Abstract
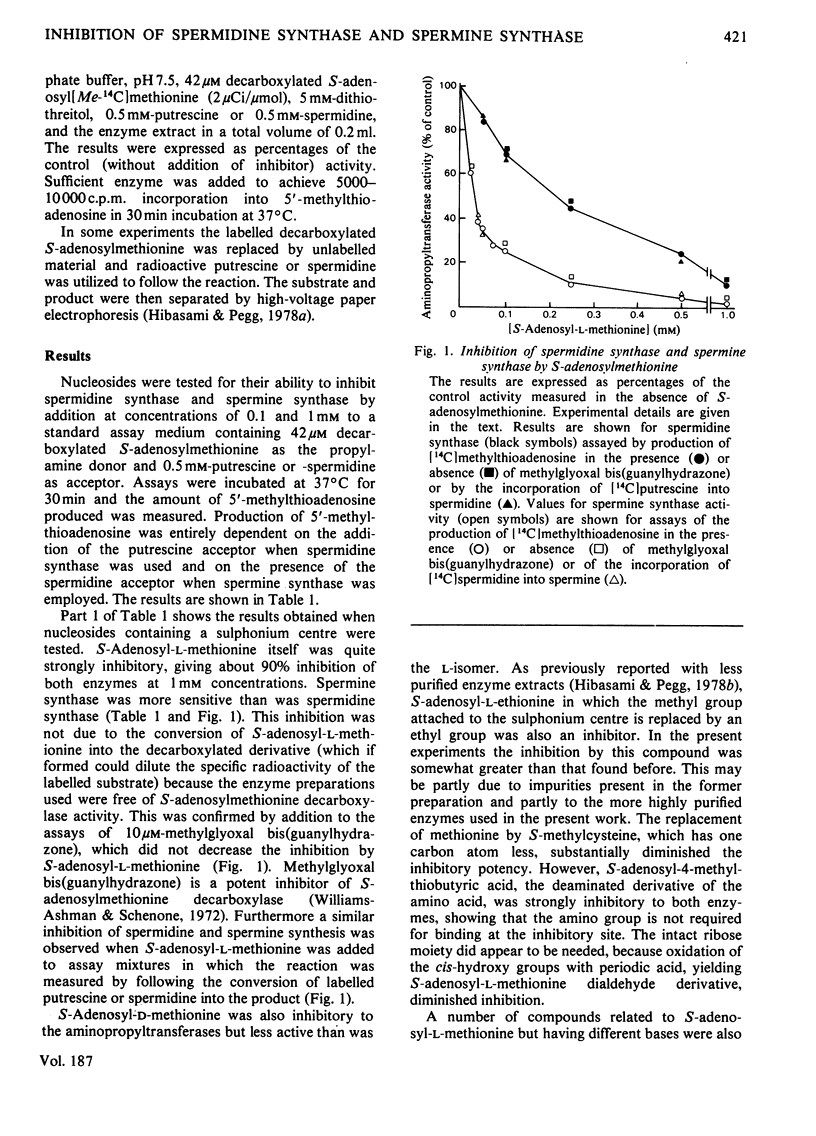
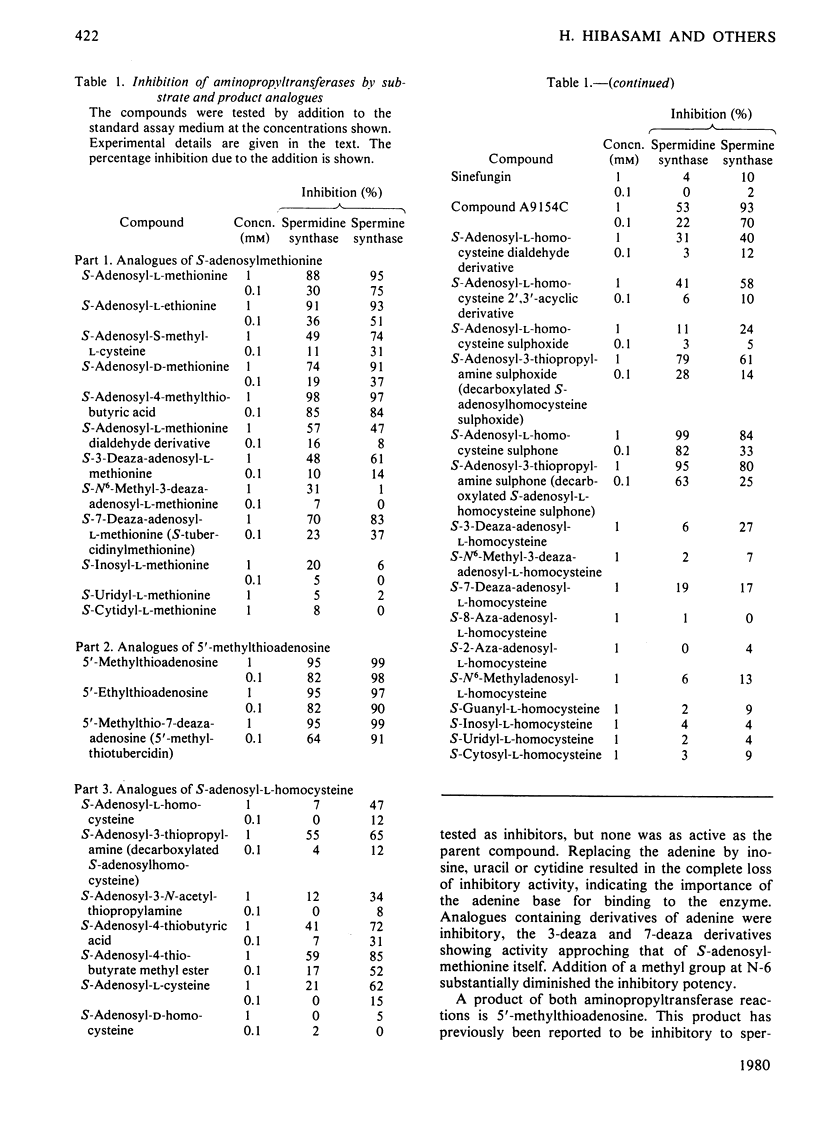
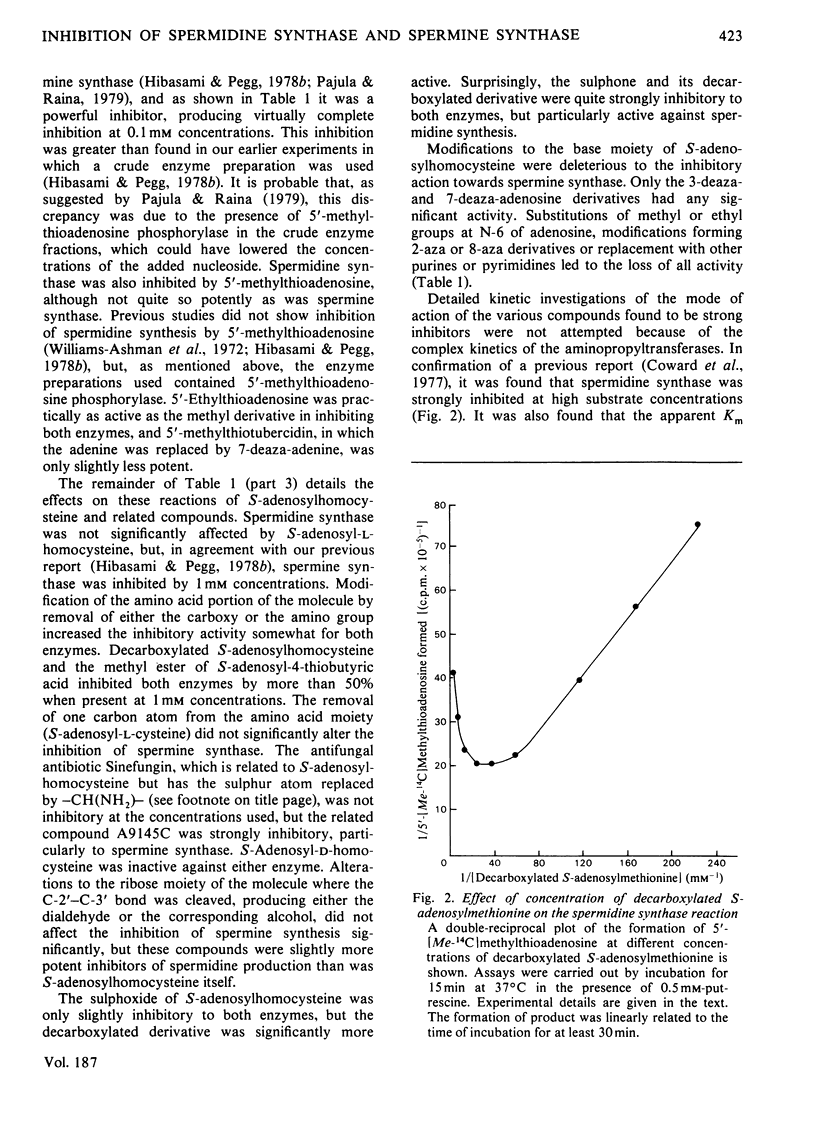
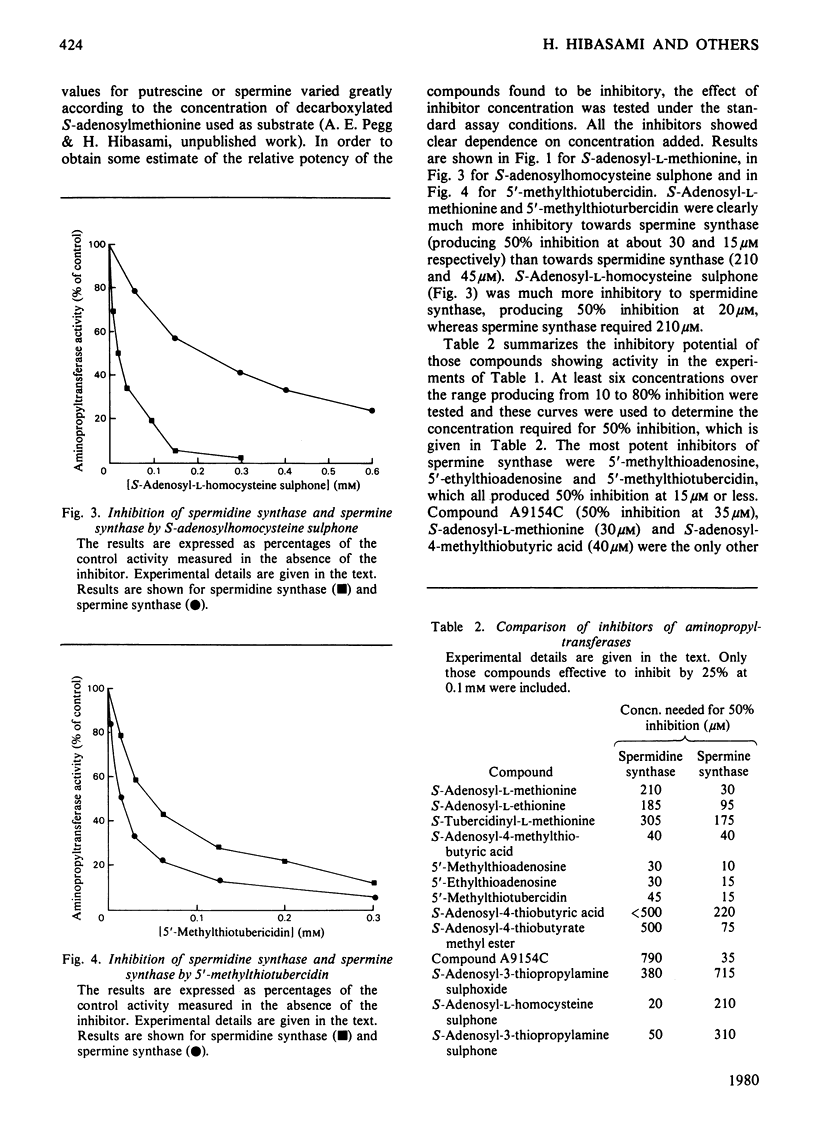
1. S-Adenosyl-l-methionine, S-adenosyl-l-homocysteine, 5′-methylthioadenosine and a number of analogues having changes in the base, sugar or amino acid portions of the molecule were tested as potential inhibitors of spermidine synthase and spermine synthase from rat ventral prostate. 2. S-Adenosyl-l-methionine was inhibitory to these reactions, as were other nucleosides containing a sulphonium centre. The most active of these were S-adenosyl-l-ethionine, S-adenosyl-4-methylthiobutyric acid, S-adenosyl-d-methionine and S-tubercidinylmethionine, which were all comparable in activity with S-adenosylmethionine itself, producing 70–98% inhibition at 1mm concentrations. Spermine synthase was somewhat more sensitive than spermidine synthase. 3. 5′-Methylthioadenosine, 5′-ethylthioadenosine and 5′-methylthiotubercidin were all powerful inhibitors of both enzymes, giving 50% inhibition of spermine synthase at 10–15μm and 50% inhibition of spermidine synthase at 30–45μm. 4. S-Adenosyl-l-homocysteine was a weak inhibitor of spermine synthase and practically inactive against spermidine synthase. Analogues of S-adenosylhomocysteine lacking either the carboxy or the amino group of the amino acid portion were somewhat more active, as were derivatives in which the ribose ring had been opened by oxidation. The sulphoxide and sulphone derivatives of decarboxylated S-adenosyl-l-homocysteine and the sulphone of S-adenosyl-l-homocysteine were quite potent inhibitors and were particularly active against spermidine synthase (giving 50% inhibition at 380, 50 and 20μm respectively). 