Abstract
The circadian rhythm of the immune system helps to protect against pathogens1–3; however, the role of circadian rhythms in immune homeostasis is less well understood. Innate T cells are tissue-resident lymphocytes with key roles in tissue homeostasis4–7. Here we use single-cell RNA sequencing, a molecular-clock reporter and genetic manipulations to show that innate IL-17-producing T cells—including γδ T cells, invariant natural killer T cells and mucosal-associated invariant T cells—are enriched for molecular-clock genes compared with their IFNγ-producing counterparts. We reveal that IL-17-producing γδ (γδ17) T cells, in particular, rely on the molecular clock to maintain adipose tissue homeostasis, and exhibit a robust circadian rhythm for RORγt and IL-17A across adipose depots, which peaks at night. In mice, loss of the molecular clock in the CD45 compartment (Bmal1∆Vav1) affects the production of IL-17 by adipose γδ17 T cells, but not cytokine production by αβ or IFNγ-producing γδ (γδIFNγ) T cells. Circadian IL-17 is essential for de novo lipogenesis in adipose tissue, and mice with an adipocyte-specific deficiency in IL-17 receptor C (IL-17RC) have defects in de novo lipogenesis. Whole-body metabolic analysis in vivo shows that Il17a−/−Il17f−/− mice (which lack expression of IL-17A and IL-17F) have defects in their circadian rhythm for de novo lipogenesis, which results in disruptions to their whole-body metabolic rhythm and core-body-temperature rhythm. This study identifies a crucial role for IL-17 in whole-body metabolic homeostasis and shows that de novo lipogenesis is a major target of IL-17.
Subject terms: Innate lymphoid cells, Interleukins
Innate IL-17-producing T cells—in particular, adipose γδ17 T cells—are enriched in molecular-clock genes, and the circadian expression of IL-17A and RORγt by these cells has a role in maintaining local homeostasis and regulating lipogenesis.
Main
Circadian rhythms allow organisms to anticipate and adjust to predictable changes in the external environment, such as light8, temperature9 and feeding behaviour10. At the cellular level, circadian rhythms are dictated by the molecular clock, an auto-regulatory system of transcriptional activators (BMAL1 and CLOCK) and repressors (PER1–PER3, CRY1 and CRY2 and REV-ERBα and REV-ERBβ), expressed by all mammalian cells11. Cellular rhythms in a tissue are kept in synchrony by a combination of neuronal, hormonal and metabolic cues. These, in turn, give rise to circadian rhythms in physiological processes such as endocrine signalling12, immune cell function1 and systemic metabolism13.
Maintenance of core body temperature is one such physiological process, which oscillates daily over 24 h and is governed by metabolic rate. In mice, body temperature is highest at night while active and lowest during the day while inactive, and is important for optimal whole-body homeostasis14. White adipose tissue (WAT) and brown adipose tissue (BAT) are crucial for whole-body metabolic homeostasis, and are influenced by daily fluctuations in energy demands such as feeding, fasting and activity15. Light and feeding govern the circadian rhythm of adipose lipid metabolism, including lipolysis, lipogenesis and thermogenesis. Lipolysis peaks during the inactive phase to maintain body temperature, whereas lipogenesis peaks during feeding for lipid uptake and storage from the diet16,17. The molecular clock tightly regulates lipid metabolism in adipose tissue, and circadian disruption can lead to impaired lipid homeostasis and subsequent metabolic disorder. For example, circadian disruption, often observed in shift workers, can impair lipid homeostasis and increase the risk of metabolic disease18. Thus, it is important to understand how circadian rhythms interact, especially in the setting of metabolic disease.
Adipose tissue has a unique immune environment that is enriched for innate T cells, including γδ T cells, mucosal-associated invariant T (MAIT) cells and invariant natural killer T (iNKT) cells4,5. The adipose tissue immune system is crucial for maintaining homeostasis, and holds the potential for treating metabolic disease6,7. Cytokines, including TNF19, IL-6 (ref. 20), IL-17A21 and IL-17F22, from adipose immune cells regulate local homeostasis and systemic metabolic processes. We have previously found that clonal IL-17-producing Vγ6+ γδ T cells reside in adipose tissue, and that mice lacking γδ T cells or IL-17A are susceptible to hypothermia21. A subsequent study also showed that IL-17RC signalling in adipocytes was important for thermogenesis22. These studies highlight members of the IL-17 family as key mediators of adipose thermogenesis.
The molecular clock regulates both T helper 17 (TH17) cells and type-3 innate lymphoid cells (ILC3s) in the gut, which produce IL-17 and IL-22 (refs. 23,24). Here we investigated whether type-17 innate T cells are also regulated by the molecular clock. We show that type-17 innate T cells (γδ17, MAIT17 and NKT17) are enriched for molecular-clock genes, compared with type-1 innate T cells (γδIFNγ, MAIT1 and NKT1), particularly in adipose tissue, and that this mediates the circadian fluctuation of RORγt and IL-17A, which peaks at night. We identify adipose de novo lipogenesis (DNL) as a major target of diurnal IL-17 production in adipose tissue. Furthermore, loss of IL-17 signalling in adipocytes disrupts circadian DNL, ultimately impairing systemic metabolic rhythm and circadian body-temperature regulation. We identify a crucial role for adipose IL-17, which is controlled by the molecular clock. In turn, IL-17 regulates circadian physiological processes to support whole-body metabolism. This pathway could provide a therapeutic target for metabolic disease.
Molecular-clock enrichment in innate IL-17+ T cells
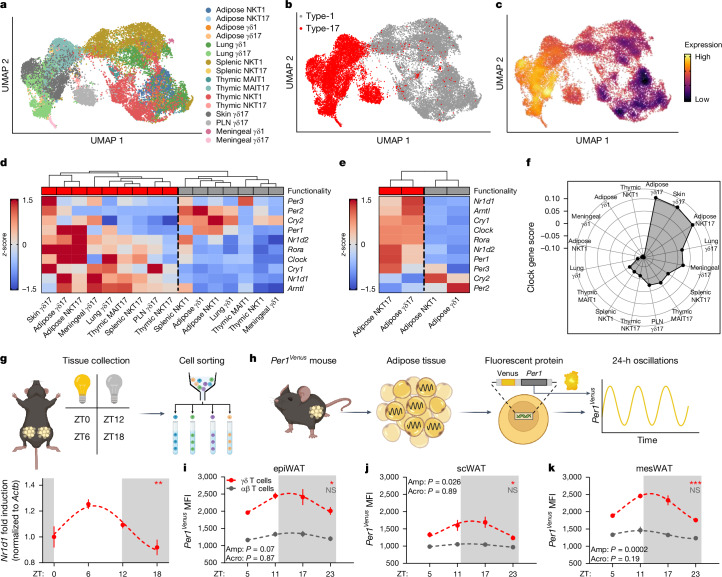
Adaptive CD4+ TH17 cells and innate gut ILC3s, which produce IL-17, express a rhythmic molecular clock23–26. Whether this is a specific trait of type-17 cells, or a trait of innate T cells, remains unknown. We performed single-cell RNA sequencing (scRNA-seq) in innate T cells from epididymal adipose tissue (epiWAT), and compiled publicly available scRNA-seq data on innate T cells from the lungs, spleen, thymus, skin and lymph nodes of wild-type mice, including iNKT, MAIT and γδ T cells (Fig. 1a). Notably, uniform manifold approximation and projection (UMAP) analysis first separated innate T cells into IL-17-producing (type-17) or IFNγ-producing (type-1) cells, and then separated on the basis of tissue origin and cell subtype (Fig. 1a,b). This highlights the distinct transcriptional program of TH17-like cells, regardless of tissue or cell type. We then generated a ‘molecular-clock score’ using the average expression of the molecular-clock genes Arntl (also known as Bmal1), Clock, Nr1d1, Nr1d2, Cry1, Cry2, Per1, Per2, Per3 and Rora (Fig. 1c and Extended Data Fig. 1a), and found enriched expression in type-17 innate T cells compared with their type-1 counterparts (Fig. 1d). In addition, tissue-resident innate T cells (adipose and lung) had higher expression of molecular-clock genes than innate T cells in lymphoid organs (spleen, lymph nodes and thymus; Fig. 1d and Extended Data Fig. 1b).
Fig. 1. Innate IL-17+ T cells are enriched for molecular-clock genes.
a, UMAP of mouse innate T cells across several organs, reanalysed from GSE142845, GSE141895, E-MTAB-7704, E-MTAB-8732, GSE123400 and GSE147262 (all publicly available scRNA-seq datasets). PLN, peripheral lymph node. b, UMAP of mouse innate T cells subclustered on the basis of functionality (type 1 (TH1-like) or type 17 (TH17-like)). c, Density plot of the cumulative expression of all molecular-clock genes across innate T cell subsets, designated the molecular-clock score. d, Heat map of the averaged gene expression of molecular-clock genes with hierarchal clustering of data from a. e, Heat map of the averaged gene expression of molecular-clock genes from adipose tissue innate lymphocytes with hierarchal clustering of data generated in this laboratory. f, Spider plot showing that adipose γδ17 T cells have the highest cumulative expression of molecular-clock genes. g, Top, diagram of the isolation and PCR analysis of adipose γδ T cells. Bottom, relative expression of Rev-erba (also known as Nr1d1) in epiWAT γδ T cells across 24 h. h, Diagram of Per1Venus oscillations in cells isolated from adipose tissue. i–k, Circadian time plots of Per1Venus expression by αβ (grey) and γδ (red) T cells across 24 h in epiWAT (i), scWAT (j) and mesWAT (k). MFI, mean fluorescence intensity. g,i–k, White and grey panels represent light and dark periods, respectively. i–k, Data are representative of two independent experiments. g,i–k, Data are mean ± s.e.m., n = 4–5 mice per group. g,i–k, Significance was calculated using cosinor analysis, with cosine fitted curves; amplitude (amp) and acrophase (acro) were extracted from the cosinor model. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Fig. 1. Innate IL-17+ T cells are enriched for molecular-clock genes within tissues.
a, Density plot expression of individual molecular-clock genes across innate T cell subsets. b, Violin plot showing expression of the molecular-clock score of type-17 versus type-1 innate lymphocytes, further divided by tissue-resident or lymphoid. c, Violin plot showing expression of the molecular-clock score of adipose innate lymphocytes. d, Violin plot showing expression of the molecular-clock score of thymic innate lymphocytes (left), heat map of averaged gene expression of molecular-clock genes with hierarchal clustering of data generated from GSE141895 and E-MTAB-7704 (right). e, Violin plot showing expression of the molecular-clock score of lung innate lymphocytes (left), heat map of averaged gene expression of molecular-clock genes with hierarchal clustering of data generated from E-MTAB-8732 (right). f, Violin plot showing expression of the molecular-clock score of meningeal innate lymphocytes (left), heat map of averaged gene expression of molecular-clock genes with hierarchal clustering of data generated from GSE147262 (right). g, Violin plot showing expression of the molecular-clock score of splenic innate lymphocytes (left), heat map of averaged gene expression of molecular-clock genes with hierarchal clustering of data generated from GSE142845 (right). h, Representative gating strategy for adipose tissue T cells.
