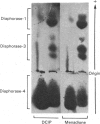
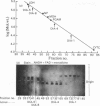
Abstract
A newly discovered human diaphorase, designated diaphorase-4, which accounts for a major part of the diaphorase activity of most tissues but does not occur in erythrocytes, is described. In contrast with other human diaphorases, it is dependent on FAD for activity after electrophoresis, inhibited by low concentrations of dicoumarol and shows a marked affinity for Cibacron Blue. The molecular weight was estimated to be 49000 +/- 1800 by gel filtration. Diaphorase-4 appears to show person-to-person quantitative variation, so that about 4% of the population lack appreciable enzyme activity, but it is not yet clear whether this variation is of genetic or non-genetic origin.
Full text
PDF


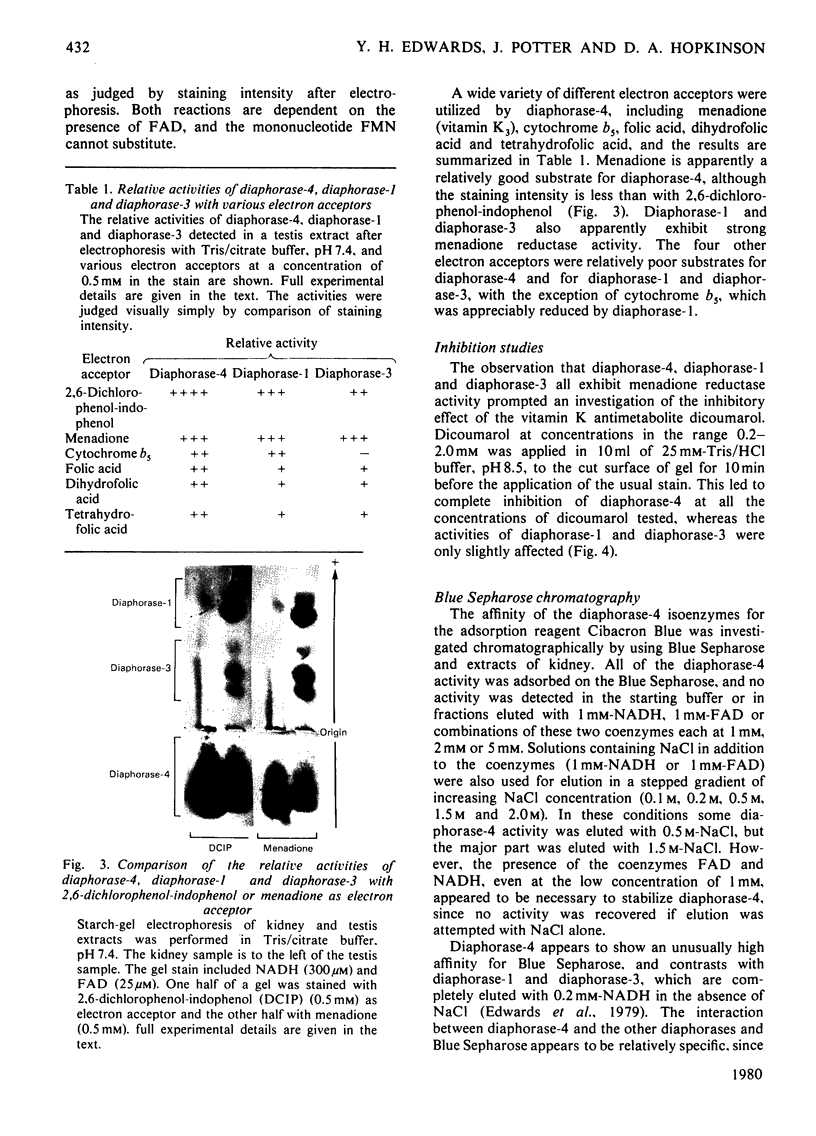
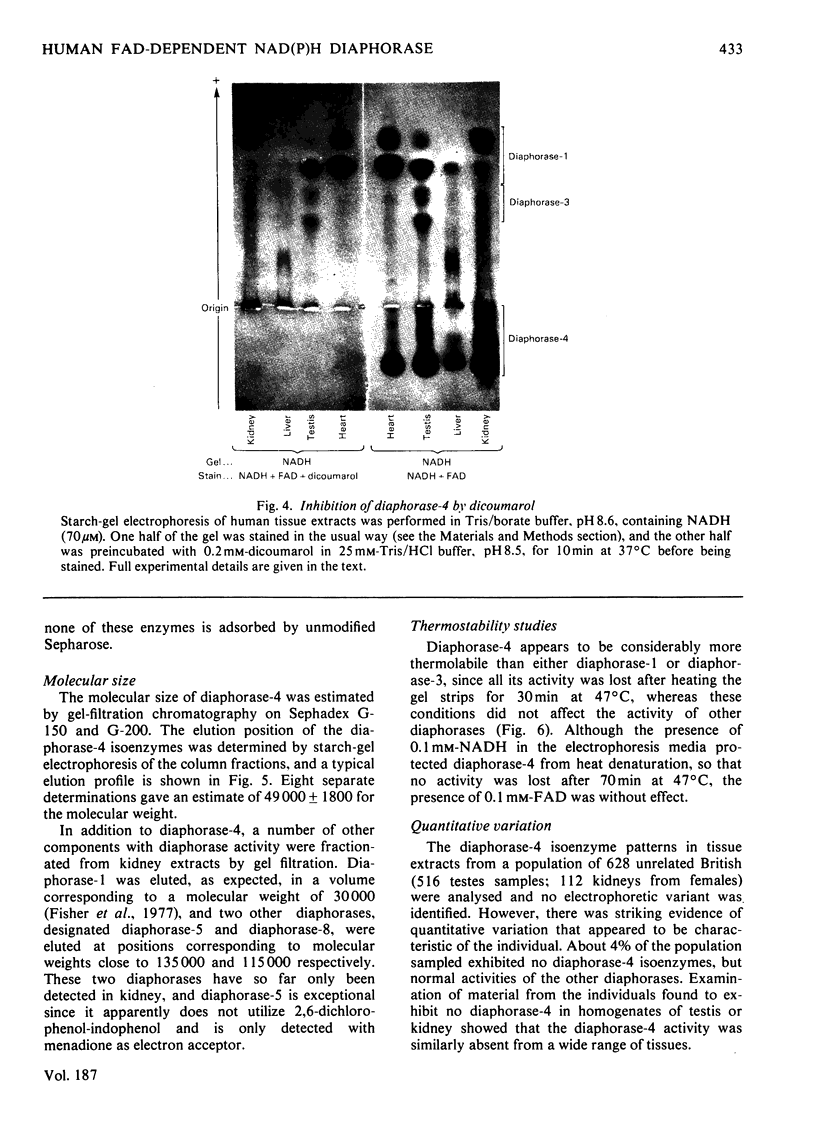
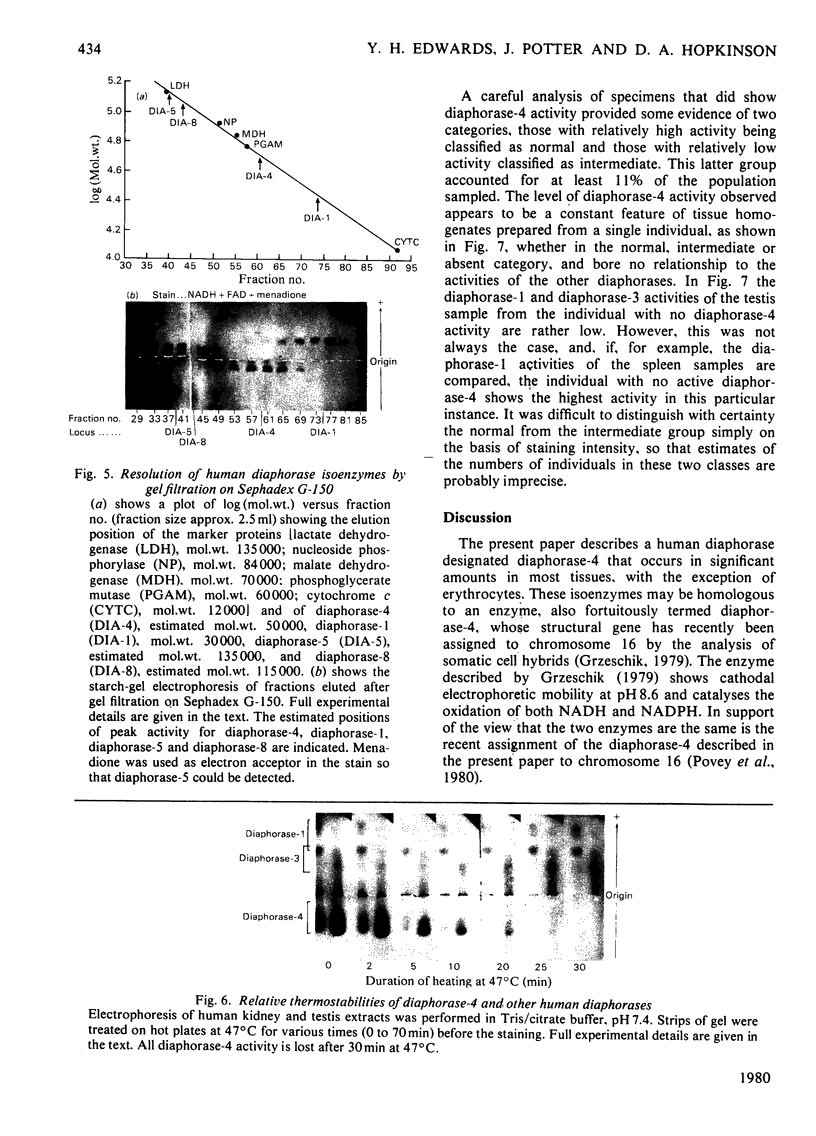
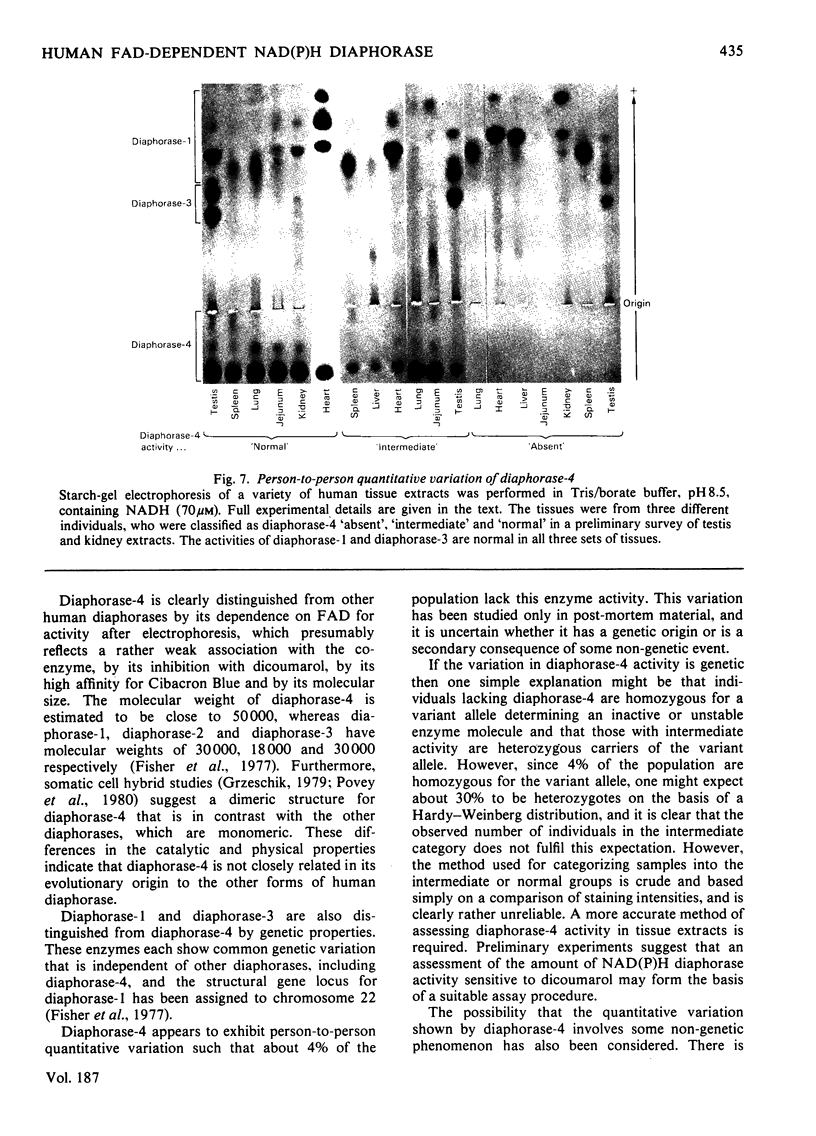

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell K., Blake E. T., Sensabaugh G. F. Sperm diaphorase: genetic polymorphism of a sperm-specific enzyme in man. Science. 1976 Mar 19;191(4232):1185–1187. doi: 10.1126/science.3851. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Potter J. E., Hopkinson D. A. A comparison of the biochemical properties of the human diaphorase (DIA3) isozymes determined by the common alleles DIA13, DIA23 and DIA33. Ann Hum Genet. 1979 Jan;42(3):293–302. doi: 10.1111/j.1469-1809.1979.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Ernster L., Lind C., Rase B. A study of the DT-diaphorase activity of warfarin-resistant rats. Eur J Biochem. 