5. These results are discussed in terms of the possible regulation of polyamine synthesis by endogenous nucleosides and the possible value of some of the inhibitory substances in experimental manipulations of polyamine concentrations. It is suggested that 5′-methylthiotubercidin and the sulphone of S-adenosylhomocysteine or of S-adenosyl-3-thiopropylamine may be particularly valuable in this respect.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borchardt R. T., Huber J. A., Wu Y. S. Potential inhibitor of S-adenosylmethionine-dependent methyltransferases. 2. Modification of the base portion of S-adenosylhomocysteine. J Med Chem. 1974 Aug;17(8):868–873. doi: 10.1021/jm00254a017. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T., Huber J. A., Wu Y. S. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 4. Further modifications of the amino and base portions of S-adenosyl-L-homocysteine. J Med Chem. 1976 Sep;19(9):1094–1099. doi: 10.1021/jm00231a003. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T., Shiong Y., Huber J. A., Wycpalek A. F. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 6. Structural modifications of S-adenosylmethionine. J Med Chem. 1976 Sep;19(9):1104–1110. doi: 10.1021/jm00231a005. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T., Wu Y. S. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 1. Modification of the amino acid portion of S-adenosylhomocysteine. J Med Chem. 1974 Aug;17(8):862–868. doi: 10.1021/jm00254a016. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T., Wu Y. S. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 3. Modifications of the sugar portion of S-adenosylhomocysteine. J Med Chem. 1975 Mar;18(3):300–304. doi: 10.1021/jm00237a018. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T., Wu Y. S., Wu B. S. Affinity labeling of histamine N-methyltransferase by 2',3'-dialdehyde derivatives of S-adenosylhomocysteine and S-adenosylmethionine. Kinetics of inactivation. Biochemistry. 1978 Oct 3;17(20):4145–4152. doi: 10.1021/bi00613a007. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T., Wu Y. S., Wu B. S. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 7. Role of the ribosyl moiety in enzymatic binding of S-adenosyl-L-homocysteine and S-adenosyl-L-methionine. J Med Chem. 1978 Dec;21(12):1307–1310. doi: 10.1021/jm00210a026. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T., Wu Y. S-Aristeromycinyl-L-homocysteine, a potent inhibitor of S-adenosylmethionine-dependent transmethylations. J Med Chem. 1976 Jan;19(1):197–198. doi: 10.1021/jm00223a043. [DOI] [PubMed] [Google Scholar]
- Bowman W. H., Tabor C. W., Tabor H. Spermidine biosynthesis. Purification and properties of propylamine transferase from Escherichia coli. J Biol Chem. 1973 Apr 10;248(7):2480–2486. [PubMed] [Google Scholar]
- Cornforth J. W., Reichard S. A., Talalay P., Carrell H. L., Glusker J. P. Determination of the absolute configuration at the sulfonium center of S-adenosylmethionine. Correlation with the absolute configuration of the diastereomeric S-carboxymethyl-(S)-methionine salts. J Am Chem Soc. 1977 Oct 26;99(22):7292–7300. doi: 10.1021/ja00464a032. [DOI] [PubMed] [Google Scholar]
- Coward J. K., Motola N. C., Moyer J. D. Polyamine biosynthesis in rat prostate. Substrate and inhibitor properties of 7-deaza analogues of decarboxylated S-adenosylmethionine and 5'-methylthioadenosine. J Med Chem. 1977 Apr;20(4):500–505. doi: 10.1021/jm00214a008. [DOI] [PubMed] [Google Scholar]
- Garbers D. L. Demonstration of 5'-methylthioadenosine phosphorylase activity in various rat tissues. Some properties of the enzyme from rat lung. Biochim Biophys Acta. 1978 Mar 14;523(1):82–93. doi: 10.1016/0005-2744(78)90011-6. [DOI] [PubMed] [Google Scholar]
- Hannonen P., Jänne J., Raina A. Separation and partial purification of S-adenosylmethionine decarboxylase and spermidine and spermine synthases from rat liver. Biochem Biophys Res Commun. 1972 Jan 31;46(2):341–348. doi: 10.1016/s0006-291x(72)80144-x. [DOI] [PubMed] [Google Scholar]