To account for time-of-day variation in the scRNA-seq datasets, we analysed each dataset separately for in-run comparisons, comparing innate T cells collected from the same mice at the same time of day only (Fig. 1e). Regardless of the time at which the sequencing was run, type-17 innate T cells had higher expression of molecular-clock genes (Fig. 1e and Extended Data Fig. 1c–g), with adipose γδ T cells showing the highest expression overall (Fig. 1f). Therefore, to investigate the role of the molecular clock in innate type-17 cells, we focused on adipose γδ17 T cells. To confirm that γδ T cells have rhythmic expression of circadian clock genes, we isolated adipose γδ17 T cells across 24 h, and found robust circadian expression of Nr1d1, which peaked before the active phase (Fig. 1g). Next, we used Venus reporter mice to observe Per1 oscillations (Per1Venus; Fig. 1h) in adipose T cells over 24 h. Adipose γδ T cells have rhythmic expression of Per1, with peak expression as the mouse dark cycle is entered (Fig. 1i–k), which was not observed in adipose αβ T cells (Fig. 1i–k). In addition, adipose γδ17 T cells had higher expression of Per1 than adipose αβ T cells in all depots throughout the entire 24-h period, providing further evidence for higher levels of molecular-clock expression in innate type-17 adipose T cells (Fig. 1i–k).
The molecular clock regulates γδ IL-17A
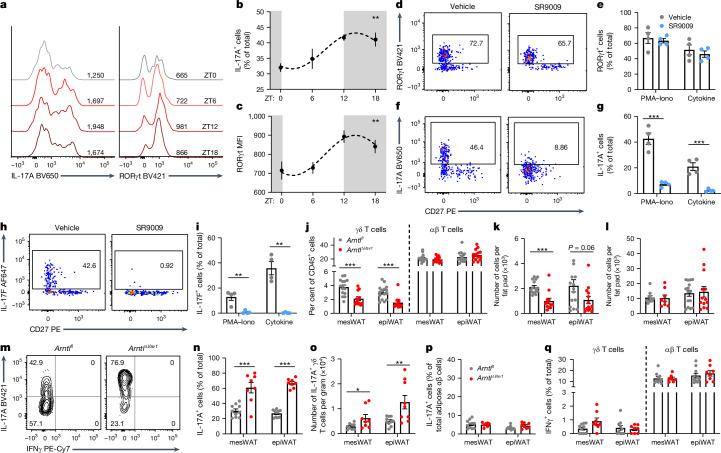
Having established molecular-clock enrichment in γδ T cells, we investigated whether the production of IL-17A and its transcriptional regulator RORγt exhibit a circadian rhythm over 24 h. We first measured the circadian production of IFNγ and IL-17A ex vivo by collecting lymph-node γδ T cells at zeitgeber time (ZT) 0, then splitting them into eight groups for stimulation at 3-h intervals over 24 h (Extended Data Fig. 2a). Ex vivo stimulated γδ17 T cells had a robust circadian rhythm for IL-17A production, which peaked during the night (Extended Data Fig. 2b–e). By contrast, γδIFNγ T cells had an opposing diurnal rhythm that peaked during the day (Extended Data Fig. 2f–i). Previous studies on CD4+ T cells showed that NFIL3 antagonizes IL-17 circadian rhythm23. NFIL3 was expressed almost exclusively in γδ17 T cells, not in γδIFNγ T cells, and exhibited a circadian rhythm in γδ17 T cells (Extended Data Fig. 2j–l). Although lymph-node γδ17 T cells express higher levels of molecular-clock genes than γδIFNγ T cells, adipose tissue γδ17 T cells have the highest expression of these genes (Fig. 1d,f). Therefore, we investigated whether adipose γδ17 T cells expressed a circadian rhythm for IL-17A. There was no change in the total number of γδ17 T cells over a 24-h period (Extended Data Fig. 3a,b). Although adipose γδ17 T cells exhibit a circadian rhythm to their proportions, no rhythm was observed in absolute cell number (Extended Data Fig. 3a,b), suggesting that the fluctuations in proportions are due to changes in circulating γδIFNγ T cells, which are higher in the blood. We found a robust circadian rhythm for IL-17A production, and RORγt expression by adipose γδ17 T cells (Fig. 2a–c). Together, these data show that γδ17 T cells have a circadian rhythm for IL-17A production, which can be maintained intrinsically for at least 24 h ex vivo.
Extended Data Fig. 2. Lymph-node γδ T cells express IL-17A and IFNγ rhythmically.
a, Diagrammatic representation of experimental set-up. b, Representative flow plots of IL-17A expression at nadir (ZT5), and peak (ZT15). c, IL-17A expression by lymph node (LN) γδ T cells stimulated ex vivo for 3 h with PMA/Ionomycin, d, displaying diurnal rhythm. e, Circadian fluctuation of IL-17A production by LN γδ T cells over 24 h. f, Representative flow plots of IFNγ expression at peak (ZT6), and nadir (ZT18). g,h, IFNγ expression by LN γδ T cells stimulated ex vivo for 3 h with PMA/Ionomycin, displaying diurnal rhythm (h). i, Circadian fluctuation of IFNγ production by LN γδ T cells over 24 h. j, Representative flow plots of NFIL3 expression at nadir (ZT12), and peak (ZT0). k, NFIL3 expression by LN γδ T cells stimulated ex vivo for 3 h with PMA/Ionomycin. l, Circadian fluctuation of NFIL3 expression (blue), overlaid with IL-17A production (red), by LN γδ T cells over 24 h. Data are representative of two independent experiments. White and grey panels represent light and dark periods respectively. c-e,g-i,k,l, Data shown as mean ± SEM, n = 4 mice per group. c,d,g,h,k, Significance was calculated using two-tailed unpaired students t-test, e,i,l, Cosinor analysis, with cosine fitted curves, l, amplitude (Amp) and acrophase (Acro) were extracted from the cosinor model. NS = Non-Significant, * p < 0.05, ** p < 0.01.
Extended Data Fig. 3. SR9009 dose-dependently decreases IL-17A and IL-17F expression by lymph-node γδ T cells.
a, Circadian time plot of percentage CD27- and CD27+ γδ T cell subsets in adipose tissue, with b, subset cell numbers of 24 h. c, Lymph-node lymphocyte viability with increasing concentration of SR9009. d, Percentage of lymph-node γδ T cell of total lymphocytes with increasing concentrations of SR9009. e, Representative flow plots of RORγt expression by lymph-node γδ T cell with increasing concentration of SR9009, with f, percentage and g, MFI of RORγt expressed by lymph-node γδ T cells. h, Representative flow plots of IL-17A expression by lymph-node γδ T cell with increasing concentration of SR9009, with i, percentage and j, MFI of IL-17A expressed by lymph-node γδ T cells. k, Percentage and l, MFI of IL-17AF expressed by lymph-node γδ T cells with increasing concentration of SR9009. m, Percentage of IL-17A expressed by lymph-node γδ T cells with increasing concentration of vehicle. n, γδ T cell and o, αβ T cell percentage of total lymphocytes in Arntlfl (grey) and Arntl∆Vav1 (red) mice. p, IL-7R histogram of adipose T cells. q, γδ T cell percentage of total lymphocytes in Arntlfl (grey) and Arntl∆Il7r (red) mice. r, Percentage IL-17A of adipose γδ T cells from Arntlfl (grey) and Arntl∆Vav1 (red) mice. a,b, White and grey panels represent light and dark periods respectively. a,b,n–r, Data shown as mean ± SEM, n = 4–8 mice per group. c,d,f,g,i-o, Data shown as mean ± SEM, c–m, n = 9 technical replicates from 3 mice per group. a,b, Significance was calculated using cosinor analysis, with cosine fitted curves, amplitude (Amp) and acrophase (Acro) were extracted from the cosinor model, c,d,f,g,i-m,p, One-way ANOVA, s,t, Two-tailed unpaired students t-test. NS = Non-Significant, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Fig. 2. IL-17A expression by adipose γδ T cells is regulated by the molecular clock.
a, IL-17A and RORγt histograms from adipose (epiWAT) γδ T cells across 24 h. b, Percentage of IL-17A+ cells. c, RORγt MFI. d, RORγt flow plots from adipose (epiWAT) γδ T cells stimulated with PMA–ionomycin and 40 µM SR9009. e, Percentage of RORγt+ cells. f, IL-17A flow plots from adipose (epiWAT) γδ T cells stimulated with PMA–ionomycin and 40 µM SR9009. g, Percentage of IL-17A+ cells. h, IL-17F flow plots from adipose (epiWAT) γδ T cells stimulated with IL-1β, IL-23 and 40 µM SR9009. i, Percentage of IL-17F+ cells. j, Percentage of γδ T cells and αβ T cells among total lymphocytes in Arntlfl (grey) and Arntl∆Vav1 (red) mice. k,l, Absolute cell numbers of γδ (k) and αβ (l) T cells in adipose tissue of Arntlfl (grey) and Arntl∆Vav1 (red) mice. m, IL-17A flow plots from Arntlfl and Arntl∆Vav1 adipose γδ T cells. n, Percentage of IL-17A+ cells. o, IL-17A+ γδ T cell numbers per gram of adipose tissue in Arntlfl (grey) and Arntl∆Vav1 (red) mice. p, IL-17A+ cells as a percentage of the total adipose αβ T cells. q, IFNγ expression by adipose αβ and γδ T cells from Arntlfl (grey) and Arntl∆Vav1 (red) mice. b,c, White and grey panels represent light and dark periods, respectively. Data are representative of three (a–c,j–q) or two (d–i) independent experiments. b,c,e,g,i–l,n–q, Data are mean ± s.e.m., n = 4–15 mice per group. b,c, Significance was calculated using cosinor analysis, with cosine fitted curves, e,g,i–l,n–q, Two-tailed unpaired student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine the influence of the molecular clock on IL-17A expression by adipose γδ17 T cells, we used SR9009, a REV-ERBα agonist that inhibits RORγt-mediated transcription by competing for DNA-binding sites27. We observed that SR9009 has no effect on RORγt expression by adipose or lymph-node γδ17 T cells (Fig. 2d,e and Extended Data Fig. 3e–g). However, SR9009 completely inhibited IL-17A and IL-17F expression by adipose γδ17 T cells (Fig. 2f–i). SR9009 did not affect lymph-node lymphocyte viability or γδ17 T cell proportions (Extended Data Fig. 3c,d), but it caused a dose-dependent decrease in the production of IL-17A and IL-17F by lymph-node γδ17 T cells (Extended Data Fig. 3h–m). These data demonstrate the molecular-clock modulation of IL-17A expression by γδ17 T cells, particularly in adipose tissue.
