without releasing the substrate.
In cells, the Htz1–H2B variant is enriched at the +1 nucleosome at transcription start sites, which is typically flanked by a long (approximately 140 bp) nucleosome-free region (NFR) on one side30. SWR1 has been found, by crosslinking, to reside on the NFR-proximal side of the +1 nucleosome, which led the authors to question how SWR1 might exchange NFR-distal dimers31. At certain genes, chromatin immunoprecipitation-exo data from yeast cells have shown a preference for Htz1 insertion into the NFR-distal side of the nucleosome32; however, at the genome-wide level, the NFR-distal preference was closer to approximately 60:40, suggesting the opposite to be the case at other transcription start sites, despite the location of the SWR1 complex on the opposite face of the nucleosome31. In vitro, linker-distal or linker-proximal dimer preference for the first-exchange reaction has been somewhat controversial, as discussed18. Our initial studies12 have shown a weak exchange preference (between 50:50 and 60:40) for the linker-distal (dye-proximal) dimer. Our new single-molecule exchange data (Fig. 1c) are consistent with those results (approximately 55:45 linker-distal). Data from other laboratories are also consistent with a weak linker-distal preference, particularly at physiological temperatures29. Conversely, others have reported a stronger preference for the linker-distal dimer based on more frequent and faster kinetics for linker-distal exchange18,19. The origin of these differences is not clear. The nature of the enzyme or histone source (for example, recombinant versus native, and yeast versus frog/Drosophila) or variations of the nucleosome positioning sequence could, in principle, explain these differences, but the data presented here do not resolve this issue.
Finally, we speculate that nucleosome flipping might have a role in other nucleosome remodelling activities. Flipping could be used to recognize and modify different histone components on both faces of nucleosomes or to monitor the histone composition of different faces of nucleosomes as part of regulation processes. Furthermore, the sliding directionality of nucleosomes on DNA could be swapped by such a flipping mechanism, as proposed for Chd1 (ref. 33), allowing processive sliding of nucleosomes to space them evenly along DNA or to position them in gene regulation and/or DNA repair.
Methods
Purification of wild-type SWR1
Recombinant SWR1 was produced as previously described12,16 with minor modifications. Baculoviruses encoding SWR1 genes were initially amplified in Sf9 cells, before using the amplified baculoviruses to infect BTI-TN-5B1-4 (High Five) cells for expression, which were harvested after 72 h. Cells were lysed by sonication in 50 mM HEPES (pH 8.0), 0.5 M NaCl, 1 mM TCEP, 10% glycerol, 1 mM benzamidine-HCL supplemented with 1 protease inhibitor tablet and 10 µl of benzonase per litre of cell culture. Lysate was clarified by centrifugation at 30,000g for 60 min at 4 °C. The supernatant was filtered before being injected onto a StrepTrap HP (Cytiva) column. The column was washed with buffer A (25 mM HEPES (pH 7.5), 0.3 M NaCl, 1 mM TCEP and 10% glycerol) before being eluted with buffer A supplemented with 5 mM desthiobiotin. The eluted protein was combined and diluted 1:1 with buffer B (25 mM HEPES (pH 7.5), 0.1 M NaCl, 1 mM TCEP and 10% glycerol) to dilute the salt before being loaded onto a HiTrap Q HP (Cytiva) column. The protein was eluted with a linear gradient from buffer B to buffer C (25 mM HEPES (pH 7.5), 2 M NaCl, 1 mM TCEP and 10% glycerol). The relevant fractions were pooled and diluted again 1:1 with buffer B to reduce the salt before being injected onto a Heparin HP (Cytiva) column. Protein was eluted with a linear gradient from buffer B to buffer C. Finally, the protein was concentrated, snap frozen in liquid nitrogen and stored at −80 °C.
Purification of fluorescently labelled SWR1
To site specifically label the SWR1 complex, we made use of the ybbR-labelling approach34,35. The 11-amino acid ybbR tag was fused to the N terminus of the Arp6 subunit of SWR1. The ybbR–Arp6 mutant was used in place of the wild-type Arp6 gene when assembling the SWR1 genes using the MultiBac system16. The SWR1(ybbR–Arp6) complex was expressed and purified in an analogous way to wild-type SWR1 with the ybbR-labelling reaction taking place after elution from the HiTrap Q HP column. The labelling reaction was carried out overnight at 4 °C. Typically, SWR1(ybbR–Arp6; approximately 1 µM) was labelled with CoA-Atto647N (approximately 10 µM) using recombinant Sfp transferase (approximately 0.2 µM) in buffer B supplemented with 10 mM MgCl2. The labelled SWR1 complex was separated from free dye and Sfp transferase using a Heparin HP (Cytiva) column, eluting with a linear gradient from buffer B to buffer C. Finally, SWR1(Atto647N–Arp6) (referred to as SWR1(647N) in the text) was concentrated, snap frozen in liquid nitrogen and stored at −80 °C.
Purification of S. cerevisiae histones
All nucleosomes or hexasomes used in this study were composed of S. cerevisiae histones assembled on DNA containing the 601 Widom sequence.