- Hibasami H., Pegg A. E. Differential inhibition of mammalian aminopropyltransferase activities. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1398–1405. doi: 10.1016/0006-291x(78)91291-3. [DOI] [PubMed] [Google Scholar]
- Hibasami H., Pegg A. E. Rapid and convenient method for the assay of aminopropyltransferases. Biochem J. 1978 Mar 1;169(3):709–712. doi: 10.1042/bj1690709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Leboy P. S., Glick J. M., Steiner F. G., Haney S., Borchardt R. T. S-adenosylhomocysteine analogues as inhibitors of specific tRNA methylation. Biochim Biophys Acta. 1978 Aug 23;520(1):153–163. doi: 10.1016/0005-2787(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Montgomery J. A., Shortnacy A. T., Thomas H. J. Analogs of 5'-deoxy-5'-(methylthio)adenosine. J Med Chem. 1974 Nov;17(11):1197–1207. doi: 10.1021/jm00257a014. [DOI] [PubMed] [Google Scholar]
- Pajula R. L., Raina A., Kekoni J. Purification of spermine synthase from bovine brain by spermine-Sepharose affinity chromatography. FEBS Lett. 1978 Jun 1;90(1):153–156. doi: 10.1016/0014-5793(78)80319-6. [DOI] [PubMed] [Google Scholar]
- Pajula R. L., Raina A. Methylthioadenosine, a potent inhibitor of spermine synthase from bovine brain. FEBS Lett. 1979 Mar 15;99(2):343–345. doi: 10.1016/0014-5793(79)80988-6. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Investigation of the turnover of rat liver S-adenosylmethionine decarboxylase using a specific antibody. J Biol Chem. 1979 May 10;254(9):3249–3253. [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Enzymic synthesis of spermine in rat prostate. Arch Biochem Biophys. 1970 Mar;137(1):156–165. doi: 10.1016/0003-9861(70)90422-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Phosphate-stimulated breakdown of 5'-methylthioadenosine by rat ventral prostate. Biochem J. 1969 Nov;115(2):241–247. doi: 10.1042/bj1150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh C. S., Borchardt R. T., Stone H. O. Sinefungin, a potent inhibitor of virion mRNA(guanine-7-)-methyltransferase, mRNA(nucleoside-2'-)-methyltransferase, and viral multiplication. J Biol Chem. 1978 Jun 25;253(12):4075–4077. [PubMed] [Google Scholar]
