Abstract
Purpose
We aimed to evaluate the efficacy of moderate doses of bevacizumab in combination with TAS-102 for the treatment of refractory metastatic colorectal cancer.
Methods
A total of 261 patients with refractory mCRC were enrolled and categorized into two groups: TAS-102 combined with bevacizumab and TAS-102 alone. Patients in the bevacizumab combination group were divided into two subgroups based on a median dose of 3.3 mg/kg. Categorical variables were compared using the chi-square or Fisher’s exact test, and continuous variables were assessed using the t-test. The Cox proportional hazards model was used to adjust covariates. Survival analysis was performed using the log-rank test and Kaplan–Meier curves. Specific survival was evaluated using restricted mean survival time (RMST) and landmark analysis.
Result
The median progression-free survival (PFS) was 3.7 months in the TAS-102 combined with the bevacizumab group and 2.2 months in the non-bevacizumab group, showing significance in favor of the bevacizumab combination. Median overall survival (OS) was 9.4 months in the bevacizumab combination group and 10.3 months in the group that did not receive combination therapy. A survival benefit was observed within 9.5 months in both the RMST and landmark analyses. The PFS benefit was consistent across different doses of bevacizumab, while no significant difference in OS was observed compared to TAS-102 monotherapy. Both PFS and OS did not significantly differ between the different doses of bevacizumab.
Conclusion
Moderate doses of bevacizumab and TAS-102 provided satisfactory efficacy over the standard dose within a limited timeframe of 9.5 months.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-024-04767-9.
Keywords: Bevacizumab, TAS-102, Colorectal cancer, Chemotherapy
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death worldwide and is one of the most prevalent types of cancer [1]. Unfortunately, approximately half of all patients eventually develop metastatic CRC (mCRC). At this stage, salvage chemotherapy options include 5-fluorouracil (5-FU), oxaliplatin, irinotecan, anti-VEGF agents (such as bevacizumab), anti-EGFR agents (such as cetuximab and panitumumab), regorafenib, and TAS-102, either alone or in combination. However, patients undergoing salvage chemotherapy may experience adverse effects such as neutropenia following TAS-102 treatment, requiring dosage adjustments to maintain a balance between quality of life and efficacy.
Efficacy, sustained response to treatment, and maintenance of quality of life are crucial aspects of managing refractory mCRC. TAS-102 improved overall survival (OS) in refractory mCRC compared to placebo in the phase III RECOURSE trial [2]. Additionally, Van Cutsem et al. investigated 800 patients from the RECOURSE study, demonstrating the effectiveness of TAS-102 across various subgroups, regardless of age, geographic region, or KRAS status. However, of the 38% of patients who received TAS-102, 4% experienced grade 3 or greater neutropenia, and an additional 4% developed febrile neutropenia despite treatment with granulocyte colony-stimulating factor. Consequently, patients may face risks that could profoundly affect their quality of life [3].
In the SUNLIGHT study, combining bevacizumab with TAS-102 demonstrated significant OS benefits compared to TAS-102 monotherapy [4]. However, serious adverse events (grade ≥ 3) were reported more frequently in the combination group, with neutropenia being the most dominant. Severe (grade ≥ 3) neutropenia was noted in 43.1% of the combination group, and 29.3% of patients in the combination group received concurrent treatment with granulocyte colony-stimulating factor. Moreover, in a retrospective analysis (n = 24), Cann et al. demonstrated that alternative biweekly dosing of TAS-102 can reduce toxicity while maintaining efficacy, with a median PFS of 2.3 months [5]. However, survival data for patients with mCRC remains unsatisfactory. In the RECOURSE trial, OS was 7.1 months in the TAS-102 group and 5.3 months in the placebo group. The OS was 10.8 months in the combination group and 7.5 months in the TAS-102 group.
In this study, we assessed the effectiveness of a moderate dose of bevacizumab in combination with TAS-102. We hypothesized that patients receiving moderate doses of bevacizumab would have poorer survival outcomes.
Method
Patient characteristics and assessment
This retrospective cohort study included patients with mCRC who had developed resistance to prior therapies, including 5-FU, oxaliplatin, irinotecan, anti-VEGF, or anti-EGFR agents (if RAS wild-type). Eligibility criteria required that patients had received at least one cycle of TAS-102 therapy. Patients were classified as receiving concomitant bevacizumab if they had received three or more infusions, regardless of bevacizumab dose or timing in relation to TAS-102 treatment.