1972 Jan 31;25(1):198–206. doi: 10.1111/j.1432-1033.1972.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Edwards Y. H., Putt W., Potter J., Hopkinson D. A. An interpretation of human diaphorase isozymes in terms of three gene loci DIA1, DIA2 and DIA3. Ann Hum Genet. 1977 Oct;41(2):139–149. doi: 10.1111/j.1469-1809.1977.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Hultquist D. E., Passon P. G. Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nat New Biol. 1971 Feb 24;229(8):252–254. doi: 10.1038/newbio229252a0. [DOI] [PubMed] [Google Scholar]
- Leroux A., Kaplan J. C. Presence of red cell type NADH-methemoglobin reductase (NADH-diaphorase) in human non erythroid cells. Biochem Biophys Res Commun. 1972 Nov 15;49(4):945–950. doi: 10.1016/0006-291x(72)90303-8. [DOI] [PubMed] [Google Scholar]
- MOTULSKY A. G. PHARMACOGENETICS. Prog Med Genet. 1964;23:49–74. [PubMed] [Google Scholar]
- McAlpine P. J., Hopkinson D. A., Harris H. Thermostability studies on the isozymes of human phosphoglucomutase. Ann Hum Genet. 1970 Jul;34(1):61–71. doi: 10.1111/j.1469-1809.1970.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Povey S., Wilson D., Edwards Y. H. Assignment of a human diaphorase (DIA4) to chromosome 16. Ann Hum Genet. 1980 May;43(4):349–353. doi: 10.1111/j.1469-1809.1980.tb01569.x. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of dicumarol levels in man. J Clin Invest. 1968 Dec;47(12):2657–2663. doi: 10.1172/JCI105949. [DOI] [PMC free article] [PubMed] [Google Scholar]