S. cerevisiae octamers with and without Alexa Fluor 555 on H2A K119C were prepared as previously described16.
S. cerevisiae H2A–H2B, Htz1–H2B (with and without Alexa Fluor 555 on Htz1 K125C) or Htz1–H2B(3×Flag) histone dimers were expressed in E. coli and purified as soluble dimers. Cells were lysed by sonication in buffer D (20 mM Tris (pH 7.5), 0.5 M NaCl, 0.1 mM EDTA and 1 mM TCEP) plus protease inhibitor tablets (Roche; 2 tablets per 100 ml). Dimers were purified by loading the cleared lysate onto tandem HiTrap Q FF and HiTrap Heparin HP columns in buffer E (20 mM Tris (pH 7.5), 0.5 M NaCl, 1 mM EDTA and 1 mM TCEP). The HiTrap Q FF column was removed before elution from the HiTrap Heparin HP column via a gradient to buffer F (20 mM Tris (pH 7.5), 2 M NaCl, 1 mM EDTA and 1 mM TCEP), followed by gel filtration on a Superdex S200 in buffer F.
S. cerevisiae histone H3(Q120M, K121P and K125Q) and histone H4 were co-expressed in E. coli and purified as soluble tetramers. Cells were lysed by sonication in buffer D plus protease inhibitor tablets (Roche; 2 tablets per 100 ml). Tetramers were purified using a HiTrap Heparin HP column in buffer E and eluted via a gradient to buffer F, followed by gel filtration on a Superdex S200 in buffer F.
Preparation of nucleosomes
Biotinylated DNA containing the Widom 601 sequence was generated as previously described12. Salt gradient dialysis of the S. cerevisiae octamers with DNA was carried out to form a ‘core’ nucleosome. A biotinylated DNA overhang was ligated to the core nucleosome as previously described12. This resulted in nucleosomes with one long overhang of 113 bp and a short overhang of 2 bp, which we refer to as 113N2 (‘N’ representing the Widom 601 nucleosome positioning sequence). The biotin was present on the long 113-bp linker. For nucleosomes where the DNA was labelled, the fluorophore was attached at the end of the 2-bp short overhang.
Preparation of hexasomes
To facilitate the formation of yeast hexasomes, three amino acid substitutions were introduced into the S. cerevisiae H3 histone (Q120M, K121P and K125Q)36. These substitutions (MPQ) are the corresponding amino acids found in human and Xenopus laevis H3.
To form hexasomes, S. cerevisiae H2A–H2B dimers were mixed with S. cerevisiae H3(MPQ)–H4 tetramers. The amount of H2A–H2B dimers used was limited to 0.6× the amount of tetramers to ensure only partial H2A–H2B occupancy. Hexasomes were assembled onto the same DNA that was used for nucleosomes by salt gradient dialysis to generate ‘core’ hexasomes. Core hexasomes were separated from tetrasomes, nucleosomes and free DNA using a MonoQ column, loaded in buffer G (20 mM Tris (pH 7.5), 1 mM EDTA, 1 mM TCEP and 200 mM NaCl) eluting with a gradient into buffer H (as buffer G with 2 M NaCl). The fractions were immediately diluted into 4× volume of 20 mM Tris (pH 7.5) to reduce the salt concentration. A biotinylated DNA overhang was ligated to the core hexasome in the same way as was used for nucleosomes. This resulted in a hexasome with one long overhang of 113 bp and a short overhang of 2 bp, which we refer to as 113H2 (‘H’ representing a hexasome assembled on the Widom 601 sequence). The biotin was present on the long 113-bp linker. For hexasomes where the DNA was labelled, the fluorophore was attached at the end of the 2-bp short overhang.
As is the case for hexasomes prepared with X. laevis histones37, yeast hexasomes prepared in this way exploit the inherent asymmetry of the Widom 601 sequence. Because of this asymmetry, the H2A–H2B dimer present in a hexasome is preferentially located on the ‘TA-rich’ side of the Widom 601 sequence, leaving the vacant site on the ‘TA-poor’ side. We orientated our Widom 601 sequence with the TA-rich side closest to the 2-bp short overhang. This resulted in the vacant H2A–H2B site being located next to the 113-bp linker.
Preparation of heterotypic nucleosomes
Core hexasomes, prepared as described above, were mixed with S. cerevisiae Htz1–H2B dimers to form heterotypic nucleosomes. Htz1–H2B dimers were added at an amount equal to 0.3× the amount of hexasome present. Core heterotypic nucleosomes were then purified in the same way as canonical nucleosomes. A biotinylated DNA overhang was ligated to the core heterotypic nucleosomes as described above. Resulting heterotypic nucleosomes contain the Htz1–H2B dimer next to the long 113-bp overhang and the conical H2A–H2B dimer next to the short 2-bp overhang.
Bulk histone exchange assay
SWR1 (100 nM; wild type or SWR1(647N)), 200 nM nucleosomes and 400 nM Htz1–H2B(3×Flag) were mixed in exchange buffer (25 mM Tris-HCl (pH 7.8), 100 mM KCl, 0.2 mM EDTA and 2 mM MgCl2), with or without 1 mM ATP. The exchange reaction was carried out at 30 °C. At the indicated time points, 8 µl of the reaction was removed and quenched by the addition of 4 µl of a stopping solution (0.5 mg ml−1 salmon sperm DNA, 30 mM EDTA and 3× ficoll loading buffer) and placed on ice. The ‘no ATP’ control was taken at the longest indicated time point. After all time points had been taken, the reaction products were separated by 6% native PAGE, run at 110 V in 0.5× TBE at 4 °C and visualized using fluorescence of the nucleosome.