Baseline patient data were collected, including demographics (age, sex), body surface area (BSA) calculated using the Mosteller formula, primary tumor location, historical pathology, TAS-102 dosage (mg/m²/day), and relevant molecular data (RAS, BRAF mutation status, and microsatellite stability status). Additionally, clinical history data included the time interval between the diagnosis of metastatic disease and TAS-102 initiation, the number of prior therapy lines before TAS-102, and the presence of metastases in liver, lymph nodes, peritoneum, lung, or brain. This study was approved by the Institutional Review Board (IRB) of Taipei Veteran General Hospital, with a waiver of informed consent.
Bevacizumab dosing stratification
Among study participants, most received moderate doses of bevacizumab, while 11 patients were treated with a dose of 5 mg/kg. To analyze the effect of bevacizumab dosage, patients were stratified into two groups based on the median bevacizumab dose (3.3 mg/kg): a high-dose group (> 3.3 mg/kg) and a low-dose group (≤ 3.3 mg/kg).
Treatment response and survival outcomes
Treatment response was evaluated through imaging (CT and MRI) according to the RECIST criteria. PFS was calculated as the time from TAS-102 initiation to imaging-confirmed disease progression. OS was defined as the interval from TAS-102 initiation to death due to cancer.
Statistical analyses
Patient characteristics were reported with means and standard deviations for continuous variables, and medians with interquartile ranges where appropriate. Categorical variables were presented as frequencies and percentages. Group differences for categorical variables were assessed with chi-square or Fisher’s exact tests, while continuous variables were evaluated with independent t-tests or non-parametric equivalents where appropriate.
Survival analysis was conducted using the Kaplan–Meier method, with log-rank tests comparing survival curves. Cox proportional hazards models were employed for univariate and multivariate analyses to identify predictors of OS. Variables with P < 0.1 in univariate analysis were included in the multivariate model. Additionally, restricted mean survival time (RMST) and a landmark method were used to assess average survival at specific time points. All statistical tests were two-sided, and P-values < 0.05 were considered significant.
Results
Patient characteristics
We retrospectively reviewed 261 patients with histologically confirmed mCRC between January 1, 2010, and February 19, 2023. Of these, 175 received TAS-102 treatment without bevacizumab, and 86 received combination therapy. Baseline characteristics did not differ significantly between the two groups (Table 1).
Table 1.
Patient characteristics (n = 261)
| without bevacizumab n = 175 (%) |
bevacizumab n = 86 (%) |