To further investigate the role of the molecular clock in IL-17A expression, mice were generated with a specific deletion of Arntl in CD45-expressing cells (Arntl∆Vav1 mice). We found that deletion of Arntl decreased the percentage and number of γδ T cells in mesenteric adipose tissue (mesWAT) and epiWAT (Fig. 2j,k), but did not have this effect in γδ intraepithelial lymphocytes (IELs) (Extended Data Fig. 3n). Deletion of Arntl in CD45+ cells also did not affect the proportion or number of adipose αβ T cells, or IELs (Fig. 2j,l and Extended Data Fig. 3o). Notably, loss of Arntl increased both the proportion and the cell number of adipose IL-17A+ γδ T cells, compared with Arntlfl controls (Fig. 2m–o), but had no effect on IL-17A or IFNγ expression in adipose αβ T cells (Fig. 2p,q). γδ17 T cells have the highest expression of IL-7R in adipose tissue (Extended Data Fig. 3p), so we specifically deleted Arntl in IL-7R expressing cells (Arntl∆Il7r mice). Similarly to the Arntl∆Vav1 mice, there were decreased levels of adipose γδ T cells in the Arntl∆Il7r mice, but no difference in the lungs and spleen (Extended Data Fig. 3q). Again, adipose γδ T cells lacking Arntl had increased IL-17A expression (Extended Data Fig. 3r). These data suggest that the molecular clock is a major regulator of IL-17A and IL-17F, whereby activation of Rev-erba (also known as Nr1d1) decreases IL-17 expression, whereas loss of Bmal1 (Arntl) increases it. Together, these data show that the molecular clock regulates the homeostatic function of adipose γδ17 T cells.
Environmental factors regulate γδ IL-17A
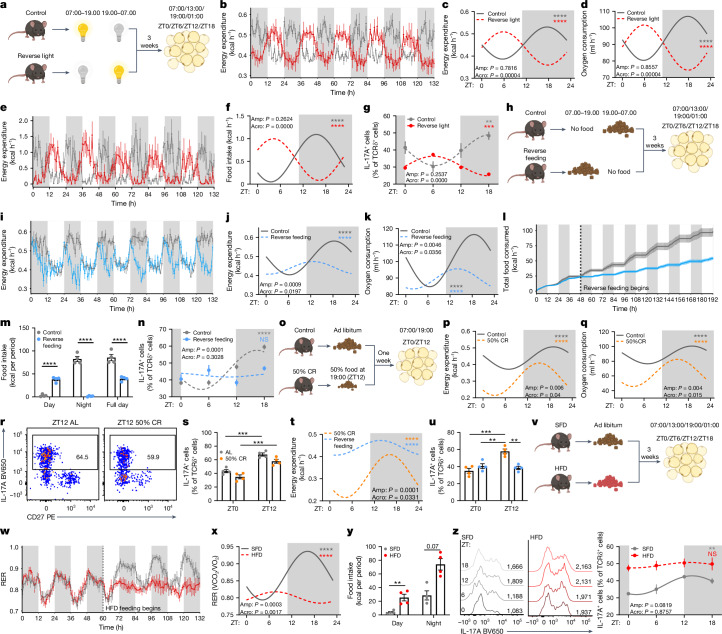
Light is a major entrainment signal for the circadian regulation of whole-body metabolic rhythm. Therefore, we investigated whether light is an entrainment signal for circadian IL-17A production by adipose γδ T cells. Using metabolic cages, mice were housed under normal light conditions (lights on 07:00–19.00; control) or on a reversed light–dark cycle (lights on 19:00–07:00 for three weeks; Fig. 3a). After three weeks of inverted light, energy expenditure and metabolic rate rhythms were completely reversed (Fig. 3b,d and Extended Data Fig. 4a). Adipose tissue was then collected from both groups at ZT0, ZT6, ZT12 and ZT18 (Fig. 3a). IL-17A production by adipose γδ17 T cells had a circadian rhythm, which was reversed in the light-inverted group (Fig. 3g). Thus, light is a major entrainment signal for IL-17A expression by γδ17 T cells.
Fig. 3. Light entrains whole-body metabolism and IL-17A production by adipose γδ T cells.
a, Reverse-light-cycle experimental set-up. b, Energy expenditure of regular (grey) and inverted-light-cycle (red) mice after three weeks. c,d, Circadian plots of energy expenditure (c) and oxygen consumption (d). e, Food consumption of regular (grey) and inverted-light-cycle (red) mice after three weeks. f, Circadian plot of food consumption. g, Percentage of IL-17A+ cells among adipose γδ T cells of normal (grey) and reverse-light-cycle (red) mice over 24 h. h, Reverse-feeding experimental set-up. i, Energy expenditure of regular (grey) and reverse-fed (blue) mice after three weeks. j,k, Circadian plots of energy expenditure (j) and oxygen consumption (k). l, Cumulative food consumption of regular (grey) and reverse-fed (blue) mice after three weeks. m, Averaged food consumption. n, Percentage of IL-17A+ cells among adipose γδ T cells of regular (grey) and reverse-fed (blue) mice over 24 h. o, Experimental set-up for 50% CR feeding. p,q, Energy expenditure (p) and oxygen consumption (q) of ad-libitum-fed (grey) and 50%-CR-fed (orange) mice over 24 h. r, IL-17A flow plots of adipose γδ T cells from mice fed ad libitum (AL) and mice fed a 50% CR diet. s, Percentage of IL-17A+ cells. t, Energy expenditure from 50%-CR-fed (orange) and reverse-fed (blue) mice over 24 h. u, Percentage of IL-17A+ cells among adipose γδ T cells. v, HFD experimental set-up. w, RER SFD-fed (grey) and HFD-fed (red) mice after three weeks. x, Circadian plot of RER. y, Bar plot of food intake. z, Left, IL-17A histograms of adipose γδ T cells from SFD-fed and HFD-fed mice. Right, percentage of IL-17A+ cells. White and grey panels represent light and dark periods, respectively. a–u, Data are representative of three independent experiments; w–z, two independent experiments. c,d,f,j,k,p,q,t,x, Data are mean; b,e,g,i,l–n,s,u,w,y,z, Data are mean ± s.e.m.; n = 4–6 mice per group. b–g,i–l,n,p,q,t,x,z, Significance was calculated using cosinor analysis, with cosine fitted curves; amplitude (amp) and acrophase (acro) were extracted from the cosinor model. m, Two-group analysis with ANCOVA. s,u, Two-way ANOVA. y, Two-tailed unpaired student’s t-test. NS, not significant, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 4. Whole-body metabolic rate is entrained by light and disrupted by irregular feeding.
a, Time plot of metabolic rate parameter (oxygen consumption) of mice on a regular light cycle (grey; lights on 7am – 7pm), versus mice on an inverted light cycle (red; lights on 7pm – 7am) after 3 weeks on these light cycles. b, Time plot of metabolic rate parameter (oxygen consumption) of mice on a regular feeding cycle (grey; feeding 7pm – 7am), versus mice on a reverse feeding cycle (blue; feeding 7am – 7pm) after 3 weeks on these feeding regimens. c, Time plot of metabolic rate parameter (oxygen consumption) of mice on an ad libitum (grey), versus mice on a 50% CR feeding regimen (orange) after 1 week on these feeding regimens. d, Time plot of energy expenditure of mice on an ad libitum (grey), versus mice on a 50% CR feeding regimen (orange) after 1 week on these feeding regimens. e, Per cent feeding during day or night of SFD and HFD-fed mice. f, Representative histograms (left), of adipose γδ T cells from SFD (grey) or HFD (red) fed mice with circadian time plots of RORγt MFI (right). g, Diagrammatic representation of experimental set-up for long-term HFD feeding regimen experiments. h, Time plot of weight gain over a 16-week HFD feeding regime. White and grey panels represent light and dark periods respectively. Data are representative of a–d three independent experiments, or e,f, two independent experiments. a–h, data shown as mean ± SEM, n = 4–9 mice per group. f, Significance was calculated using cosinor analysis, with cosine fitted curves, amplitude (Amp) and acrophase (Acro) were extracted from the cosinor model, e, Two-tailed unpaired students t-test, h, two-way repeated measures ANOVA followed by Bonferroni’s multiple comparisons test. NS, Non-Significant, ** p < 0.01, *** p < 0.001.
Previous studies have shown an increase in circulating IL-17A in both mice and humans after eating28. Adipose tissue is sensitive to feeding behaviour; it expands and contracts during feeding (active) and fasting (inactive) periods, respectively29. A caveat of reversing the light cycle of mice is that the feeding cycle is also reversed (Fig. 3e,f); thus, we investigated whether the adipose IL-17A rhythm was entrained by light as a result of reverse feeding. Mice were placed into metabolic cages under normal light conditions but with reversed feeding schedules, with access to food from 07:00–19.00 (reverse feeding), or from 19.00–07:00 (control) for three weeks (Fig. 3h). Of note, energy expenditure and metabolic rate rhythms did not completely reverse, but rather, were disrupted in reverse-fed mice compared with controls (Fig. 3i–k and Extended Data Fig. 4b). Furthermore, reverse-fed mice had disrupted circadian expression of IL-17A by adipose γδ17 T cells, with a decreased amplitude and an altered acrophase compared with controls (Fig. 3n), suggesting that adipose IL-17 is linked with feeding. However, reverse-fed mice consumed approximately 50% fewer calories than the control mice did (Fig. 3l,m), which could have affected adipose IL-17 production. To investigate whether the disruption to IL-17A expression was due to decreased food intake or the time of feeding, mice were fed ad libitum or fed a 50% calorie-restricted diet (50% CR), reducing the amount of food consumed to match the amount of food eaten by the reverse-fed mice (Fig. 3o). Although the calorie-restricted diet decreased energy expenditure and metabolic rate at all times of day, compared with the ab-libitum-fed mice, the calorie-restricted group maintained the rhythm (amplitude) of energy expenditure and metabolic rate (Fig. 3p,q and Extended Data Fig. 4c,d). Likewise, calorie restriction reduced IL-17 expression (Fig. 3r,s), but did not affect the rhythm of IL-17 production (Fig. 3r,s). Comparing the circadian rhythm of energy expenditure in calorie-restricted and reverse-fed mice, both consuming the same amount of food, revealed differences in amplitude and acrophase between the feeding regimens (Fig. 3t). Furthermore, this was associated with a loss of IL-17 rhythm by adipose γδ17 T cells only in the reverse-fed group (Fig. 3u). Together, these data suggest that light entrains the production of IL-17A by adipose γδ17 T cells, and that this effect occurs mostly through feeding behaviour, because disrupted feeding behaviour disturbs IL-17A expression in adipose γδ17 T cells.