Two-colour smFRET microscope
smFRET measurements looking at the flipping of nucleosomes by SWR1 were performed on an Olympus IX-71 microscope equipped with a homebuilt prism-TIRF module. Excitation was provided by a 532-nm laser (Stradus, Vortran) or a 637-nm laser (Stradus, Vortran). Fluorescence was collected through a 1.2 NA, 60× water objective (Olympus) and filtered through a dual bandpass filter (FF01-577/690-25, Semrock). The fluorescence was spectrally separated using a OptoSplit II (Cairn Research) to separate donor and acceptor emission. The donor and acceptor emissions were further filtered through ET585/65M and ET700/75M (Chroma) bandpass filters, respectively. The donor and acceptor images were then projected side-by-side onto an electron-multiplying charge-coupled device (EMCCD) (Andor iXon Ultra 897). Data were collected as raw movies using a custom LabVIEW script.
Single-molecule fluorescence spots from the raw movies were localized using custom IDL scripts and converted into raw fluorescence trajectories. Raw fluorescence trajectories were corrected for bleed through of the donor fluorescence into the acceptor channel. Apparent FRET efficiencies were calculated as the ratio of acceptor intensity divided by the sum of the donor and acceptor intensities.
Two mechanical shutters (LS-3, Uniblitz, Vincent Associates) were placed in the excitation path for alternating laser excitation (Extended Data Fig. 4e–g). Frame acquisition and shutter synchronization were obtained using a homebuilt negative-edge-triggered JK flip–flop circuit (SN74LS112AN, Texas Instruments) using the ‘Fire’ output of the EMCCD as the input clock. IDL scripts were modified accordingly to locate single molecules and extract fluorescence trajectories.
Three-colour smFRET microscope
smFRET measurements looking at histone exchange coupled with SWR1 binding were performed on an Olympus IX-71 microscope equipped with a homebuilt prism-TIRF module. Alternating laser excitation was provided by a 488-nm laser (OBIS, Coherent) or a 637-nm laser (OBIS, Coherent). Alternation of the lasers and synchronization of the lasers with the camera were controlled by a custom LabVIEW script and a DAQ (USB-6341, National Instruments). Fluorescence was collected through a 1.2 NA, 60× water objective (Olympus) and filtered through ET500lp (Chroma) and NF03-642E-25 (Semrock) filters. The fluorescence was spectrally separated using a MultiSplit (Cairn Research) housing the following dichroic filters: T500lpxr UF2, T635lpxr UF2 and T725lpxr UF2 (Chroma). The separated fluorescent emission was projected onto quadrants of a sCMOS (ORCA Fusion, Hammamatsu) camera. Data were collected as raw movies using HCImage Live (Hammamatsu).
Single-molecule fluorescence spots from the raw movies were localized using custom IDL scripts and converted into raw fluorescence trajectories. Raw fluorescence trajectories were corrected for bleed through of the donor fluorescence into the acceptor channel. Apparent FRET efficiencies were calculated as the ratio of acceptor intensity divided by the sum of the donor and acceptor intensities.
Microscope slide passivation and flow chamber assembly
Quartz slides (UQC optics) and glass coverslips were aminosilinized with N-(2-aminoethyl)-3-aminopropyltrimethoxysilane, then passivated using methoxy-PEG-SVA (relative molecular mass = 5,000; Laysan Bio, Inc.) containing 5% biotin-PEG-SVA (relative molecular mass = 5,000, Laysan Bio, Inc.) in 100 mM sodium bicarbonate as previously described38 with minor modifications. Following passivation, slides and coverslips were stored under nitrogen in the dark at −20 °C. Before use, slides and coverslips were warmed to room temperature and assembled into flow chambers using 0.12-mm thick double-sided adhesive sheets (Grace Bio-Labs SecureSeal). Flow chambers were sealed with epoxy glue.
Nucleosome or hexasome immobilization
Nucleosomes or hexasomes were surface immobilized as previously described12. In brief, neutravidin (0.1 mg ml−1) in T50 buffer (50 mM Tris-HCl (pH 7.5) and 50 mM NaCl) was injected into the assembled flow chamber and incubated for 5 min to allow binding to the biotinylated PEG surface. Excess neutravidin was washed out with reaction buffer (25 mM Tris-HCl (pH 7.8), 100 mM KCl, 4% glycerol, 1 mM EDTA, 2 mM MgCl2 and 0.2 mg ml−1 BSA). Biotinylated nucleosomes or hexasomes were diluted to 10 pM in reaction buffer before injecting into the flow chamber and allowed to bind to the neutravidin for 5 min. Excess nucleosomes or hexasomes were flushed out using imaging buffer (reaction buffer with Trolox, 2.5 mM protocatechuic acid and 0.25 µM protocatechuate-3,4-dioxygenase) and imaged immediately.
smFRET between nucleosome or hexasome and SWR1 data collection
Nucleosomes or hexasomes labelled with a Cy3 donor on the short end of the DNA overhang (113N2.Cy3 or 113H2.Cy3) were immobilized in a flow chamber and imaged. SWR1(647N), 10 nM in imaging buffer (25 mM Tris-HCl (pH 7.8), 100 mM KCl, 4% glycerol, 1 mM EDTA, 2 mM MgCl2, 0.2 mg ml−1 BSA, Trolox, 2.5 mM protocatechuic acid and 0.25 µM protocatechuate-3,4-dioxygenase) was injected. Imaging was performed by first directly exciting the acceptor with a 637-nm laser for approximately 15 s to localize SWR1(647N), before switching to 532-nm excitation to observe FRET between the nucleosome or hexasome and SWR1. All single-molecule measurements were carried out at room temperature, data were acquired with a 100-ms frame time.
smFRET between nucleosome or hexasome and SWR1 data analysis
Manual inspection of the donor intensity, acceptor intensity and apparent FRET from each molecule was carried out using custom MATLAB scripts. For a molecule to be included in downstream analysis, it needed to have a constant signal from the acceptor under direct acceptor excitation to indicate that SWR1(647N) was bound and display a single step photobleaching event of either the donor or acceptor under donor excitation. All molecules that satisfied these criteria were truncated to just the FRETing region preceding the photobleaching event.