P value | |||
|---|---|---|---|---|---|
| Age | 0.22 | ||||
| ≤ 60-year-old | 56 | (32.0) | 34 | (39.5) | |
| > 60-year-old | 119 | (68.0) | 52 | (60.5) | |
| Sex | 0.27 | ||||
| Male | 100 | (57.1) | 43 | (50.0) | |
| Female | 75 | (42.9) | 43 | (50.0) | |
| BSA, median, m2 (IQR) | 1.59 | (1.45–1.75) | 1.63 | (1.45–1.79) | 0.45 |
| Primary tumor location | 0.08f | ||||
| Right side | 50 | (28.6) | 17 | (19.8) | |
| Life side | 72 | (41.1) | 33 | (38.4) | |
| Rectum | 52 | (29.7) | 34 | (39.5) | |
| Synchronous | 1 | (0.6) | 0 | (0.0) | |
| Site unknown | 0 | (0.0) | 2 | (2.3) | |
| Pathology | 0.11f | ||||
| Adenocarcinoma | 164 | (93.7) | 85 | (98.8) | |
| Mucinous adenocarcinoma | 11 | (6.3) | 1 | (1.2) | |
| TAS-102 dose (mg/m2/d) (IQR) | 53.3 | (45.7–62.1) | 57.3 | (45.8–66.3) | 0.46 |
| RAS# | |||||
| wild type | 82 | (46.8) | 39 | (45.3) | 0.34 |
| mutation | 89 | (50.8) | 47 | (54.6) | |
| BRAF+ | |||||
| mutation | 5 | (2.8) | 2 | (2.3) | 0.93 |
| Microsatellite stability status$ | |||||
| MSS | 143 | (81.7) | 71 | (82.5) | 0.60 |
| MSI-H | 2 | (1.1) | 0 | (0.0) | |
| Time from metastatic disease (%) | 0.70 | ||||
| < 18 months | 53 | (30.2) | 28 | (32.5) | |
| ≥ 18 months | 122 | (69.8) | 58 | (67.5) | |
|
Previous treatment before TAS-102 |
0.88 | ||||
| ≤3 | 51 | (29.1) | 24 | (27.9) | |
| ≥4 | 124 | (70.9) | 62 | (72.1) | |
| Metastatic site | |||||
| Liver | 112 | (64.0) | 62 | (72.0) | 0.19 |
| Lymph node | 83 | (47.4) | 34 | (39.5) | 0.22 |
| Peritoneum | 65 | (37.1) | 26 | (30.2) | 0.27 |
| Lung | 129 | (73.7) | 69 | (80.2) | 0.24 |
| Brain | 5 | (2.8) | 5 | (5.8) | 0.30f |
Chi-Square test. fFisher’s Exact test. *P < 0.05, **P < 0.01
#4 patients RAS mutation data miss
+37 patients BRAF data miss
$45 patients MSS data miss
#RAS including KRAS and NRAS
MSS Microsatellite Stable, MSI-H microsatellite instability-high
Survival outcomes
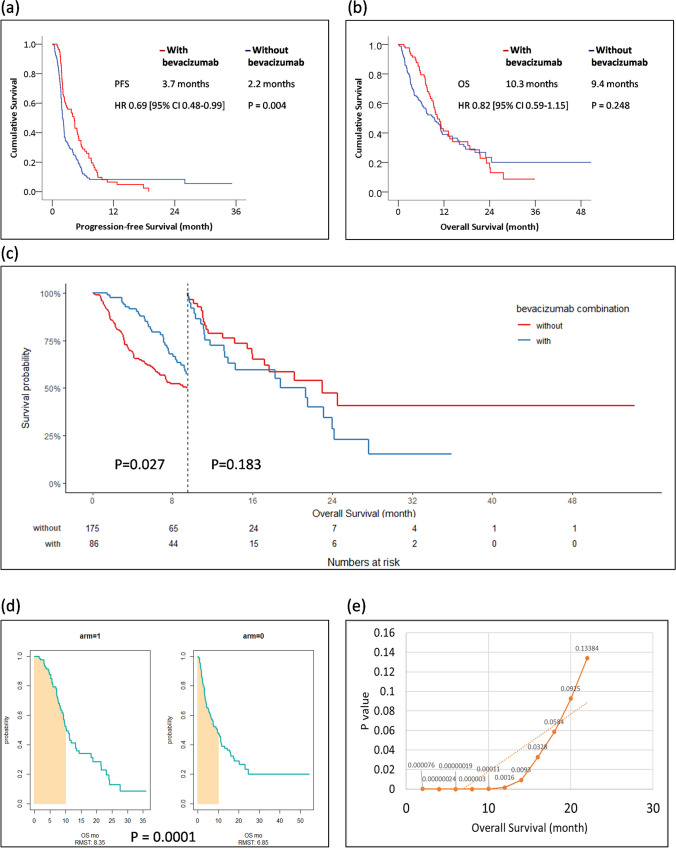
In our cohort, the Kaplan–Meier plot showed a PFS of 3.7 months in the TAS-102 group in combination with bevacizumab and 2.2 months in the group without bevacizumab (hazard ratio [HR] 0.69; 95% confidence interval [CI] 0.48–0.99), showing statistical significance in favor of the bevacizumab combination (P = 0.004) (Fig. 1a). The OS was 10.3 months in the bevacizumab combination group and 9.4 months in the group without combination therapy. No statistically significant difference was observed in OS regardless of the bevacizumab combination (P = 0.248) (Fig. 1b). In landmark analysis, survival showed statistical significance before 9.5 months rather than 9.5 months (P = 0.027) (Fig. 1c). RMST at 10 months showed a change in estimated survival probabilities for both groups (Fig. 1d). Patients in the bevacizumab combination group experienced greater benefit within 9.5 months (Fig. 1e).