A high-fat diet disrupts circadian IL-17A expression
Mice exhibit a circadian rhythm in fuel use, switching between carbohydrate and lipid burning across dark and light cycles. This can be measured in metabolic cages by the respiratory exchange ratio (RER), in which 0.7 indicates lipid burning and 1 indicates carbohydrate use. Switching to a 60% high-fat diet (HFD) for 3 weeks disrupts the RER rhythm, causing mice to use lipids throughout both light and dark cycles, as previously described30 (Fig. 3v–x). In addition, a one-week HFD was sufficient to disrupt the normal feeding rhythm of mice; it caused them to feed more during the day, indicating increased grazing, as previously reported31,32 (Fig. 3y and Extended Data Fig. 4e). We found that a three-week HFD increased the expression of IL-17A and RORγt by adipose γδ17 T cells throughout 24 h, disrupting the IL-17A rhythm (Fig. 3z and Extended Data Fig. 4f). Inhibition of the IL-17A signalling axis in adipose tissue has been shown to prevent diet-induced obesity33. We also found that Il17a/f−/− mice were resistant to weight gain after 16 weeks on a HFD (Extended Data Fig. 4g,h). Together, these results suggest that an increase in caloric intake throughout 24 h by HFD feeding increases the expression of IL-17A by adipose γδ17 T cells, and that loss of IL-17 signalling prevents diet-induced weight gain.
Previous studies have shown that IL-17A signalling has a pathogenic role in neurodegenerative disease, including multiple sclerosis34. In the mouse model of experimental autoimmune encephalomyelitis (EAE), IL-17A produced by γδ T cells has a pathological role in the development and onset of EAE34. We examined the pathological role of disrupted IL-17A from a HFD feeding regimen using the EAE mouse model. HFD-fed mice had faster disease progression, compared with controls that were fed a standard-fat diet (SFD) (Extended Data Fig. 5a–d).
Extended Data Fig. 5. A three-week HFD feeding regime increases the onset and severity of EAE.
a, EAE scores of SFD-fed (grey), and b, HFD-fed (red) mice. c, Disease progression measured by rate of change (disease onset to disease peak) between feeding regimes. d, Time to clinical end-point post EAE induction between feeding regimes. Data are representative of two independent experiments. a–d, data shown as mean ± SEM, n = 6 mice per group. Significance was calculated using b, two-way repeated measures ANOVA followed by Bonferroni’s multiple comparisons test, c, Two-tailed unpaired students t-test, d, Simple survival analysis with Gehan-Breslow-Wilcoxon test. * p < 0.05, ** p < 0.01.
γδ IL-17A regulates DNL
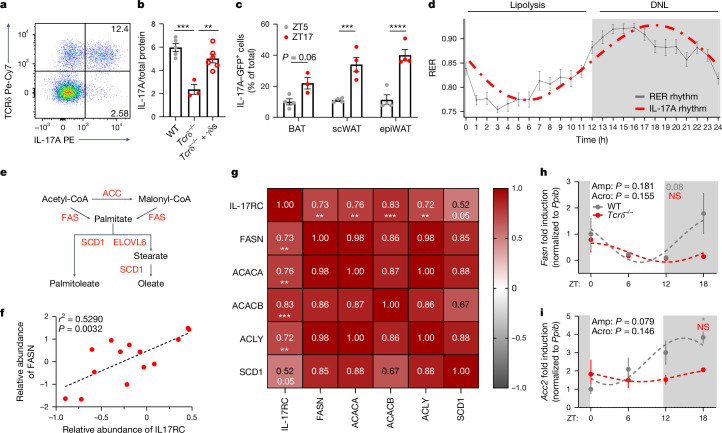
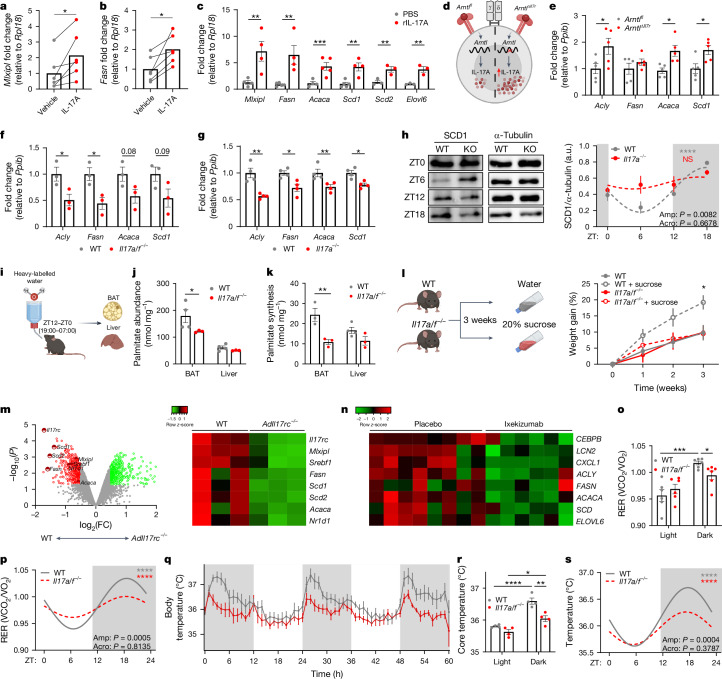
Adipose γδ T cells are the main source of IL-17A in adipose tissue, contributing 75% of the IL-17A in adipose tissue (Fig. 4a and Extended Data Fig. 6a). Loss of adipose γδ T cells decreased the levels of IL-17A protein, and the levels recovered after readministration of γδ T cells (Fig. 4b). Using IL-17A-GFP mice, we found diurnal expression of IL-17A across all adipose depots including BAT, epiWAT and subcutaneous (scWAT) adipose tissue, which peaked at night during the active phase (Fig. 4c). Studies from our laboratory and others have shown that γδ17 T cells have a key role in maintaining body temperature and thermogenesis21,22. We next wondered why fluctuations in IL-17A expression might be important in adipose tissue. Adipose lipolysis and lipogenesis are under strict circadian control, with lipolysis occurring during the day in mice, and lipogenesis happening at night. Overlaying adipose IL-17A expression, we found that IL-17 peaks when lipogenesis peaks15 (Fig. 4d). Because adipose IL-17 was influenced by food intake, we hypothesized that adipose IL-17A might have a role in DNL (Fig. 4e). Using the public BAT proteome database, OPABAT, we found that the levels of IL-17RC protein directly correlated with the abundance of the DNL proteins FASN, ACACA, ACACB, ACLY and SCD1 (Fig. 4f,g and Extended Data Fig. 6b), but not with proteins involved in lipolysis35 (Extended Data Fig. 7a). In addition, DNL protein abundance did not correlate with other cytokine receptor levels in BAT (Extended Data Fig. 7b–f). We isolated adipose tissue across 24 h from mice lacking γδ T cells (Tcrδ−/− mice), and found that the circadian expression of the DNL genes Fasn, Acc2 (also known as Acacb) and Scd1 was disrupted in Tcrδ−/− mice compared with wild-type controls (Fig. 4h,i and Extended Data Fig. 6c). We next investigated the direct effects of IL-17 on DNL, by treating primary brown adipocytes with recombinant (r)IL-17A or vehicle, and found increased expression of a transcriptional regulator of DNL genes, Mlxipl, and fatty acid synthase Fasn, by rIL-17A (Fig. 5a,b). Next, we administered IL-17A in vivo over three days, which increased the expression of all BAT DNL genes (Mlxipl, Acaca, Fasn, Scd1, Scd2 and Elovl6; Fig. 5c). These experiments support the conclusion that γδ-T-cell-derived IL-17 regulates adipose DNL.
Fig. 4. γδ T cells are the main source of adipose tissue IL-17A, and maintain adipose tissue DNL.
a, Representative flow plot showing that γδ T cells are the main producer of IL-17A in adipose tissue. b, Adipose IL-17A protein levels by enzyme-linked immunosorbent assay (ELISA) from wild-type (WT) mice (grey), Tcrδ−/− mice (red) and Tcrδ−/− mice reconstituted with wild-type γδ T cells (γδs; open circles). c, Diurnal expression of IL-17A–GFP by γδ17 T cells across the adipose tissue depots BAT, scWAT and epiWAT. d, Diagrammatic representation of circadian whole-body metabolism by RER, showing the most active metabolic processes during the day and night, with the IL-17A circadian rhythm overlaid. e, Representation of the DNL pathway. f, Correlation analysis of BAT IL-17RC protein levels with FASN abundance from OPABAT. g, Correlation heat map of IL-17RC protein levels with DNL protein abundance from OPABAT. h,i, Circadian time plots showing the relative expression of the DNL genes Fasn (h) and Acc2 (i) from BAT of wild-type (grey) and Tcrδ−/− (red) mice. d,h,i, White and grey panels represent light and dark periods, respectively. a–d,h,i, Data are representative of two independent experiments. b–d,h,i, Data are mean ± s.e.m., n = 4–12 mice per group. Significance was calculated using a one-way ANOVA (b), two-tailed unpaired student’s t-test (c), simple linear regression (f,g) or cosinor analysis, with cosine fitted curves (h,i); amplitude (amp) and acrophase (acro) were extracted from the cosinor model. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 6. γδ T cells are the main source of adipose tissue IL-17A, and maintain adipose tissue DNL.
a, Pie chart depicting adipose lymphocytes contributing to total IL-17A expression, γδ T cells (red), non-T lymphocytes (dark grey) and TH17 cells (light grey). b, Correlation analysis of BAT IL-17RC protein levels with ACLY abundance from OPABAT. c, Circadian time plots showing relative expression of the DNL gene Scd1 from BAT of wild-type (grey) and Tcrδ−/− (red) mice. c, White and grey panels represent light and dark periods respectively. c, Data are representative of two independent experiments. c, data shown as mean ± SEM, n = 4–14 mice per group. b, Significance was calculated using a simple linear regression, c, cosinor analysis, with cosine fitted curves, amplitude (Amp) and acrophase (Acro) were extracted from the cosinor model. ** p < 0.01.
Extended Data Fig. 7. BAT DNL proteins do not correlate with the expression of other cytokine receptors.
a, Correlation heat map of IL-17RC protein levels with adipocyte lipolysis-associated protein abundance from OPABAT. b, Correlation heat map of IL-10RB protein levels with DNL protein abundance from OPABAT. c, Correlation heat map of IL-1R1 protein levels with DNL protein abundance from OPABAT. d, Correlation heat map of IL-6ST protein levels with DNL protein abundance from OPABAT. e, Correlation heat map of IL-17RE protein levels with DNL protein abundance from OPABAT. f, Correlation heat map of IL-4R protein levels with DNL protein abundance from OPABAT. a–f, Significance was calculated using simple linear regression. * p < 0.05, ** p < 0.01.