Truncated FRET traces were analysed with a hidden Markov model using vbFRET, using default parameters39. The idealized FRET from vbFRET was used to generate FRET histograms, plotted using Igor Pro 8 (Wavemetrics). Dwell times from the idealized FRET trajectories were extracted using custom MATLAB scripts. Only dwell times in which the idealized FRET transitioned between proximal and distal states (or the reverse) were included. Dwell time plots were generated in MATLAB and plotted in Igor Pro 8. The lifetime of the proximal-bound and distal-bound states was determined by fitting the dwell time plots to a double exponential function in Igor Pro 8. The slow and fast exponential phases probably correspond to a fully or partially engaged SWR1 complex, respectively. The average lifetimes (τave) for proximal-bound and distal-bound states were calculated using the pre-exponential factors (A) and lifetimes (τ) determined from the double exponential fit as follows:
In all cases, we observed both static and dynamic trajectories when probing the FRET between nucleosomes or hexasomes and SWR1. Only dynamic trajectories were used for determining the kinetics. For both the canonical and the heterotypic nucleosomes, static trajectories represent a minority of the observed molecules. Short static traces may be due to dye photobleaching or SWR1 diffusion before a flipping event can take place. However, longer static traces are also observed. This heterogeneity is summarized in Extended Data Fig. 5. Long static trajectories suggest that a proportion of SWR1 molecules are stably engaged on one side of the nucleosome and not dynamically checking the histone identity of each nucleosome face. The nature of this stable SWR1 binding, compared with binding that allows nucleosome flipping, is unknown, as is the method by which SWR1 could transition from a static (stable binding) to a flipping (checking histone identity) state.
smFRET real-time imaging of histone exchange and SWR1-binding data collection
A quartz flow cell was prepared as described above. Neutravidin (0.01 mg ml−1) in T50 buffer (50 mM Tris-HCl (pH 7.5) and 50 mM NaCl) was injected into the flow chamber and incubated for 5 min to allow binding to the biotinylated PEG surface. Excess neutravidin was washed out and the flow cell further passivated by incubation with Pluronic F127 (0.5% w/v) in T50 buffer. Excess Pluronic F127 was washed out with reaction buffer (25 mM Tris-HCl (pH 7.8), 100 mM KCl, 4% glycerol, 0.2 mM EDTA, 2 mM MgCl2 and 0.2 mg ml−1 BSA).
To follow the insertion of variant histones in real time at the single-molecule level, a ‘gain of FRET’ assay was used. Nucleosomes labelled with Alexa Fluor 488 (FRET donor) on the short 2-bp overhang (113N2.AF488) were immobilized in a flow chamber and imaged. To start the reaction, 1 nM SWR1, 4 nM Chz1–Htz1(AF555)–H2B and 1 mM ATP in imaging buffer (25 mM Tris-HCl (pH 7.8), 100 mM KCl, 4% glycerol, 0.2 mM EDTA, 2 mM MgCl2, 0.2 mg ml−1 BSA, Trolox, 2.5 mM protocatechuic acid and 0.25 µM protocatechuate-3,4-dioxygenase) was injected into the chamber using a syringe pump. Exchange can be monitored by stepwise FRET increases as the AF555-labelled (FRET acceptor) Htz1–H2B dimer is exchanged into the immobilized AF488-labelled nucleosome. To reduce nonspecific binding of the Htz1(AF555)–H2B dimer, the dimer was first complexed with its natural chaperone, Chz1 (ref. 40).
For experiments that simultaneously followed exchange and SWR1 binding, the experiment was conducted as described but with SWR1(647N) using the three-colour smFRET microscope described above. The two excitation lasers (488 nm and 637 nm) were alternated at a frequency of 1 Hz. All experiments were carried out at room temperature (22 °C).
smFRET real-time imaging of histone exchange and SWR1-binding data analysis
Visualization of single-molecule trajectories was carried out using custom MATLAB scripts. For each single molecule, the intensity of the donor (Alexa Fluor 488), acceptor (Alexa Fluor 555) and corresponding FRET, along with the colocalized SWR1-binding intensity (Atto647N) were inspected. Nucleosomes that underwent exchange were identified by stepwise increases in the FRET trajectory. SWR1 binding was identified as an increase in the Atto647N intensity. Nucleosomes where the signal for SWR1 binding overlapped with at least one exchange event were included for further analysis. Dwell times were collected by manual inspection of the trajectories. Data were obtained by measuring several regions of interest from at least three independent slides. Dwell time plots were generated in MATLAB and plotted and fit in Igor Pro 8.
Single-molecule measurements of SWR1 nucleosome lifetime
Nucleosomes labelled with Alexa Fluor 488 on the short 2-bp overhang (113N2.AF488) were immobilized in a flow chamber as described above. Of SWR1(647N), 5 nM in imaging buffer (25 mM Tris-HCl (pH 7.8), 100 mM KCl, 4% glycerol, 1 mM EDTA, 2 mM MgCl2, 0.2 mg ml−1 BSA, Trolox, 2.5 mM protocatechuic acid and 0.25 µM protocatechuate-3,4-dioxygenase with 1 mM ATP) was injected. The three-colour smFRET microscope described above was used. The two excitation lasers (488 nm and 637 nm) were alternated at a frequency of 1 Hz. Experiments were carried out at room temperature (22 °C). Trajectories in which SWR1(647N) colocalized with a nucleosome were selected and further processed using tMAVEN41 to determine the time for SWR1 to bind and the time SWR1 remained bound to a nucleosome.