Fig. 1.
Panel (a) represent the survival curve by using the Kaplan–Meier method to represent the PFS by bevacizumab combination or not (n =261); panel (b) represent the survival curve of the OS by bevacizumab combination or not (n =261); panel (c) represent the specific time in 9.5-month of OS of bevacizumab combination or not in landmark test (n =261); panel (d) illustrates the RMST method for average survival at a specific time of 10 months. Arm1 stands for the bevacizumab combination, and arm2 stands for TAS-102 monotherapy; panel (e) represent the P value of OS as time goes by (n =261)
We summarized both univariate and multivariate analyses of OS. Univariate analysis revealed statistical significance for patients with BSA ≥ 1.61 m2, time from diagnosis of metastatic disease to TAS-102 administration > 18 months, and for patients with liver, lymph node, or brain metastases. In multivariate analysis, these factors remained statistically significant (Table 2).
Table 2.
Summary of univariate and multivariate analyses of overall survival
| Patient Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Bevacizumab combination | ||||
| Without bevacizumab | Reference | |||
| With bevacizumab | 0.82 (0.59–1.15) | 0.24 | ||
| Bevacizumab dose | ||||
| lower than 3.3 mg/kg | Reference | |||
| higher than 3.3 mg/kg | 1.30 (0.76–2.25) | 0.34 | ||
| Age, years | ||||
| ≤ 60-year-old | Reference | |||
| > 60-year-old | 1.11 (0.78–1.56) | 0.56 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 0.85 (0.62–1.17) | 0.30 | ||
| BSA, median, m2 | ||||
| < 1.61 | Reference | Reference | ||
| ≥ 1.61 | 0.50 (0.28–0.87) | < 0.01** | 0.45 (0.25–0.81) | < 0.01** |
| Primary tumor location | ||||
| Right side | Reference | |||
| Left side | 0.80 (0.53–1.20) | 0.27 | ||
| Rectum | 0.80 (0.53–1.20) | 0.27 | ||
| Synchronous | 1.63 (0.22–11.88) | 0.63 | ||
| Pathology | ||||
| Adenocarcinoma | Reference | |||
| Mucinous adenocarcinoma | 0.94 (0.42–2.14) | 0.89 | ||
| TAS-102 dose (mg/m2/d) | ||||
| <56 mg/m2/d | Reference | |||
| ≥56 mg/m2/d | 0.87 (0.64–1.20) | 0.40 | ||
| RAS# | ||||
| wild type | Reference | |||
| mutation | 0.86 (0.62–1.18) | 0.34 | ||
| BRAF+ | ||||
| wild type | Reference | |||
| mutation | 1.22 (0.50–3.00) | 0.66 | ||
| Microsatellite stability status$ | ||||
| MSS | Reference | |||
| MSI-H | 0.69 (0.10–4.96) | 0.90 | ||
| Time from metastatic disease | ||||
| < 18 months | Reference | Reference | ||
| ≥ 18 months | 0.60 (0.42–0.84) | 0.003** | 0.64 (0.45–0.91) | 0.013** |
| Previous treatment before TAS-102 | ||||
| ≤ 3 | Reference | |||
| ≥ 4 | 1.44 (0.97–2.12) | 0.06 | 1.43 (0.94–2.16) | 0.08 |
| Metastatic site | ||||
| Liver | 1.58 (1.10–2.26) | 0.01* | 1.58 (1.10–2.27) | 0.01* |