Fig. 5. IL-17A signalling is necessary for adipocyte DNL, and for whole-body metabolism.
a,b, Expression of Mlxipl (a) and Fasn (b) in brown adipocytes treated with IL-17A (2 µg). c, BAT DNL gene expression from PBS- or IL-17A-treated mice. d, Diagram showing the increased expression of IL-17A in Arntl∆Il7r mice. e, Acly, Fasn, Acaca and Scd1 gene expression from wild-type or Arntl∆Il7r BAT. f,g, Acly, Fasn, Acaca and Scd1 gene expression from wild-type or Il17a/f−/− BAT (f), and from wild-type or Il17a−/− BAT (g). h, Left, western blots for the circadian expression of SCD1 from wild-type (grey) and Il17a−/− (knockout; KO) BAT. Right, circadian plot. a.u., arbitrary units. i, Heavy-labelled-water protocol for palmitate labelling. j,k, Palmitate abundance (j) and palmitate synthesis (k) in wild-type (grey) and Il17a/f−/− (red) BAT and liver. l, Left, experimental set-up for high-sucrose feeding. Right, weight gain after three weeks. m, Left, volcano plot of DNL-associated gene expression in BAT from wild-type and AdIl17rc−/− mice, from GSE144255. log2(FC), log2-transformed fold change. Right, differential expression heat map of DNL-associated genes. n, DNL-associated genes from skin biopsies of patients with psoriasis who were treated with placebo or ixekizumab (150 mg). o, RER averaged by light or dark cycle. p, Circadian rhythmicity analysis. q, Wild-type (grey) and Il17a/f−/− body temperature. r, Core-temperature data averaged by light or dark cycle. s, Circadian rhythmicity analysis. White and grey panels represent light and dark periods, respectively. Data are representative of one independent experiment (h–k), two independent experiments (a–g,l) or four independent experiments, with two experiments pooled (o–s). c,e-h,j-i,o,q,r Data are mean ± s.e.m., n = 3–6 mice per group. Significance was calculated using two-tailed paired student’s t-test (a,b), two-tailed unpaired student’s t-test (c,e–g), two-way ANOVA (l), repeated measures ANOVA, (j,k,o,r) or cosinor analysis, with cosine fitted curves (h,p,s); amplitude (amp) and acrophase (acro) were extracted from the cosinor model. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001 ****P < 0.0001.
Because we found an increase in IL-17A expression in adipose γδ T cells from Arntl∆Il7r mice (Extended Data Fig. 3r and Fig. 5d), we investigated whether there was also a corresponding increase in the expression of DNL genes. Indeed, expression of Acly, Acaca and Scd1 was increased in the BAT of Arntl∆Il7r mice, compared with Arntlfl controls, specifically at ZT18, but not at ZT6 (Fig. 5e and Extended Data Fig. 8a). Previous studies have shown that Il17a−/− and Il17f−/− mice have impaired thermogenesis21,22. Because adipose γδ T cells produce both IL-17A and IL-17F, which can compensate for one another, we used IL-17A and IL-17F double-knockout mice (Il17a/f−/−). BAT taken from Il17a/f−/− mice at ZT6 and ZT18, in the middle of the inactive and the active phases, respectively, showed lower expression of DNL genes at ZT18, compared with BAT from wild-type mice, but this was not the case at ZT6 (Fig. 5f and Extended Data Fig. 8b). This effect was also observed in Il17a−/− single-knockout mice (Fig. 5g). Furthermore, we found that Il17a/f−/− mice had decreased expression of thermogenesis and lipolysis genes at ZT6 and ZT18, compared with wild-type mice (Extended Data Fig. 8c,d). However, wild-type and Il17a/f−/− mice upregulated DNL genes when administered rIL-17A or rIL-17F (Extended Data Fig. 8e–h). No differences were observed between wild-type and Il17a/f−/− groups that were treated with phosphate-buffered saline (PBS), because injections were given during the day, when DNL and IL-17A expression are low (Extended Data Fig. 8e–h). At the protein level, circadian expression of SCD1 was disrupted in Il17a−/− mice, compared with wild-type controls (Fig. 5h). Next, heavy-labelled water (2H2O) was used to determine the ability of wild-type and Il17a/f−/− BAT to synthesize palmitate. We found decreased lipid synthesis in Il17a/f−/− BAT compared with wild-type, but no significant difference in the liver (Fig. 5i–k). Owing to decreased DNL in the BAT of IL-17-deficient mice, we placed wild-type and Il17a/f−/− mice on a three-week high-sucrose regime that promotes DNL, and found that Il17a/f−/− mice were resistant to high-sucrose-induced weight gain (Fig. 5l).
Extended Data Fig. 8. IL-17A regulates DNL and lipolysis gene expression.
a, Relative expression of DNL genes Mlxipl, Fasn, Acaca, Scd1 and Elovl6 in the BAT of Arntlfl mice compared to BAT of Arntl∆Il7r mice at ZT6. b, Relative expression of DNL genes Mlxipl, Fasn, Acaca, Scd1 and Elovl6 in the BAT of wild-type mice compared to BAT of Il17a/f−/− mice at ZT6. c, Relative expression of lipolysis/thermogenesis genes Ucp1, Cidea, Ppargc1a, Atgl and Hsl in the BAT of wild-type mice compared to BAT of Il17a/f−/− mice at ZT6. d, Relative expression of lipolysis/thermogenesis genes Ucp1, Cidea, Ppargc1a, Atgl and Hsl in the BAT of Arntlfl mice compared to BAT of Arntl∆Il7r mice at ZT18. e–h, Relative expression of Mlxipl (e), Acly (f), Fasn (g) and Scd1 (h) after administration of IL-17A (1 µg) and IL-17F (1 µg) to wild-type and Il17a/f−/− mice compared to PBS administered controls. i, Expression of ACACA and SCD from skip biopsies of patients with psoriasis who were treated with placebo and brodalumab. a–i, Data shown as mean ± SEM, n = 2–5 mice per group, or n = 27–33 patients per group i. a–d,i, Significance was calculated using Two-tailed unpaired student’s t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
We next took advantage of publicly available transcriptomic data of adipocyte-specific deletion of the IL-17 receptor, IL-17RC. We found that the DNL genes Mlxipl, Acaca, Fasn, Scd1, Scd2 and Srebf1 were significantly downregulated in BAT lacking IL-17RC, compared with wild-type BAT (Fig. 5m). Furthermore, publicly available clinical data from skin biopsies of patients with psoriasis who were receiving either a placebo or anti-IL-17 therapy show that individuals who were treated with brodalumab36 (anti-IL-17RA; Extended Data Fig. 8i) or ixekizumab37 (anti-IL-17A; Fig. 5n) exhibited a decrease in the expression of DNL genes. Combined, these data show that IL-17 signalling has a role in regulating DNL in BAT and human skin.
Finally, we performed whole-body metabolic analysis in wild-type and Il17a/f−/− mice at thermoneutrality (30 °C). Thermoneutrality was chosen to remove any influence from adaptive thermogenesis, because mice that are housed at room temperature expend approximately 40% of their energy to keep warm at room temperature38,39. A previous study suggested that BAT DNL is crucial for the maintenance of body temperature in mice40. We found that Il17a/f−/− mice have an altered RER, particularly at night (Fig. 5o), and show a blunted circadian RER rhythm (Fig. 5p). Notably, the body temperature of Il17a/f−/− mice was decreased at all times of day, but especially during the active phase (Fig. 5r,s). Circadian analysis found that Il17a/f−/− mice had a significantly blunted circadian fluctuation of body temperature over 24 h (Fig. 5s). Combined, our results show that circadian IL-17A from adipose-tissue-resident γδ T cells is important for physiological whole-body metabolism and body temperature, and that it targets the adipose tissue DNL pathway.
Discussion
Here we show that type-17 innate T cells—particularly adipose γδ17 T cells—are enriched for the molecular clock. The molecular clock controls adipose γδ17 T cell maintenance and IL-17A production, which is essential for adipose tissue circadian homeostasis, whole-body metabolism and body-temperature regulation, through the circadian regulation of DNL. Tissue-resident innate lymphocytes (γδ T cells21, iNKT cells7 and ILCs41) produce effector cytokines that are important for host defence, but also for the normal maintenance of tissue physiology. We show that the molecular clock is more prominent in type-17 tissue-resident innate T cells than it is in type-1 cells, particularly in adipose tissue. The molecular clock has been shown to regulate IL-17A responses in ILC3s26 and TH17 cells23; however, circadian rhythms in innate T cells have not been described. In the small intestine, genetic deletion of Nfil3 or Rev-erba disrupted IL-17A expression, but had no effect on the expression of IFNγ by TH1 cells23. Furthermore, IL-17A-producing ILC3s also had higher expression of molecular-clock genes than ILC1s26, ILC2s25 and ILC progenitors24. Our data show that γδ17 T cells have a daily rhythm to IL-17A and RORγt expression, which is negatively regulated by REV-ERBα activity. We found that all adipose depots, including BAT, had a diurnal rhythm to IL-17A expression by γδ17 T cells. These studies, combined with our findings, suggest that enriched expression of the molecular clock is a conserved feature of IL-17A-producing lymphocytes.
Previous work by our laboratory and others have described a key thermogenic role for IL-17A—and/or IL-17F—signalling in adipose tissue21,22. However, these studies were performed in cold-challenged mice, and here we describe a circadian homeostatic role of IL-17A. Given the cold-challenge results, it was at first puzzling that we found peaks of IL-17A expression by adipose γδ17 T cells at night during feeding, rather than during the day when lipolysis is highest in mice. At night, when mice feed, energy is stored as triacylglycerides in adipose tissue through DNL42. Another study found that DNL and lipolysis in adipose tissue are intricately connected. The research showed that loss of DNL, through the BAT-specific disruption of SREBP activity, was sufficient to disrupt the circadian body temperature of mice, providing evidence that BAT DNL has an essential role in maintaining body temperature40. Therefore, loss of DNL circadian rhythm in Il17a/f−/− mice might blunt lipolysis owing to a reduction in the lipid stores that act as fuel for circadian lipolysis and body-temperature regulation. We show that disrupted DNL could be restored by administering IL-17A and IL-17F in vitro and in vivo. A reanalysis of a previously published dataset found that loss of IL-17RC specifically in adipocytes significantly reduced BAT DNL gene expression22. The authors concluded that IL-17F, rather than IL-17A, mediates the pro-thermogenic effect on adipose tissue because of the IL-17RC deletion. However, both IL-17A and IL-17F are known to signal through IL-17RC heterodimers with IL-17RA. Thus, both cytokines might act in synergistic or compensatory mechanisms to promote thermogenesis, and maintain body temperature43–45. Thus, here we analysed Il17a/f−/− double-knockout mice, and found a marked defect in the daily fluctuations of body-temperature rhythm.
