Abstract
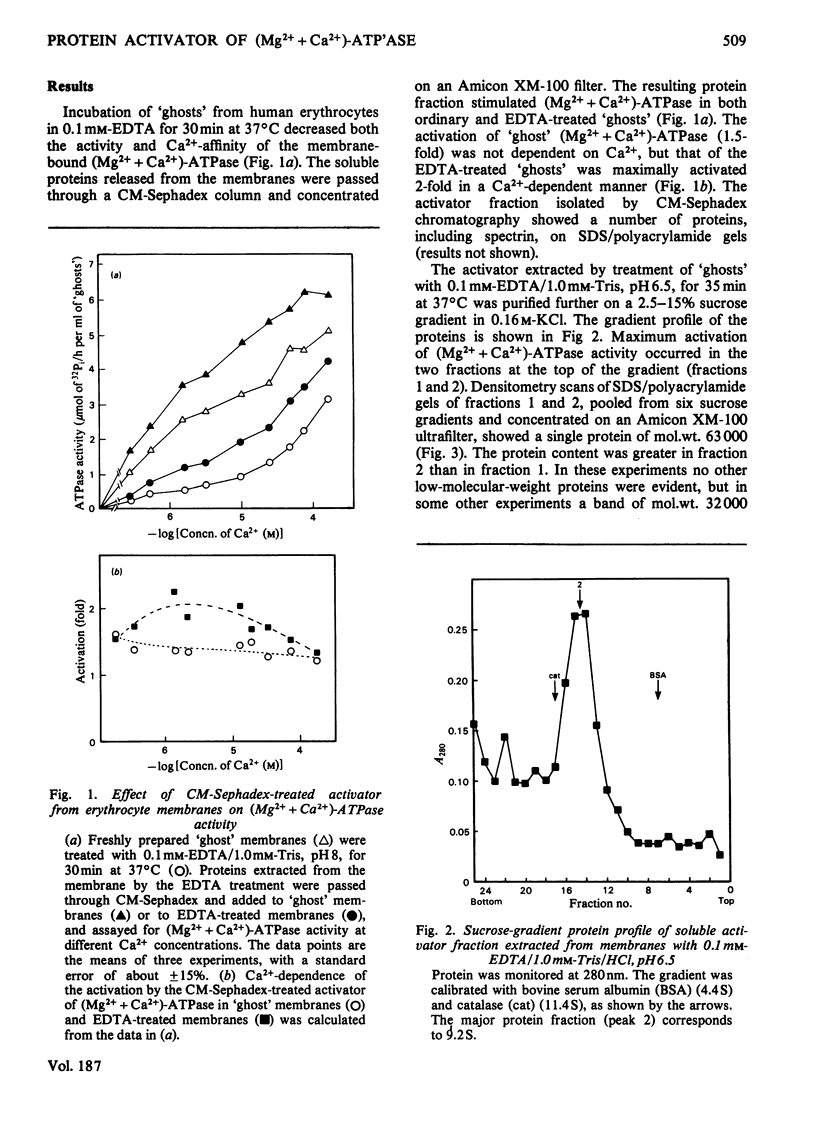
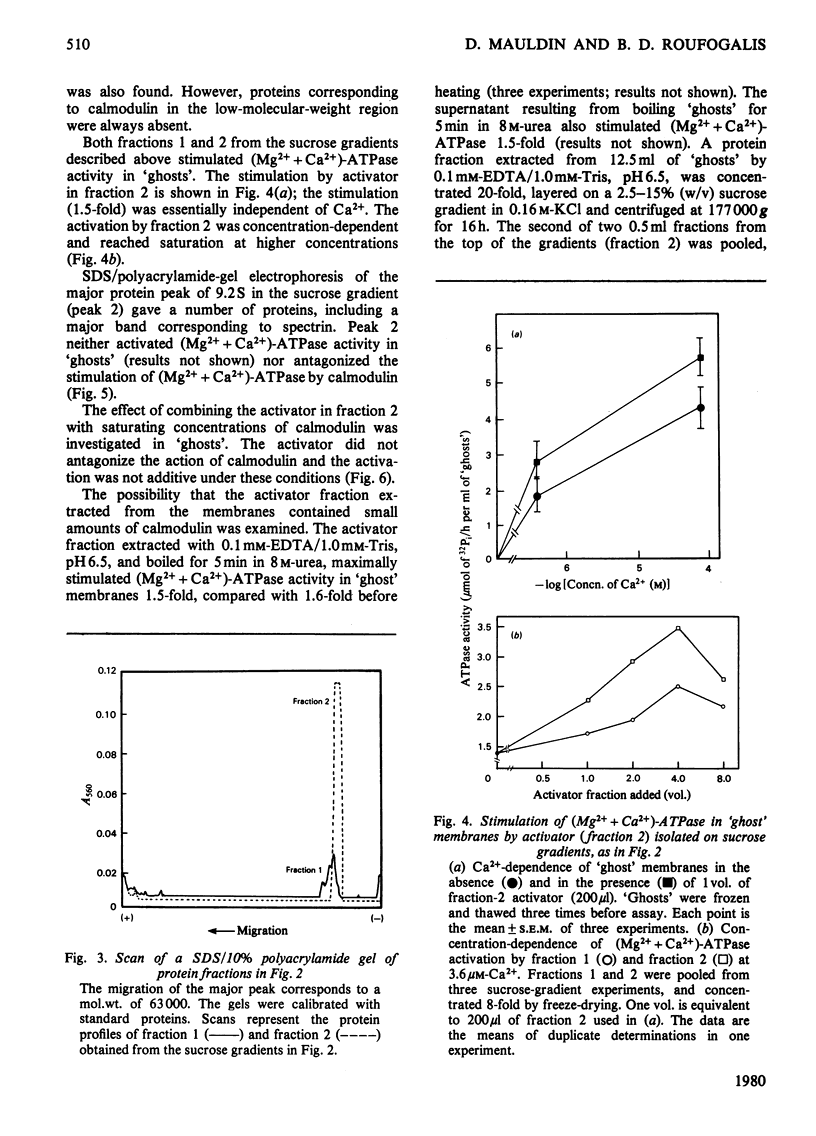
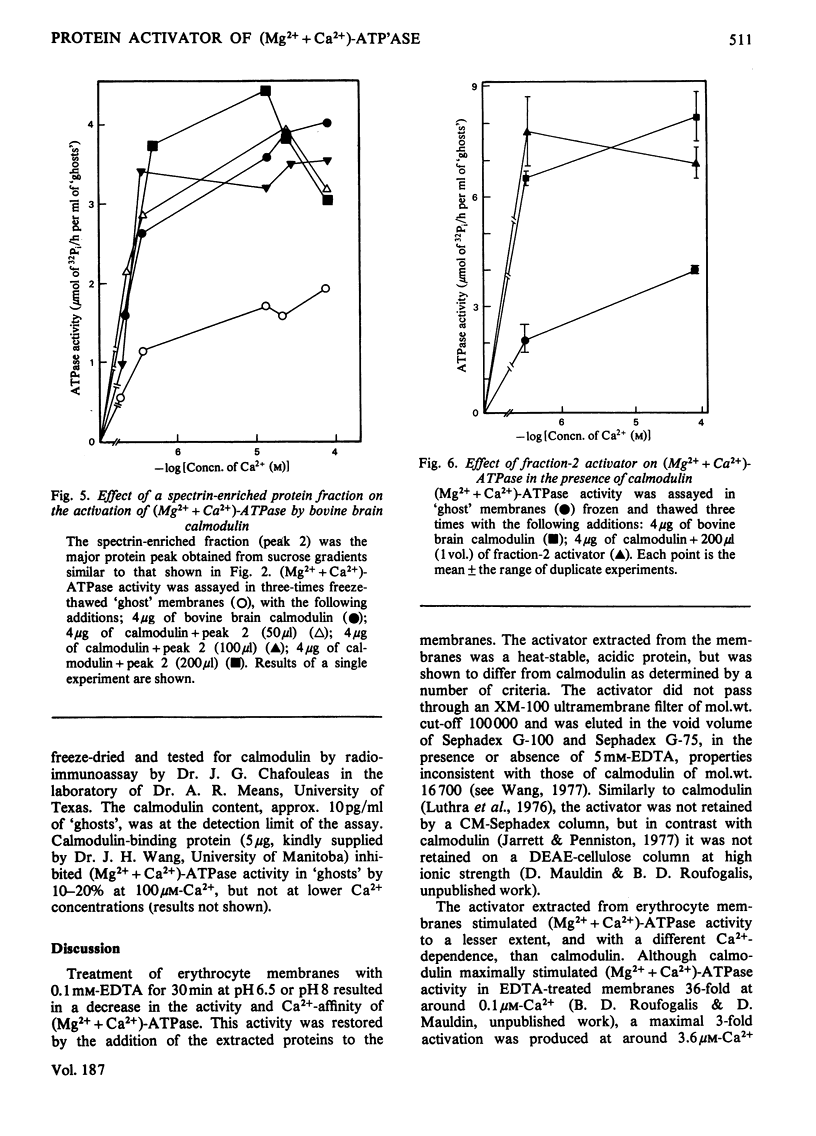
Treatment of extensively washed erythrocyte membranes with 0.1mm-EDTA decreased their Mg2+-dependent, Ca2+-stimulated ATPase [(Mg2++Ca2+)-ATPase] activity. An activator released by this treatment restored the (Mg2++Ca2+)-ATPase to its original value in a Ca2+-dependent manner. This activator was different from calmodulin, as determined by a number of criteria. It was retained on an Amicon XM-100 ultrafiltration membrane (molecular-weight cut-off 100000); it appeared in the void volume of Sephadex G-100 and G-75 columns; it was not retained on a DEAE-cellulose ion-exchange column at ionic strengths similar to those used to retain calmodulin; and it maximally activated (Mg2++Ca2+)-ATPase activity less than calmodulin and at a higher Ca2+ concentration. Like calmodulin, the activator is heat-stable. The activator fraction isolated on a 2.5–15% sucrose gradient in 0.16m-KCl showed a single band of mol.wt. 63000 and no calmodulin on 10%-polyacrylamide/sodium dodecyl sulphate gels. A trace amount of calmodulin was detected in the activator fraction by radioimmunoassay (approx. 10pg/ml of `ghosts'), but this amount was insufficient to account for the (Mg2++Ca2+)-ATPase activation. Furthermore, calmodulin-binding protein failed to inhibit (Mg2++Ca2+)-ATPase activity by more than 10–20% in the membrane preparations from which the activator was extracted. It was concluded that erythrocyte membranes contain a (Mg2++Ca2+)-ATPase activator that may attenuate the activation