| Lymph node | 1.43 (1.03–1.97) | 0.02* | 1.83 (1.30–2.57) | 0.001** |
| Peritoneum | 1.08 (0.77–1.52) | 0.64 | ||
| Lung | 1.01 (0.70–1.46) | 0.94 | ||
| Brain | 2.12 (1.08–4.18) | 0.02* | 6.51 (2.97–14.27) | < 0.001** |
*P < 0.05, **P < 0.01
#4 patients RAS mutation data miss
+37 patients BRAF data miss
$45 patients MSS data miss
#RAS including KRAS and NRAS
Impact of bevacizumab dose
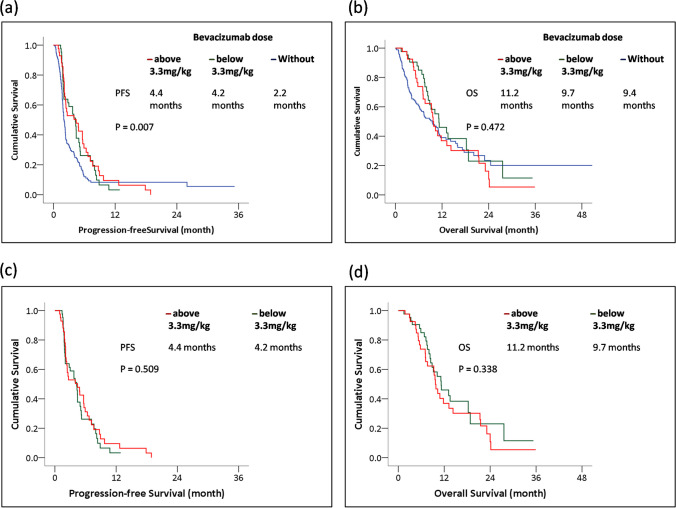
We investigated the effect of the bevacizumab dose on survival. A statistically significant improvement in PFS was noted via the log-rank test, indicating that patients experienced a survival benefit with combination therapy compared to TAS-102 monotherapy (P = 0.007) (Fig. 2a). No significant difference was observed in OS between patients not receiving the bevacizumab combination and patients receiving different doses, whether above or below 3.3 mg/kg (P = 0.472) (Fig. 2b). In patients receiving the bevacizumab combination therapy (n = 86), PFS rates were not significantly different between the two groups, regardless of dosage (Fig. 2c). Median PFS was 4.2 months in the bevacizumab group with doses less than 3.3 mg/kg and 4.4 months in the comparison group (P = 0.509). These results suggest that bevacizumab treatment is associated with improved PFS and that a combination of moderate doses of bevacizumab and TAS-102 provided comparable efficacy. No significant difference in OS was observed between different bevacizumab doses. This implies that patients receiving doses either slightly below standard (between 3.3 mg/kg and 5 mg/kg) or significantly lower (below 3.3 mg/kg) show no significant effects (P = 0.338).
Fig. 2.
Panel (a) represent the survival curve of PFS by 3 different doses of bevacizumab combination, including none, lower and above medium dose of 3.3mg/kg (n =261); panel (b) represent the OS by 3 different doses of bevacizumab combination, including none, lower and above medium dose of 3.3mg/kg (n =261); panel (c) and (d) analysis among patients using bevacizumab only. Panel (c) represent the PFS in bevacizumab combination group, including lower and above medium dose of 3.3mg/kg (n =86); panel (d) represent the OS in bevacizumab combination group, including lower and above medium dose of 3.3mg/kg (n =86)
In our cohort, the median dose of bevacizumab was 3.3 mg/kg. Therefore, we summarized the characteristics of patients in the bevacizumab combination group at this dosage. Sex exhibited statistically significant differences, with women having a higher prevalence in the bevacizumab group at doses above 3.3 mg/kg, whereas men were more predominant. Other characteristics were not statistically significant (Table 3).
Table 3.