A previous study found that BMAL1 deficiency specifically in RORγt-expressing cells (Bmal1∆Rorc) led to an increase in the expression of genes that are involved in lipid metabolism in gut epithelial cells24. This study attributed the phenotype to a loss of IL-22 in ILC3s. A similar study found that IL-17A expression was enhanced in Bmal1∆Rorc mice, suggesting that REV-ERBα negatively regulates IL-17 expression, in line with our data. Furthermore, Bmal1∆Rorc mice had an increase in the mass of gonadal and subcutaneous adipose tissue24, which supports our data linking IL-17 to lipid metabolism and to enhanced lipid synthesis and storage. This suggests that increased IL-17, rather than decreased IL-22 from gut ILC3s, drives the enhanced expression of lipid-metabolism genes in the gut epithelial cells of Bmal1∆Rorc mice, although further work is needed to investigate this. Together, these data highlight that the IL-17 cytokine family is important for lipid metabolism in both adipocytes and gut epithelial cells, where innate T cells and ILCs reside.
IL-17A derived from γδ T cells, among others, has a pathological role in the initiation of EAE34. In addition, a HFD exacerbates EAE pathology, and increases brain infiltration by TH17 cells, enhancing the production of IL-17A by TH17 cells. We found that a three-week HFD, before the onset of obesity, enhanced EAE and disrupted the circadian rhythm of IL-17A, leading to constantly elevated levels over 24 h (ref. 46). Shift workers are reported to have an increased preference for high-fat, high-calorie foods, in addition to irregular meal timings47–49. In modern living, all-day grazing and constant food availability have changed the way many people eat in the Western world. In mice, a HFD disrupts feeding behaviour, and increases grazing during the inactive phase as well as the active phase50. Furthermore, SFD-fed mice that were forced to eat during the day (inactive phase) gained weight over time51,52. Shift work has been associated with an increased risk of developing multiple sclerosis and metabolic disorders53. Our data suggest that adipose IL-17 production is regulated by food intake, which would enhance DNL and fat storage with all-day grazing. Given the rising number of shift workers54, understanding how feeding disruption and timing affect immunometabolism could help to reduce shift work-related diseases.
Our data reveal that IL-17 has a circadian rhythm, with nightly peaks. This rhythm is controlled by the molecular clock, entrained by light and affected by feeding. A major target of IL-17 in adipose tissue is DNL, and the absence of IL-17A and IL-17F results in defects in DNL and a reduced ability to store fat from food, as is also seen33 in mice with an adipocyte-specific deletion of Il17rc. It is becoming increasingly clear that immune cells have a crucial role in regulating local tissue homeostasis, which affects whole-body circadian metabolism. Given the ever-increasing burden of metabolic disease, a better understanding of the links between immune function, circadian rhythm and whole-body metabolic health is essential to combat conditions such as obesity, cachexia and metabolic syndrome. Here we provide evidence for the IL-17 family as potent metabolic modulators that could be therapeutic targets in metabolic disease.
Methods
Mice
In all experiments, male C57BL/6J-OlaHsd mice between 8 and 12 weeks of age were used. Mice were bred in-house under specific-pathogen-free conditions in accordance with Irish and European Union regulations. Il17a/f−/− mice were received from the laboratories of V. K. Kuchroo and Y. Iwakura. Il17a−/− mice were received from the laboratory of K.H.G.M. IL-17A-GFP mice were purchased from The Jackson Laboratory. The Vav1Cre, Il7rCre, Per1Venus and Arntlfl mouse lines were bred and maintained at the Champalimaud Centre for the Unknown (CCU) animal facility. All mouse work was performed in compliance with the L.L. laboratory project licence, with ethical approval from the Trinity College Dublin ethics committee and the Animal Research Ethics Committee from the Health Products Regulatory Authority (HPRA), and the Brigham and Women’s Hospital Institutional Animal Care and Use Committee guidelines. Experiments involving the Vav1Cre (ref. 55), Il7rCre, Per1Venus (ref. 56) and Arntlfl (ref. 57) mouse lines were approved by national and local institutional review boards, the Direção Geral de Veterinária and CCU ethical committees. For experiments using rIL-17A, mice were administered 100 µl of rIL-17A (2 µg per mouse) or PBS control by intraperitoneal (i.p.) injection three times over five days.
Environmental modulation
Inversion of light–dark cycles
For experiments manipulating light cycles, mice were placed inside a ventilated, light-tight cabinet at room temperature, and light was adjusted such that control mice had lights on from 07:00–19:00, and test mice had lights on from 19.00–07:00.
Diet models
For HFD feeding experiments, mice were fed a 45% or 60% HFD for 3 weeks or 16 weeks ad libitum, compared with control mice that were fed a SFD, in which 10% of the calories were from fat. For reverse-feeding experiments, mice were fed a SFD ad libitum from 07:00–19:00 during the light cycle, compared with controls that were fed a SFD ad libitum from 19:00 to 07:00 during the dark cycle. For isocaloric feeding experiments, mice were placed into metabolic cages, and food consumption was measured over two days. The total calories per day was calculated, and mice were then fed 50% of the total calories consumed per day, for one week, compared with ad-libitum-fed controls that had 24-h access to food. For high-sucrose feeding experiments, mice were provided a 20% sucrose solution as their drinking water ad libitum for three weeks.
Tissue processing
Mice were euthanized by CO2 inhalation and adipose tissue was removed, minced with a razor and digested in 1 mg ml−1 collagenase type II (Worthington) dissolved in RPMI, in a shaking incubator for 25–30 min at 37 °C. Digested cells were filtered through a 70-μm nylon mesh and centrifuged at 300g for 5 min to pellet the stromovascular fraction (SVF). Lymph nodes were disrupted through a 70-μm filter and centrifuged for 5 min at 300g to pellet. After processing, the adipose SVF and lymph-node leukocytes were stimulated with phorbol 12-myristate 13-acetate (PMA; 10 ng ml−1; Sigma), ionomycin (500 ng ml−1; Sigma) and brefeldin A (BFA; 5 µg ml−1; Sigma) in cRPMI medium, and incubated at 37 °C for 3 h. For cytokine stimulations, adipose SVF was stimulated with rIL-23 (10 ng ml−1; Miltenyi) and rIL-1β (10 ng ml−1; Immunotools) with BFA (5 µg ml−1) for 3 h.
For the circadian cytokine production of lymph-node γδ T cells, all lymph nodes were collected from mice at 07:00 (ZT0) and plated at 3-h time points over 24 h. Each time point was then stimulated with PMA and ionomycin in BFA for 3 h, leading up to the respective time point, before being collected for fluorescent staining for flow cytometry analysis.
For treatments with the REV-ERBβ agonist SR9009 (Sigma), cells were stimulated with PMA–ionomycin or with the recombinant cytokines rIL-1β and rIL-23 as described above, in the presence or absence of SR9009. For dose–response graphs, lymph-node cells were stimulated in the presence of 0 µM, 5 µM, 10 µM, 20 µM, 40 µM and 80 µM doses of SR9009. Adipose SVF was stimulated with 40 µM of SR9009 only.
ELISA
γδ T cells were pre-expanded in wild-type mice using IL-7 and IL-15 cytokines and sorted to purity. A total of 50,000 γδ T cells were adoptively transferred into Tcrδ−/− mice. After one day, wild-type, Tcrδ−/− and Tcrδ−/− age-matched mice reconstituted with 50,000 γδ T cells were euthanized and adipose tissue lysates were assayed for IL-17A protein levels by ELISA. Processed adipose SVF lysates were diluted 1:2 in reagent diluent (1% bovine serum albumin in PBS) and IL-17A protein levels were quantified using the Mouse IL-17 Quantikine ELISA kit (M1700, R&D Systems).
Culturing of mouse adipocytes
Interscapular BAT was isolated, minced and incubated in Dulbecco’s modified Eagle’s medium (DMEM) (glucose-free) supplemented with 10% fetal calf serum (FCS), 2.5 mM l-glutamine and 5 mM glucose. Tissue explants were then treated with or without rIL-17A (50 ng ml−1; R&D Systems) for 18 h at 37 °C. Once the medium was removed, explants were snap-frozen in liquid nitrogen for RNA extraction.
Flow cytometry analysis
For intracellular and intranuclear staining, cells were washed with 1 ml PBS and incubated in ZombieAqua Cell Viability Dye (Biolegend; 1:1,000 in PBS) for 20 min at room temperature. Cells were incubated with an extracellular fluorochrome-labelled antibody cocktail with Fc block (1:200; BD Biosciences) in FACS buffer (PBS + 2% FCS) for 20 min at room temperature. Cells were then washed with 2% FACS buffer resuspended in 100 µl of Foxp3 staining kit (eBioscience) and incubated at room temperature for 20 min. Cells were washed with 1× permeabilization buffer (eBioscience) and then incubated with an intracellular fluorochrome-labelled antibody cocktail in 1× permeabilization buffer (eBioscience) for 20 min at room temperature. Cells were incubated on ice for 30 min and subsequently washed in 2% FACS buffer. Cells were acquired on a FACS Canto or LSR Fortessa cytometer (BD Biosciences). Data were analysed with FlowJo v.10 software. Cell sorting was performed using a FACSAria (BD Biosciences). Sorted populations were more than 95% pure. For a list of flow cytometry antibodies, see Supplementary Table 1.
Induction and assessment of EAE
Mice were fed either ad libitum or a 60% HFD three weeks before EAE induction. EAE was induced in male mice by subcutaneous immunization with MOG35–55 peptide (150 µg per mouse; GenScript) emulsified in complete Freund’s adjuvant (Condrex) containing 4 mg ml−1 heat-killed Myocobacterium tuberculosis (MTB). Mice were injected i.p. with pertussis toxin (200 ng per mouse) (Kaketsuken) on day 0 to induce EAE development. Disease severity was monitored and assessed by clinical scores as follows: no clinical signs, 0; limp tail, 1; ataxic gait, 2; hind limb weakness, 3; hind limb paralysis, 4; tetra paralysis or moribund, 5. A weight loss of more than 20% constituted an additional humane end-point.
RNA extraction and quantitative PCR analysis
RNA extraction from isolated cells
Adipose γδ17 T cells were isolated and sorted from adipose tissue. RNA and cDNA were generated from isolated cells using the SYBR Green Fast Advanced Cells-to-CT Kit (Invitrogen) following the manufacturer’s specific instructions. To quantify the relative mRNA expression of genes of interest, quantitative PCR was performed in a 96- or 384-well plate format (Thermo Fisher Scientific) using PowerUp SYBR Green Master Mix (Invitrogen)-based detection (eBioscience). Relative mRNA levels were calculated using the ∆∆ cycle threshold (∆∆Ct) method and normalized to corresponding endogenous controls (Actb).