of the Ca2+-transport ATPase by calmodulin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond G. H. Ligand-induced conformational changes in the (Mg 2+ + Ca 2+ )-dependent ATPase of red cell membranes. Biochim Biophys Acta. 1972 Nov 2;288(2):423–433. doi: 10.1016/0005-2736(72)90263-5. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Lynch T. J., Wallace R. W. An endogenous Ca2+-dependent activator protein of brain adenylate cyclase and cyclic neucleotide phosphodiesterase. Adv Cyclic Nucleotide Res. 1978;9:233–251. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Farrance M. L., Vincenzi F. F. Enhancement of (Ca2+ + Mg2+)-ATPase activity of human erythrocyte membranes by hemolysis in isosmotic imidazole buffer. I. General properties of variously prepared membranes and the mechanism of the isosmotic imidazole effect. Biochim Biophys Acta. 1977 Nov 15;471(1):49–58. doi: 10.1016/0005-2736(77)90392-3. [DOI] [PubMed] [Google Scholar]
- Farrance M. L., Vincenzi F. F. Enhancement of (Ca2+ + Mg2+)-ATPase activity of human erythrocyte membranes by hemolysis in isosmotic imidazole buffer. II. Dependence on calcium and a cytoplasmic activator. Biochim Biophys Acta. 1977 Nov 15;471(1):59–66. doi: 10.1016/0005-2736(77)90393-5. [DOI] [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J., Ekholm J. E. The expression of optimum ATPase activities in human erythrocytes. A comparison of different lytic procedures. Arch Biochem Biophys. 1978 Apr 15;187(1):170–179. doi: 10.1016/0003-9861(78)90020-6. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Kyte J. Human erythrocyte calmodulin. Further chemical characterization and the site of its interaction with the membrane. J Biol Chem. 1979 Sep 10;254(17):8237–8244. [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1210–1216. doi: 10.1016/s0006-291x(77)80108-3. [DOI] [PubMed] [Google Scholar]
- Katz S., Roufogalis B. D., Landman A. D., Ho L. Properties of (Mg2 + Ca2+)-ATPase of erythrocyte membranes prepared by different procedures: influence of Mg2+, Ca2+, ATP, and protein activator. J Supramol Struct. 1979;10(2):215–225. doi: 10.1002/jss.400100211. [DOI] [PubMed] [Google Scholar]
- Luthra M. G., Hildenbrandt G. R., Hanahan D. J. Studies on an activator of the (Ca2+ plus Mg2+)-ATPase of human erythrocyte membranes. Biochim Biophys Acta. 1976 Jan 8;419(1):164–179. doi: 10.1016/0005-2736(76)90380-1. [DOI] [PubMed] [Google Scholar]
- Lynch T. J., Cheung W. Y. Human erythrocyte Ca2+-Mg2+-ATPase: mechanism of stimulation by Ca2+. Arch Biochem Biophys. 1979 Apr 15;194(1):165–170. doi: 10.1016/0003-9861(79)90606-4. [DOI] [PubMed] [Google Scholar]
- Quist E. E., Roufogalis B. D. Calcium transport in human erythrocytes. Separation and reconstitution of high and low Ca affinity (Mg mca)-AT Pase activities in membranes prepared at low ionic strength. Arch Biochem Biophys. 1975 May;168(1):240–251. doi: 10.1016/0003-9861(75)90247-7. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. Reversible shift between two states of Ca2+-ATPase in human erythrocytes mediated by Ca2+ and a membrane-bound activator. Biochim Biophys Acta. 1978 May 4;509(1):67–77. doi: 10.1016/0005-2736(78)90008-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