Patient characteristics, combination with bevacizumab
| bevacizumab lower than 3.3 mg/kg n = 43 (%) |
bevacizumab higher than 3.3 mg/kg n = 43 (%) |
P value | |||
|---|---|---|---|---|---|
| Age, median, years(IQR) | 64 | (55–72) | 63 | (54–71) | 0.34 |
| ≤60-year-old | 18 | (41.8) | 16 | (37.2) | |
| >60-year-old | 25 | (58.2) | 27 | (62.8) | |
| Sex | 0.01* | ||||
| Male | 27 | (62.8) | 16 | (37.2) | |
| Female | 16 | (37.2) | 27 | (62.8) | |
| BSA, median, m2 | 1.75 | (1.56–1.94) | 1.55 | (1.42–1.65) | 0.09 |
| Primary tumor location | 0.12 | ||||
| Right side | 6 | (14.0) | 11 | (25.6) | |
| Life side | 20 | (46.5) | 13 | (30.2) | |
| Rectum | 17 | (39.5) | 17 | (39.5) | |
| Site unknown | 0 | (0.0) | 2 | (4.7) | |
| Pathology | 0.31 | ||||
| Adenocarcinoma | 43 | (100.0) | 42 | (97.6) | |
| Mucinous adenocarcinoma | 0 | (0.0) | 1 | (2.4) | |
| TAS-102 dose (mg/m2/) (IQR) | 54.8 | (47.9–63.6) | 59.9 | (52.6–69.4) | 0.59 |
| RAS | 0.12 | ||||
| wild type | 23 | (53.4) | 16 | (37.2) | |
| mutation | 20 | (46.5) | 27 | (62.8) | |
| BRAF | 0.95 | ||||
| wild type | 42 | (97.6) | 42 | (97.6) | |
| mutation | 1 | (2.4) | 1 | (2.4) | |
| Microsatellite stability status | 0.77 | ||||
| MSS | 36 | (83.7) | 35 | (81.4) | |
| MSI-H | 0 | (1.1) | 0 | (0.0) | |
| Time from metastatic disease | 0.64 | ||||
| < 18 months | 13 | (30.2) | 15 | (34.8) | |
| ≥ 18 months | 30 | (69.8) | 28 | (65.2) | |
|
Previous treatment before TAS-102 |
0.27 | ||||
| ≤ 3 | 10 | (23.2) | 14 | (32.5) | |
| ≥ 4 | 33 | (76.8) | 29 | (67.5) | |
| Metastatic site | |||||
| Liver | 29 | (67.4) | 33 | (76.8) | 0.33 |
| Lymph node | 21 | (48.8) | 13 | (30.2) | 0.07 |
| Peritoneum | 16 | (37.2) | 33 | (76.8) | 0.27 |
| Lung | 35 | (81.4) | 34 | (79.0) | 0.78 |
| Brainf | 4 | (9.3) | 1 | (2.4) | 0.16 |
Mann-Whitney U test. Chi-Square test. fFisher’s Exact test. *P < 0.05, **P < 0.01
Subgroup analysis of PFS in bevacizumab combination
Our results demonstrate the potential PFS benefits of combining TAS-102 with bevacizumab, particularly in specific subgroups (Table 4). These subgroups included patients > 60 years old, men, individuals with a BSA < 1.61 m2, patients with adenocarcinoma, those who received a TAS-102 dose > 56 mg/m2/d, individuals with RAS mutation, wild-type BRAF, microsatellite stable status, patients with a duration of < 18 months from diagnosis of metastatic disease to initiation of TAS-102, heavily pretreated patients, and those with liver, lymph node, or lung metastases. Although the data are limited, PFS benefits were observed in patients with BRAF mutations. PFS in patients with microsatellite instability status was not analyzed because no patient in the bevacizumab combination group had microsatellite instability status. These findings suggest that TAS-102, in combination with bevacizumab, may lead to improved PFS in patients with mCRC with the above characteristics.
Table 4.
Subgroup analysis of progression-free survival in combination with bevacizumab
| Subgroup | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age, median, years | |||
| ≤60-year-old | 0.66 | 0.41–1.08 | 0.10 |
| >60-year-old | 0.63 | 0.44–0.91 | 0.01* |
| Sex | |||
| Male | 0.62 | 0.42–0.93 | 0.02* |
| Female | 0.69 | 0.45–1.05 | 0.08 |
| BSA, median, m2 | |||
| <1.61 | 0.57 | 0.38–0.87 | 0.01* |
| ≥1.61 | 0.73 | 0.49–1.10 | 0.14 |
| Primary tumor location | |||
| Right side | 0.61 | 0.32–1.13 | 0.12 |
| Life side | 0.68 | 0.43–1.08 | 0.10 |
| Rectum | 0.68 | 0.42–1.12 | 0.13 |
| Pathology | |||
| Adenocarcinoma | 0.63 | 0.47–0.85 | 0.003** |