Preparation of the SWR1–nucleosome complex for cryo-EM
Recombinant SWR1 was produced in BTI-TN-5B1-4 (High Five) insect cells, and the SWR1–nucleosome complex was assembled as previously described12. SWR1–nucleosome grids were prepared as previously described, except instead of glow discharge, the grids were cleaned by washing with water and ethyl acetate. Cryo-EM data acquisition, image acquisition and structure reconstruction were conducted using a similar procedure as previously described12. Data processing and refinement statistics for the two cryo-EM structures are summarized in Extended Data Table 1.
Cryo-EM data collection
A total of 35,076 micrographs were collected using a Titan KRIOS microscope operated at 300 kV. Images were collected on a Falcon IV direct electron detector with a pixel size of 1.1 Å px−1. Images were collected with a defocus range of −0.7 to −1.9 µm, with 1.0 s exposure time and a total dose of 40 e− Å−2 fractionated over 39 frames.
Cryo-EM data processing
Movie frames were aligned using MotionCor2 (ref. 42), as previously described12. Contrast transfer function parameters were determined using Gctf43 as previously described12. Particle picking was performed in cryoSPARC44, as previously described12. Global-resolution and local-resolution estimates were calculated based on the gold-standard Fourier shell correlation (FSC = 0.143) criterion.
The cryo-EM processing workflow for the 3.8 Å SWR1–nucleosome map in configuration I is summarized in Extended Data Fig. 6. First, in the recently collected SWR1–nucleosome dataset, 2D classification in cryoSPARC for 2D classes containing density for SWR1 or the nucleosome resulted in a working particle pool of 1,918,312 particles44. These were subdivided into three classes via heterogeneous refinement in cryoSPARC, resulting in class 1 (SWR1–nucleosome complex (15%)), class 2 (SWR1-apo (55%)) and class 3 (nucleosome only (30%)). The subset of 268,805 particles in class 1 (SWR1–nucleosome) was then further classified into five classes via heterogeneous refinement in cryoSPARC, resulting in class 1.1 (SWR1–nucleosome in configuration I (68%)), class 1.2 (SWR1–nucleosome configuration II (17%)), class 1.3 (poorly aligned class (9%)), class 1.4 (poorly aligned class (2%)) and class 1.5 (poorly aligned class (4%)). The particles in class 1.1 were then imported and subjected to 3D refinement in RELION before one round of 3D classification without alignment (T = 30), with a soft mask overlapping the Swc2–bottom gyre DNA interface45. This generated two classes: class 1.1.1 (no density for bottom gyre DNA (63%)) and class 1.1.2 (clear density for bottom gyre DNA (37%)). Particles in class 1.1.2 were further selected for 3D refinement in RELION.
Next, in the previously collected dataset, 2D classification in cryoSPARC for 2D classes containing density for SWR1 or the nucleosome resulted in a working particle pool of 296,061 particles. These were subdivided into three classes via heterogeneous refinement in cryoSPARC, resulting in a class 1.1 (SWR1–nucleosome complex (33%)), class 1.2 (SWR1-apo (39%)) and class 1.3 (nucleosome only (28%))44. The subset of 96,648 SWR1–nucleosome particles were then further classified into five classes via heterogeneous refinement in cryoSPARC, resulting in class 1.1 (SWR1–nucleosome in configuration I (68%)), class 1.2 (SWR1–nucleosome configuration II (23%)), class 1.3 (poorly aligned class (5%)), class 1.4 (poorly aligned class (2%)) and class 1.5 (poorly aligned class (2%)). Particles in class 1.1 were imported and refined in RELION before one round of 3D classification without alignment (T = 30), with a soft mask overlapping the Swc2–bottom gyre DNA interface. This generated two classes: class 1.1.1 (no density for bottom gyre DNA (16%)) and class 1.1.2 (clear density for bottom density (84%)). Particles in class 1.1.2 were further selected for 3D refinement in RELION45. Particles from classes 1.1.2 in the recently collected dataset and 1.1.2 in the previously collected dataset were then merged to generate a working pool of 123,591 particles. The resulting particles were then subjected to 3D refinement and contrast transfer function refinement in RELION with a mask corresponding to the SWR1 subcomplex of Swr1, Arp6, Swc6, Swc2, RuvBL1 and RuvBL2, and the nucleosome to generate the final 3.8 Å SWR1–nucleosome map in configuration I45.
The cryo-EM processing workflow for the 4.7 Å SWR1–nucleosome map in configuration II is summarized in Extended Data Fig. 6. First, in the recently collected SWR1–nucleosome dataset, particles in class 1.2 were selected, generating a working pool of 35,102 particles. The subset of particles was further classified into two classes in RELION using 3D classification with alignment (T = 6) in the absence of a mask45. This generated class 1.2.1 (SWR1–nucleosome with poor density for the upper gyre DNA (39%)) and class 1.2.2 (SWR1–nucleosome with clearer density of upper gyre DNA (61%)). The particles in class 1.2.2 were selected, generating a working pool of 20,990 particles for 3D refinement in RELION.