- RHODES J. B., WILLIAMS-ASHMAN H. G. OBSERVATIONS ON POLYAMINES IN MALE ACCESSORY GLANDS OF REPRODUCTION. Med Exp Int J Exp Med. 1964;10:281–285. doi: 10.1159/000135428. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Methylthio group cleavage from methylthioadenosine. Description of an enzyme and its relationship to the methylthio requirement of certain cells in culture. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1273–1280. doi: 10.1016/0006-291x(77)91430-9. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Methylthioadenosine nucleoside phosphorylase deficiency in methylthio-dependent cancer cells. Biochem Biophys Res Commun. 1978 Jul 14;83(1):27–35. doi: 10.1016/0006-291x(78)90393-5. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Polyamines in mammalian biology and medicine. Perspect Biol Med. 1979 Spring;22(3):421–453. doi: 10.1353/pbm.1979.0013. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Corti A., Tadolini B. On the development of specific inhibitors of animal polyamine biosynthetic enzymes. Ital J Biochem. 1976 Jan-Feb;25(1):5–32. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Jänne J., Coppoc G. L., Geroch M. E., Schenone A. New aspects of polyamine biosynthesis in eukaryotic organisms. Adv Enzyme Regul. 1972;10:225–245. doi: 10.1016/0065-2571(72)90016-7. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- Zappia V., Oliva A., Cacciapuoti G., Galletti P., Mignucci G., Cartení-Farina M. Substrate specificity of 5'-methylthioadenosine phosphorylase from human prostate. Biochem J. 1978 Dec 1;175(3):1043–1050. doi: 10.1042/bj1751043. [DOI] [PMC free article] [PubMed] [Google Scholar]