| Mucinous adenocarcinoma | 0.74 | 0.08–6.54 | 0.79 |
| TAS-102 dose (mg/m2/d) | |||
| <56 mg/m2/d | 0.70 | 0.45–1.08 | 0.11 |
| ≥56 mg/m2/d | 0.60 | 0.41–0.90 | 0.01** |
| RAS | |||
| wild type | 0.70 | 0.45–1.08 | 0.11 |
| mutation | 0.56 | 0.38–0.85 | 0.006** |
| BRAF | |||
| wild type | 0.65 | 0.47–0.89 | 0.008** |
| mutation¶f | 6.30 | 1.42–27.93 | 0.01* |
| Microsatellite stability status§ | |||
| MSS | 0.67 | 0.49–0.93 | 0.01* |
| Time from metastatic disease | |||
| <18 months | 0.40 | 0.22–0.71 | 0.002** |
| ≥18 months | 0.72 | 0.51–1.02 | 0.06 |
| Previous treatment before TAS-102 | |||
| ≤3 | 0.73 | 0.42–1.28 | 0.28 |
| ≥4 | 0.62 | 0.44–0.87 | 0.006** |
| Metastatic site | |||
| Liver | 0.63 | 0.45–0.89 | 0.009** |
| Lymph node | 0.60 | 0.39–0.94 | 0.02* |
| Peritoneum | 0.86 | 0.51–1.45 | 0.58 |
| Lung | 0.60 | 0.43–0.84 | 0.003** |
| Brainf | 0.52 | 0.07–3.94 | 0.53 |
Chi-Square test. fFisher’s Exact test. *P < 0.05, **P < 0.01
§There were no patients with MSI-H in the bevacizumab combination group
¶There were 2 patients with BRAF mutation in the bevacizumab combination group
Discussion
Our report is the first to show that a moderate dose of bevacizumab in combination with TAS-102 provides satisfactory survival benefits that have not been directly compared in clinical studies. This survival benefit was limited to 9.5 months.
In the RECOURSE study, TAS-102 demonstrated proven efficacy with acceptable toxicities compared to the placebo. Patients were administered TAS-102 at a dose of 35 mg/m2/d two times daily, following a 5-day-on and 2-day-off schedule each week for 2 weeks, followed by a 14-day rest period [2]. However, 53% experienced a delay of ≥ 4 d in commencing their next cycle owing to toxicity, with approximately half of this subgroup experiencing a longer delay of ≥ 8 d. In addition, 3.6% of patients discontinued treatment due to adverse reactions, and 14% required dose reduction [3]. In clinical practice, patients often experience gastrointestinal symptoms, leading to the postponement of treatment due to grade 3 or 4 neutropenia.
The rationale for using this combination was based on preliminary studies considering the patients who had been diagnosed with colorectal cancer [6–9]. Compared to the SUNLIGHT study, OS was 10.8 months in the bevacizumab combination group, while in our cohort it was 10.3 months with a moderate dose of bevacizumab. Survival was similar to that observed with relatively low doses of bevacizumab. Moreover, RMST analysis limited OS to a period of 10 months. However, patients who underwent TAS-102 treatment experienced greater survival benefits with early bevacizumab combination therapy [4].
The median TAS-102 dose in our retrospective review was 56 mg/m2/d. Our institution has reduced TAS-102 due to intolerance. In other words, most patients initially received the standard dose in their prescription and experienced a dose reduction after the first cycle of administration. Once patients received a reduced dose, it was not titrated back to the standard dose. We observed a trend of an increased TAS-102 dose in the bevacizumab combination group compared to the others; however, the difference was not statistically significant.
No significant difference was observed in the OS between the bevacizumab combination groups. This may have been caused by complications, as well as the lack of TAS-102 and bevacizumab underdosing in our cohort. Furthermore, the majority of patients had refractory disease, with 71.2% (n = 186) receiving TAS-102 as their fourth line of treatment or beyond [10].