Next, in the previously collected SWR1–nucleosome dataset, particles in class 1.2 were selected, generating a working pool of 21,054 particles. The subset of particles was further classified in two classes in RELION using 3D classification with alignment (T = 6) in the absence of a mask45. This generated class 1.2.1 (SWR1–nucleosome with poor density for the upper gyre DNA (40%)) and class 1.2.2 (SWR1–nucleosome with clearer density of upper gyre DNA (60%)). The particles in class 1.2.2 were selected, generating a working pool of 12,605 particles for 3D refinement in RELION45. Particles from classes 1.2.2 in the recently collected dataset and 1.2.2 in the previously collected dataset were then merged to generate a working pool of 33,595 particles. The resulting particles were then subjected to 3D refinement and contrast transfer function refinement in RELION with a mask corresponding to the SWR1 subcomplex of Swr1, Arp6, Swc6, Swc2, RuvBL1 and RuvBL2, and the nucleosome to generate the final 4.7 Å SWR1–nucleosome map in configuration II.
Model building
For the Swc2 subunit, an initial template was generated using AlphaFold25. Different regions corresponding to secondary structures of the template were manually truncated and docked separately into the recently generated 3.8 Å SWR1–nucleosome map in configuration I in Chimera12,46, before being further built in Coot47. The final coordinates were subjected to real-space refinement in Phenix48.
For the 3.8 Å SWR1–nucleosome configuration I map, first the SWR1–nucleosome complex from the previously solved 3.6 Å SWR1–nucleosome structure (Protein Data Bank (PDB) ID 6GEJ) was docked into the density using Chimera12,46. The coordinates for the DNA were then omitted. Next, the SWR1–nucleosome complex from the previously solved 4.5 Å SWR1–nucleosome structure (PDB ID 6GEN) was superimposed onto the docked structure using RuvBL1 and RuvBL2 as a reference. Coordinates for the superimposed structure were then omitted, with exception to the coordinates for the DNA, which was kept and docked into the 3.8 Å SWR1–nucleosome configuration I map in Chimera, before merging the two PDB models: SWR1–nucleosome DNA omitted and DNA only together. The coordinates corresponding to the previously built Swc2 subunit were then omitted, and the coordinates for the newly built Swc2 model were docked into the map. Additional DNA overhang was then built manually in Coot12,46,47. The final coordinates were then subjected to real-space refinement in Phenix48.
For the 4.7 Å SWR1–nucleosome configuration II map, SWR1 from the previously solved 3.6 Å SWR1–nucleosome structure (PDB ID 6GEJ) was docked into the density using Chimera46. The coordinates corresponding to Swc2 were omitted, and the recently built Swc2 was docked together into the density using Chimera and further built in Coot46,47. The additional DNA overhang was then built manually in Coot. The final coordinates were then subjected to real-space refinement in Phenix48.
2D classification of SWR1-mediated nucleosome flipping
First, in the recently collected SWR1–nucleosome dataset, particles in class 2 (SWR1-apo (55%)) were selected, generating a working pool of 594,100 particles. The subset of particles was then further classified into four classes via heterogeneous refinement in cryoSPARC, resulting in class 2.1 (RuvBL1–RuvBL2 only (21%)), class 2.2 (a poorly aligned class (20%)), class 2.3 (SWR1-apo with additional density underneath SWR1 (38%)) and class 2.4 (a poorly aligned class (21%)). Particles in class 2.3 were then selected for 2D classification in RELION45.
Next, in the previously collected SWR1–nucleosome dataset, particles in class 2 (SWR1-apo (39%)) were selected, generating a working pool of 115,463 particles. The subset of particles was then further classified into four classes via heterogeneous refinement in cryoSPARC, resulting in class 2.1 (RuvBL1–RuvBL2 only (25%)), class 2.2 (a poorly aligned class (20%)), class 2.3 (SWR1-apo with additional density underneath SWR1 (30%)) and class 2.4 (a poorly aligned class (25%)). Particles in class 2.3 were then selected for 2D classification in RELION. The particles in class 2.3 in the recently collected SWR1–nucleosome dataset and the particles in class 2.3 in the previously collected SWR1–nucleosome dataset were then merged and subjected to multiple rounds of 2D classification in RELION to obtain 2D classes of SWR1-mediated nucleosome flipping.
Statistics and reproducibility
For data relating to Fig. 1, the total number of traces used in each dataset is indicated in each panel and was derived from three independent experiments. For data relating to Fig. 2, the total number of traces used for each dataset is indicated in each panel and was derived from four independent experiments. For data relating to Figs. 3 and 4, two independent experiments were performed, one of which is shown. The total number of traces used for each dataset is indicated in each panel. All gels were independently and successfully repeated twice.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-024-08152-y.
Supplementary information
Uncropped raw gels for Extended Data Figs. 2a, 2b and 4j and legends for Supplementary Videos
180° rocking movie of SWR1–nucleosome complex in configuration I coordinates fitted into the 3.8 Å volume. The atomic coordinates are colored as in Extended Data Fig. 7h, and the 3.8 Å volume is colored to match the fitted atomic coordinates.
180° rocking movie of SWR1–nucleosome complex in configuration II coordinates fitted into the 4.7 Å volume. The atomic coordinates are colored as in Extended Data Fig. 7i, and the 4.7 Å volume is colored to match the fitted atomic coordinates.