RNA extraction from tissues
Tissues were snap-frozen in liquid nitrogen, defrosted at room temperature and transferred to a 2-ml tube containing a 5-mm stainless steel bead. Tissues were homogenized in 1 ml trizol reagent (Thermo Fisher Scientific) in a tissue lyser for 2.5 min, 25 pulses per second. Then, 200 µl chloroform was added to each tube, and they were inverted once and left at room temperature for 2–3 min, before centrifuging at 12,000g for 15 min. The aqueous phase containing RNA was transferred into a new Eppendorf tube and 500 µl isopropanol was added to precipitate the RNA. Tubes were inverted ten times and left at room temperature for 10 min, and then centrifuged at 12,000g for 10 min. Supernatants were discarded and RNA pellets were washed in 1 ml 75% ethanol, diluted in RNAse free dH2O. Tubes were centrifuged at 12,000g for 5 min and supernatants were discarded by inverting the tube. The RNA pellet was left to dry at room temperature for 20–30 min, until transparent, and the pellets were resuspended in 50 µl RNAse free water. RNA was left on ice for 30 min, then in a heat block set at 55 °C for 15 min. RNA quality and concentration were determined using a Nanodrop 2000 UV spectrophotometer (Thermo Fisher Scientific). Twenty microlitres of cDNA was synthesized from 2 µg of isolated RNA using the High-Capacity cDNA Reverse Transcription Kit (Biosciences) in a MiniAmp Thermal Cycler (BD Biosciences). To quantify the relative mRNA expression of genes of interest, quantitative PCR was performed in a 96- or 384-well plate format (Thermo Fisher Scientific) using SYBR Green-based detection (eBioscience). Relative mRNA levels were calculated using the ∆∆ cycle threshold (∆∆Ct) method and normalized to corresponding endogenous controls (Ppib or Rpl18). For a list of primers, see Supplementary Table 2.
Protein analysis by western blotting
BAT from wild-type and Il17a−/− mice was collected over 24 h and snap-frozen. The BAT was then lysed and centrifuged at 14,000g, and the pellet was discarded. The amount of protein was quantified using a BCA kit. Tissue lysates were subsequently boiled at 95 °C for 5 min to denature the proteins. Proteins were resolved, on the basis of their molecular weight, through SDS–PAGE gels in 1× running buffer. Proteins were electro-transferred onto PVDF membranes (Merck) in 1× transfer buffer containing 10% methanol. Membranes were blocked in 5% milk in 1× Tris-buffered saline with Tween-20 and incubated with SCD1, or α-tubulin primary antibodies (Cell Signaling Technologies) overnight at 4 °C and HRP-conjugated secondary antibodies (Jackson Immunoresearch). Membranes were incubated in ECL substrate (BioRad), and images were developed on a Biorad Gel Doc imaging system. Images were quantified using ImageJ. For raw uncropped blots, see Supplementary Fig. 1.
Plasma 2H2O enrichment analysis and DNL calculations
For measurements of DNL in vivo, wild-type or Il17a/f−/− mice were placed overnight on 8% enriched D2O drinking water, with subsequent collection and snap-freezing of BAT and liver. The 2H labelling of water from samples or standards was determined by deuterium acetone exchange, and using equations as previously described58.
Metabolic cage analysis
Indirect calorimetry experiments were performed with the Promethion metabolic cage system, or the comprehensive lab animal monitoring system (CLAMS), essentially as described59. Mice were singly housed and allowed at least 12 h of acclimatization to the new environment. O2 consumption, CO2 emission, energy expenditure, body weight, food and water intake and locomotor activity were monitored throughout the experiment. For experiments involving the manipulation of light cycles, mice were placed inside the metabolic cages at room temperature, and light was adjusted such that the control mice had lights on from 07:00–19:00, and test mice had lights on from 19:00–07:00. Analysis was performed using the online indirect calorimetry vignettes CalR (ref. 60); online software is available at https://calrapp.org/.
Bulk RNA-seq and scRNA-seq data analysis
The bulk RNA-seq dataset of BAT from wild-type and AdIl17rc−/− mice was downloaded from the GEO repository GSE144255 as cuff gene counts22. The bulk RNA-seq datasets of skin biopsies from patients with psoriasis who were receiving brodalumab36 and ixekizumab37 were downloaded from the GEO repositories GSE117468 and GSE31652, respectively. Heat maps were generated using the online platform Heatmapper61 (http://heatmapper.ca/).
scRNA-seq was performed on single-cell suspensions of sorted γδ T cells and iNKT cells from the visceral adipose tissue of C57BL/6J mice using the 10x Genomics platform. A total of five visceral adipose tissue deposits from five independent biological replicates were pooled for sequencing. Single-cell suspensions were loaded onto a 10x Chromium Controller to generate gel beads-in-emulsion (GEMS), and GEMs were processed to generate unique molecular identifier (UMI)-based libraries according to the 10X Genomics Chromium Single Cell 3’ protocol. Libraries were sequenced using a NextSeq 500 sequencer. Raw BCL files were demultiplexed using Cell Ranger v.3.0.2 mkfastq to generate fastq files with default parameter. Fastq files were aligned to the mm10 genome (v.1.2.0) and feature reads were quantified simultaneously using the Cell Ranger count for feature barcoding. Filtered feature-barcode UMI count matrices containing quantification of gene expression were used for downstream analysis.
Downstream scRNA-seq data analysis
A total of 22,748 cells mouse γδ T cells, iNKT cells and MAIT cells expressing a median of 1,423 genes and 3,556 UMIs per cell were loaded from feature-barcode UMI count matrices using the Seurat v.4.1.0 package62. Adipose and splenic iNKT cells were reanalysed from GSE142845 (ref. 63), thymic iNKT cells were reanalysed from GSE141895 (ref. 64), thymic MAIT cells were reanalysed from E-MTAB-7704 (ref. 65), pulmonary γδ T cells were reanalysed from E-MTAB-8732 (ref. 66), peripheral lymph node (PLN) and dermal γδ T cells were reanalysed from GSE123400 (ref. 67) and meningeal γδ T cells were reanalysed from GSE147262 (ref. 68). Cells expressing more than 11% of mitochondrial genes as a percentage of total gene counts were considered to represent apoptotic or dead cells and were therefore removed from the analysis. Cells were also filtered on the basis of total UMI counts and total gene counts on a per-sample basis to remove empty droplets, poor quality cells and doublets, with a minimum cut-off of at least 300 genes per cell across all samples. UMI counts were normalized using regularized negative binomial regression using sctransform v.0.3.3 (ref. 69).
Dimensionality reduction was performed using principal component analysis (PCA) with n = 100 dimensions and 2,000 or 3,000 variable features, and an elbow plot was used to determine the number of PCA dimensions used as input for UMAP70. For collective analysis of cells from different batches and cells sequenced using different scRNA-seq technologies the Harmony v.1.0 package71 was used with default settings to remove batch effects, and batch-corrected harmony embeddings were used for UMAP. UMAP was performed using a minimum distance of 0.3 and a spreading factor of 1. Shared nearest neighbour graphs were calculated using k = 20 nearest neighbours. Graph-based clustering was performed using the Louvain algorithm. In some cases, overclustering was performed and clusters were manually collapsed, and/or the first two dimensions of the UMAP reduction were used as input for graph-based clustering instead of PCA or harmony embeddings. Adipose γδ T cells were distinguished from adipose iNKT cells by expression of Trdc and graph-based clustering, and analysed separately for γδ1 versus γδ17 cell identification. Type-1 and type-17 innate T cells were individually identified in each dataset using graph-based clustering and gene-expression analysis, and cycling cells were excluded. For thymic innate T cells, CD44− progenitor populations were also excluded. Raw counts from type-1 and type-17 cell populations were then merged and normalized together into a single file for comparative analysis.
Gene-expression analysis was performed using the FindMarkers() or FindAllMarkers() Seurat functions and the Wilcoxon rank sum test. Heat maps were generated using the Complex Heatmap v.2.7.11 and circlize v.0.4.14 packages72, and module scores were calculated using the AddModuleScore() Seurat function with n = 10 control features. Density plots were produced using the Nebulosa v.1.1.1 package73. Log-normalized RNA counts were used for all gene-expression analysis and visualization. All computational analysis was performed using R v.4.1.2 and RStudio Desktop v.1.4.1712 on an Ubuntu 20.04 Linux GNU (64 bit) system.
Statistical analysis
GraphPad Prism 9.3.0 was used for statistical analysis. For all experiments, a 95% confidence interval was used and P ≤ 0.05 was considered statistically significant. A D’Agostino–Pearson omnibus normality test was first performed to test whether the data were normally distributed (Gaussian distribution). If data were normally distributed, parametric testing was performed. If data were not normally distributed, non-parametric testing was performed. When comparing two groups, an unpaired or paired two-tailed student’s t-test was used. When comparing more than two groups, an ordinary one-way ANOVA with Dunnett’s test was used. When comparing data with two variables, a two-way ANOVA with Bonferroni test was used. Cosine curves were fitted in GraphPad Prism and circadian rhythmicity was evaluated using the cosinor regression model using the cosinor and cosinor2 R packages in RStudio. The circadian period was assumed to be 24 h for all analysis and the significance of the circadian fit was assessed by a zero-amplitude test with 95% confidence. Amplitude and acrophase were extracted from the cosinor model. In all figures, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Graphical representations
All diagrams and graphical representations were created using BioRender.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-024-08131-3.
Supplementary information
Supplementary Fig. 1 and Supplementary Data Tables 1 and 2.
Source data
Source Data Fig. 1–5 and Source Data Extended Data Fig. 2–8
Acknowledgements
The authors acknowledge the work of B. Moran of the flow cytometry facility, in addition to R. Rakhmatullin and all other staff of the animal facilities at our institutions. We thank the past and present members of the laboratories, who have provided invaluable input over the years, and A. Muñoz Zamora, C. Ortega-de San Luis, E. Stewart and J. O’Leary for proofreading and feedback on the manuscript. This work was funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (StG_679173 to L.L.), Science Foundation Ireland (SFI) (16/FRL/3865 to L.L.) and the NIH (R01 AI134861 and metabolic core grant S10 OD020100 to L.L.). This work was also supported by the Irish Research Council (grant GOIPG/2019/421 to A.D.)