SWR1-mediated nucleosome flipping. Distal (orange) and proximal (blue) H2A–H2B. Two intermediate coordinates of SWR1–nucleosome flipping were modelled using the 2D classes (Figure 5f and Extended data Fig. 9b). A third modelled intermediate of SWR1–nucleosome complex in configuration I (before DNA at SHL6-7 stabilization by Arp6–Swc6) was built with the nucleosome coordinates inverted, and DNA matching that in configuration II. Two morphs were generated: First between configuration II and the first modelled coordinates. Second, between the first modelled coordinates, followed by the second modelled coordinates and ending with the third modelled configuration I. Morphs were spliced to generate the summary movie.
Source data
Acknowledgements
We thank the Wigley and Rueda laboratories for comments and critical reading of the manuscript; Diamond for access and support of the cryo-EM facilities at the UK National Electron Bio-imaging Centre (eBIC), funded by the Wellcome Trust, Medical Research Council and BBSRC, and LonCEM facility; and N. Cronin for assistance with data collection at the LonCEM facility. The work was funded by the Wellcome Trust (095519/Z/11/Z and 209327/Z/17/Z (to D.B.W.)), Cancer Research UK (C6913/A21608 (to D.B.W.)), the Medical Research Council (MR/N009258/1 and MR/R009023/1 (to D.B.W.)), and a core grant from the MRC Laboratory of Medical Sciences (UKRIMC-A658-5TY10 (to D.S.R.)).
Extended data figures and tables
Author contributions
P.G., A.S.B.J., D.S.R. and D.B.W. designed the studies. P.G. conducted and analysed the single-molecule experiments. A.S.B.J. performed the cryo-EM analysis. A.S.B.J., P.G., E.A.C., M.T.S. and C.L.K. prepared the samples. P.G., A.S.B.J., D.B.W. and D.S.R. analysed the data and wrote the manuscript with input from all the authors.
Peer review
Peer review information
Nature thanks Blaine Bartholomew, Zhucheng Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Electron density maps have been deposited at the Electron Microscopy Database (accession codes EMDB-18471 and EMDB-18472), and atomic coordinates have been deposited at the PDB (PDB ID codes 8QKU and 8QKV). Initial models used for model building include PDB ID 6GEN and 6GEJ, as well as an AlphaFold-generated model of Swc2. Correspondence and requests for materials should be addressed to D.B.W. or D.S.R. All unique materials are available on request with completion of a standard Materials Transfer Agreement. Source data are provided with this paper.
Code availability
Code for the single-molecule data analysis is freely available (https://github.com/singlemoleculegroup).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Paul Girvan, Adam S. B. Jalal
Change history
11/19/2024
In the version of the article initially published, the label “Vacant H2A–H2B site” pointed to the solid orange section, and has now been amended to point at the orange line in the HTML and PDF versions of the article.
Contributor Information
Dale B. Wigley, Email: d.wigley@imperial.ac.uk
David S. Rueda, Email: david.rueda@imperial.ac.uk
Extended data
is available for this paper at 10.1038/s41586-024-08152-y.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-024-08152-y.
References
- 1.Krogan, N. J. et al. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell12, 1565–1576 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Mizuguchi, G. et al. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science303, 343–348 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Kobor, M. S. et al. A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLoS Biol.2, e131 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature389, 251–260 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Talbert, P. B. & Henikoff, S. Histone variants at a glance. J. Cell Sci.134, jcs244749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruhl, D. D. et al. Purification of a human SRCAP complex that remodels chromatin by incorporating the histone variant H2A.Z into nucleosomes. Biochemistry45, 5671–5677 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Kusch, T. et al. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science306, 2084–2087 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Giaimo, B. D., Ferrante, F., Herchenröther, A., Hake, S. B. & Borggrefe, T. The histone variant H2A.Z in gene regulation. Epigenetics Chromatin12, 37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clapier, C. R. & Cairns, B. R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem.78, 273–304 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Eustermann, S. et al. Structural basis for ATP-dependent chromatin remodelling by the INO80 complex. Nature556, 386–390 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayala, R. et al. Structure and regulation of the human INO80–nucleosome complex. Nature556, 391–395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willhoft, O. et al. Structure and dynamics of the yeast SWR1–nucleosome complex. Science362, eaat7716 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Willhoft, O. & Wigley, D. B. INO80 and SWR1 complexes: the non-identical twins of chromatin remodelling. Curr. Opin. Struct. Biol.61, 50–58 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranjan, A. et al. H2A histone-fold and DNA elements in nucleosome activate SWR1-mediated H2A.Z replacement in budding yeast. eLife10.7554/eLife.06845.001 (2015). [DOI] [PMC free article] [PubMed]
- 15.Luk, E. et al. Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell143, 725–736 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, C. L. et al. Functional characterization and architecture of recombinant yeast SWR1 histone exchange complex. Nucleic Acids Res.45, 7249–7260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe, S., Radman-Livaja, M., Rando, O. J. & Peterson, C. L. A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science340, 195–199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poyton, M. F. et al. Coordinated DNA and histone dynamics drive accurate histone H2A.Z exchange. Sci. Adv.8, eabj5509 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan, J., Moreno, A. T., Baier, A. S., Loparo, J. J. & Peterson, C. L. H2A.Z deposition by SWR1C involves multiple ATP-dependent steps. Nat. Commun.13, 7052 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh, R. K. et al. Transient kinetic analysis of SWR1C-catalyzed H2A.Z deposition unravels the impact of nucleosome dynamics and the asymmetry of histone exchange. Cell Rep.27, 374–386.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carcamo, C. C. et al. ATP binding facilitates target search of SWR1 chromatin remodeler by promoting one-dimensional diffusion on DNA. eLife11, e77352 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopfield, J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc. Natl Acad. Sci. USA71, 4135–4139 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ninio, J. Kinetic amplification of enzyme discrimination. Biochimie57, 587–595 (1975). [DOI] [PubMed] [Google Scholar]
- 24.Jalal, A. S. B. et al. Stabilization of the hexasome intermediate during histone exchange by yeast SWR1 complex. Mol. Cell84, 3871–3884.e9 (2024). [DOI] [PubMed]
- 25.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu, W. H. et al. Swc2 is a widely conserved H2AZ-binding module essential for ATP-dependent histone exchange. Nat. Struct. Mol. Biol.12, 1064–1071 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Liang, X. et al. Structural basis of H2A.Z recognition by SRCAP chromatin-remodeling subunit YL1. Nat. Struct. Mol. Biol.23, 317–323 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Ranjan, A. et al. Nucleosome-free region dominates histone acetylation in targeting SWR1 to promoters for H2A.Z replacement. Cell154, 1232–1245 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun, L., Pierrakeas, L., Li, T. & Luk, E. Thermosensitive nucleosome editing reveals the role of DNA sequence in targeted histone variant deposition. Cell Rep.30, 257–268.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang, C. & Pugh, B. F. Nucleosome positioning and gene regulation: advances through genomics. Nat. Rev. Genet.10, 161–172 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen, K., Vinayachandran, V. & Pugh, B. F. SWR-C and INO80 chromatin remodelers recognize nucleosome-free regions near +1 nucleosomes. Cell154, 1246–1256 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee, H. S., Bataille, A. R., Zhang, L. & Pugh, B. F. Subnucleosomal structures and nucleosome asymmetry across a genome. Cell159, 1377–1388 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu, Y. et al. The Chd1 chromatin remodeler shifts nucleosomal DNA bidirectionally as a monomer. Mol. Cell68, 76–88.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belan, O. et al. Single-molecule analysis reveals cooperative stimulation of Rad51 filament nucleation and growth by mediator proteins. Mol. Cell81, 1058–1073.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin, J., Lin, A. J., Golan, D. E. & Walsh, C. T. Site-specific protein labeling by Sfp phosphopantetheinyl transferase. Nat. Protoc.1, 280–285 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Leung, A. et al. Unique yeast histone sequences influence octamer and nucleosome stability. FEBS Lett.590, 2629–2638 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Levendosky, R. F., Sabantsev, A., Deindl, S. & Bowman, G. D. The Chd1 chromatin remodeler shifts hexasomes unidirectionally. eLife5, e21356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenlla, A., Markiewicz, R. P., Rueda, D. & Romano, L. J. Nucleotide selection by the Y-family DNA polymerase Dpo4 involves template translocation and misalignment. Nucleic Acids Res.42, 2555–2563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bronson, J. E., Fei, J., Hofman, J. M., Gonzalez, R. L. & Wiggins, C. H. Learning rates and states from biophysical time series: a Bayesian approach to model selection and single-molecule FRET data. Biophys. J.97, 3196–3205 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luk, E. et al. Chz1, a nuclear chaperone for histone H2AZ. Mol. Cell25, 357–368 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Verma, A. R., Ray, K. K., Bodick, M., Kinz-Thompson, C. D. & Gonzalez, R. L. Increasing the accuracy of single-molecule data analysis using tMAVEN. Biophys. J. 10.1016/j.bpj.2024.01.022 (2024). [DOI] [PMC free article] [PubMed]
- 42.Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, K. Gctf: CTF real-time determination and correction. J. Struct. Biol.193, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol.180, 519–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettersen, E. F. et al. UCSF Chimera — a visualization system for exploratory research and analysis. J. Comput. Chem.25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D74, 531–544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Uncropped raw gels for Extended Data Figs. 2a, 2b and 4j and legends for Supplementary Videos
180° rocking movie of SWR1–nucleosome complex in configuration I coordinates fitted into the 3.8 Å volume. The atomic coordinates are colored as in Extended Data Fig. 7h, and the 3.8 Å volume is colored to match the fitted atomic coordinates.
180° rocking movie of SWR1–nucleosome complex in configuration II coordinates fitted into the 4.7 Å volume. The atomic coordinates are colored as in Extended Data Fig. 7i, and the 4.7 Å volume is colored to match the fitted atomic coordinates.
SWR1-mediated nucleosome flipping. Distal (orange) and proximal (blue) H2A–H2B. Two intermediate coordinates of SWR1–nucleosome flipping were modelled using the 2D classes (Figure 5f and Extended data Fig. 9b). A third modelled intermediate of SWR1–nucleosome complex in configuration I (before DNA at SHL6-7 stabilization by Arp6–Swc6) was built with the nucleosome coordinates inverted, and DNA matching that in configuration II. Two morphs were generated: First between configuration II and the first modelled coordinates. Second, between the first modelled coordinates, followed by the second modelled coordinates and ending with the third modelled configuration I. Morphs were spliced to generate the summary movie.
Data Availability Statement
Electron density maps have been deposited at the Electron Microscopy Database (accession codes EMDB-18471 and EMDB-18472), and atomic coordinates have been deposited at the PDB (PDB ID codes 8QKU and 8QKV). Initial models used for model building include PDB ID 6GEN and 6GEJ, as well as an AlphaFold-generated model of Swc2. Correspondence and requests for materials should be addressed to D.B.W. or D.S.R. All unique materials are available on request with completion of a standard Materials Transfer Agreement. Source data are provided with this paper.
Code for the single-molecule data analysis is freely available (https://github.com/singlemoleculegroup).