Extended data figures and tables
Author contributions
L.L. provided funding for the study. L.L., L.A.J.O., K.H.G.M., C.M.M. and H.V.-F. supervised the study. A.D. and L.L. conceived and designed the original study. A.D. and L.L. managed the project administration. A.D., B.S. and M.R. performed most of the experiments. E.A.D., C.S., H.P., A.E.G., B.K., A.C.K. and K.W.-R. performed some experiments. M.B., K.O. and E.L. provided technical assistance in some experiments. H.K. and A.D., performed bioinformatic analysis. A.D. analysed and interpreted most of the data. B.S., C.S., E.A.D., B.K. and M.R. analysed some of the data. A.D. and L.L. wrote and edited the manuscript.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The data supporting the findings of this study are publicly available through the Gene Expression Omnibus (GEO) repository at GSE144255, GSE117468 and GSE31652 for the bulk RNA-seq datasets and GSE142845, GSE141895, GSE123400, GSE147262, or from ArrayExpress at E-MTAB-7704 and E-MTAB-8732 for the scRNA-seq datasets. Raw data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41586-024-08131-3.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-024-08131-3.
References
- 1.Scheiermann, C., Gibbs, J., Ince, L. & Loudon, A. Clocking in to immunity. Nat. Rev. Immunol.18, 423–437 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Man, K., Loudon, A. & Chawla, A. Immunity around the clock. Science354, 999–1003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ince, L. M. et al. Influence of circadian clocks on adaptive immunity and vaccination responses. Nat. Commun.14, 476 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trim, W. V. & Lynch, L. Immune and non-immune functions of adipose tissue leukocytes. Nat. Rev. Immunol.22, 371–386 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Kane, H. & Lynch, L. Innate immune control of adipose tissue homeostasis. Trends Immunol.40, 857–872 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Goldberg, E. L. et al. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat. Metab.2, 50–61 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch, L. et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity37, 574–587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, T. M. & Piggins, H. D. Electrophysiology of the suprachiasmatic circadian clock. Prog. Neurobiol.82, 229–255 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Buhr, E. D., Yoo, S. H. & Takahashi, J. S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science330, 379–385 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panda, S. Circadian physiology of metabolism. Science354, 1008–1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet.18, 164–179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastings, M., O’Neill, J. S. & Maywood, E. S. Circadian clocks: regulators of endocrine and metabolic rhythms. J. Endocrinol.195, 187–198 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Adamovich, Y. et al. Oxygen and carbon dioxide rhythms are circadian clock controlled and differentially directed by behavioral signals. Cell Metab.29, 1092–1103 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Seebacher, F. Responses to temperature variation: integration of thermoregulation and metabolism in vertebrates. J. Exp. Biol.212, 2885–2891 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Kiehn, J.-T., Koch, C. E., Walter, M., Brod, A. & Oster, H. Circadian rhythms and clocks in adipose tissues: current insights. Chronophysiol. Ther.7, 7–17 (2017). [Google Scholar]
- 16.Yamamuro, D. et al. Peripheral circadian rhythms in the liver and white adipose tissue of mice are attenuated by constant light and restored by time-restricted feeding. PLoS One15, e0234439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray, M. S. & Young, M. E. Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes. Rev.8, 169–181 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Antunes, L. C., Levandovski, R., Dantas, G., Caumo, W. & Hidalgo, M. P. Obesity and shift work: chronobiological aspects. Nutr. Res. Rev.23, 155–168 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Sethi, J. K. & Hotamisligil, G. S. Metabolic messengers: tumour necrosis factor. Nat. Metab.3, 1302–1312 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Qing, H. et al. Origin and function of stress-induced IL-6 in murine models. Cell182, 372–387 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlgruber, A. C. et al. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol.19, 464–474 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, B. et al. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature578, 610–614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu, X. et al. TH17 cell differentiation is regulated by the circadian clock. Science342, 727–730 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godinho-Silva, C. et al. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature574, 254–258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng, F. et al. A circadian clock is essential for homeostasis of group 3 innate lymphoid cells in the gut. Sci. Immunol.4, eaax1215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Q. et al. Circadian rhythm-dependent and circadian rhythm-independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci. Immunol.4, eaay7501 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang, C. et al. The nuclear receptor REV-ERBΑ modulates Th17 cell-mediated autoimmune disease. Proc. Natl Acad. Sci. USA116, 18528–18536 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogueira, G. et al. Interleukin-17 acts in the hypothalamus reducing food intake. Brain. Behav. Immun.87, 272–285 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal, A. et al. The circadian clock regulates adipogenesis by a Per3 crosstalk pathway to Klf15. Cell Rep.21, 2367–2375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes, N. et al. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat. Immunol.22, 179–192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohsaka, A. et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab.6, 414–421 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Chrobok, L. et al. Rhythmic neuronal activities of the rat nucleus of the solitary tract are impaired by high-fat diet—implications for daily control of satiety. J. Physiol.600, 751–767 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Teijeiro, A., Garrido, A., Ferre, A., Perna, C. & Djouder, N. Inhibition of the IL-17A axis in adipocytes suppresses diet-induced obesity and metabolic disorders in mice. Nat. Metab.3, 496–512 (2021). [DOI] [PubMed] [Google Scholar]
- 34.McGinley, A. M. et al. Interleukin-17A serves a priming role in autoimmunity by recruiting IL-1β-producing myeloid cells that promote pathogenic T cells. Immunity52, 342–356 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Xiao, H. et al. Architecture of the outbred brown fat proteome defines regulators of metabolic physiology. Cell185, 4654–4673 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomalin, L. E. et al. Short-term transcriptional response to IL-17 receptor-A antagonism in the treatment of psoriasis. J. Allergy Clin. Immunol.145, 922–932 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Krueger, J. G. et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol.130, 145–154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kokolus, K. M. et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl Acad. Sci. USA110, 20176–20181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer, A. W., Cannon, B. & Nedergaard, J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol. Metab.7, 161–170 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adlanmerini, M. et al. Circadian lipid synthesis in brown fat maintains murine body temperature during chronic cold. Proc. Natl Acad. Sci. USA116, 18691–18699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talbot, J. et al. Feeding-dependent VIP neuron–ILC3 circuit regulates the intestinal barrier. Nature579, 575–580 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ameer, F., Scandiuzzi, L., Hasnain, S., Kalbacher, H. & Zaidi, N. De novo lipogenesis in health and disease. Metabolism63, 895–902 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Goepfert, A., Lehmann, S., Wirth, E. & Rondeau, J. M. The human IL-17A/F heterodimer: a two-faced cytokine with unique receptor recognition properties. Sci. Rep.7, 8906 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGeachy, M. J., Cua, D. J. & Gaffen, S. L. The IL-17 family of cytokines in health and disease. Immunity50, 892–906 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douglas, A., Stevens, B. & Lynch, L. Interleukin-17 as a key player in neuroimmunometabolism. Nat. Metab.5, 1088–1100 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji, Z. et al. Obesity promotes EAE through IL-6 and CCL-2-mediated T cells infiltration. Front. Immunol.10, 1881 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cain, S. W., Filtness, A. J., Phillips, C. L. & Anderson, C. Enhanced preference for high-fat foods following a simulated night shift. Scand. J. Work Environ. Health41, 288–293 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Farías, R., Sepúlveda, A. & Chamorro, R. Impact of shift work on the eating pattern, physical activity and daytime sleepiness among Chilean healthcare workers. Saf. Health Work11, 367–371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samhat, Z., Attieh, R. & Sacre, Y. Relationship between night shift work, eating habits and BMI among nurses in Lebanon. BMC Nurs.19, 25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pendergast, J. S. et al. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci.37, 1350–1356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arble, D. M., Bass, J., Laposky, A. D., Vitaterna, M. H. & Turek, F. W. Circadian timing of food intake contributes to weight gain. Obesity17, 2100–2102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stucchi, P. et al. Circadian feeding drive of metabolic activity in adipose tissue and not hyperphagia triggers overweight in mice: is there a role of the pentose-phosphate pathway? Endocrinology153, 690–699 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Alfredsson, L. & Olsson, T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb. Perspect. Med.9, a028944 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puttonen, S., Viitasalo, K. & Härmä, M. Effect of shiftwork on systemic markers of inflammation. Chronobiol. Int.28, 528–535 (2011). [DOI] [PubMed] [Google Scholar]
- 55.de Boer, J. et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol.33, 314–325 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Cheng, H. Y. M. et al. Segregation of expression of mPeriod gene homologs in neurons and glia: possible divergent roles of mPeriod1 and mPeriod2 in the brain. Hum. Mol. Genet.18, 3110–3124 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Storch, K. F. et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell130, 730–741 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muta, Y. et al. Enhanced SREBP2-driven cholesterol biosynthesis by PKCλ/ι deficiency in intestinal epithelial cells promotes aggressive serrated tumorigenesis. Nat. Commun.14, 8075 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okada, K. et al. Thioesterase superfamily member 1 suppresses cold thermogenesis by limiting the oxidation of lipid droplet-derived fatty acids in brown adipose tissue. Mol. Metab.5, 340–351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mina, A. I. et al. CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metab.28, 656–666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babicki, S. et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res.44, W147–W153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell184, 3573–3587 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LaMarche, N. M. et al. Distinct iNKT cell populations use IFNγ or ER stress-induced IL-10 to control adipose tissue homeostasis. Cell Metab.32, 243–258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baranek, T. et al. High dimensional single-cell analysis reveals iNKT cell developmental trajectories and effector fate decision. Cell Rep.32, 108116 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Legoux, F. et al. Molecular mechanisms of lineage decisions in metabolite-specific T cells. Nat. Immunol.20, 1244–1255 (2019). [DOI] [PubMed] [Google Scholar]
- 66.McIntyre, C. L. et al. 2β2 Integrins differentially regulate γδ T cell subset thymic development and peripheral maintenance. Proc. Natl Acad. Sci. USA117, 22367–22377 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan, L. et al. Single-cell transcriptomics identifies the adaptation of Scart1+ Vγ6+ T Cells to skin residency as activated effector cells. Cell Rep.27, 3657–3671 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Alves de Lima, K. et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol.21, 1421–1429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol.20, 296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McInnes, L. et al. UMAP: uniform manifold approximation and projection. J. Open Source Softw.3, 861 (2018).
- 71.Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods16, 1289–1296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics32, 2847–2849 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Alquicira-Hernandez, J. & Powell, J. E. Nebulosa recovers single-cell gene expression signals by kernel density estimation. Bioinformatics37, 2485–2487 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 and Supplementary Data Tables 1 and 2.
Source Data Fig. 1–5 and Source Data Extended Data Fig. 2–8
Data Availability Statement
The data supporting the findings of this study are publicly available through the Gene Expression Omnibus (GEO) repository at GSE144255, GSE117468 and GSE31652 for the bulk RNA-seq datasets and GSE142845, GSE141895, GSE123400, GSE147262, or from ArrayExpress at E-MTAB-7704 and E-MTAB-8732 for the scRNA-seq datasets. Raw data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.