The lack of OS benefits at the higher standard dose of the bevacizumab group (> 3.3 mg/kg) may be due to several factors. First, men dominated the lower bevacizumab dose group. Second, the relatively small sample size may have influenced the results. Finally, the higher proportion of left-side-origin tumors in the lower bevacizumab group (46.5%) compared to their counterparts (30.2%) in our cohort may have had an impact. In the Monitoring of Cancer Incidence in Japan (MCIJ) project, which examined 62,350 participants with colon cancer from 2006 to 2008, the authors reported that the five-year net survival rate for left- and right-sided colon cancer was 74.0% (95% CI, 73.4–74.7%) and 70.4% (95% CI, 69.7–71.0%), respectively [11]. This suggests that patients with left-sided colon cancer have better survival rates than those with other cancers. No precise explanation exists for the better OS trend in the lower-dose group. We observed a significant PFS difference with TAS-102 in combination with bevacizumab, regardless of the bevacizumab dose. Patients receiving TAS-102 treatment experienced extended PFS after more than three bevacizumab infusions. Additionally, we observed that in certain individuals, including those > 60, men with a BSA less than 1.61, adenocarcinoma (versus mucinous adenocarcinoma), receiving a TAS-102 dose above 56 mg/m2/d, having RAS mutations, diagnosed with metastatic disease < 18 months before starting TAS-102, heavily pretreated, or with liver, lymph nodes, or lung metastases, may experience prolonged PFS.
Subgroups of patients with factors indicating a good prognosis have already been described. In a combined analysis of two phase III studies, mucinous adenocarcinomas exhibited worse OS, PFS, and overall response rates than adenocarcinomas [12]. This suggests that mucinous adenocarcinoma may be an independent factor for poor prognosis. Although RAS mutations conferred a PFS benefit in our cohort, the role of KRAS mutations as a treatment-independent prognostic factor seems unlikely; however, this remains controversial [13]. BRAF mutation has different clinical characteristics and prognoses [13–15]; however, limited data exists on this aspect.
Our study had several limitations. First, it was retrospective in nature. Further prospective randomized clinical trials are required to validate the role of different doses in combination therapy. Second, our cohort received different doses of TAS-102, making it difficult to accurately determine the efficacy of TAS-102 or bevacizumab. We could not determine the most beneficial dose combination. Factors such as limited BRAF, microsatellite stable data, and a small mucinous adenocarcinoma population made statistical significance questionable. Various salvage chemotherapy regimens were used before or after bevacizumab combination therapy.
Conclusion
This retrospective study highlights the survival benefits of TAS-102 combined with a moderate dose of bevacizumab in patients with refractory mCRC. Further prospective studies are essential to determine the optimal combination of moderate doses of bevacizumab and TAS-102 in heavily treated patients with CRC in real-world practice.
Supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Part of the data for this study was obtained from the Cancer Registry Database of Taipei Veterans General Hospital.
Author contributions
Kuan-Yu Tseng collected and analyzed the data, wrote, and submitted the manuscript. Mu-Ying Yang, Wei-Shone Chen, Jeng-Kai Jiang, Huann-Sheng Wang, Shih-Ching Chang, Yuan-Tzu Lan, Chun-Chi Lin, Hung-Hsin Lin, Sheng-Chieh Huang, Hou-Hsuan Cheng, Yu-Zu Lin, Che-Yuan Chang, and Yi-Wen Yang provided data. Hao-Wei Teng designed the study and approved the manuscript and bioinformatics.
Funding information
No.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This retrospective study was conducted using population-based data from Taipei Veterans General Hospital, Taiwan, following the guidelines of the Declaration of Helsinki. Approval was obtained from the Ethics Committee and Institutional Review Board of Taipei Veterans General Hospital, Taiwan. Due to the retrospective nature of the study, the requirement for written informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hossain MS, Karuniawati H, Jairoun AA et al (2022) Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers 14:1732. 10.3390/cancers14071732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer RJ, Van Cutsem E, Falcone A et al (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372:1909–1919. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Falcone A, Garcia-Carbonero R et al (2017) Proxies of quality of life in metastatic colorectal cancer: analyses in the RECOURSE trial. ESMO Open 2:e000261. 10.1136/esmoopen-2017-000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prager GW, Taieb J, Fakih M et al (2023) Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med 388:1657–1667. 10.1056/NEJMoa2214963 [DOI] [PubMed] [Google Scholar]
- 5.Cann CG, Cimino S, Grieb B et al (2022) Alternative biweekly dosing schedule of trifluridine-tipiracil (TAS-102) reduces rates of myelosuppression while maintaining therapeutic efficacy in patients (pts) with previously treated metastatic colorectal cancer (mCRC). Am Soc Clin Oncol 40:3559–3559. 10.1200/JCO.2022.40.16_suppl.3559 [Google Scholar]
- 6.Kuboki Y, Nishina T, Shinozaki E et al (2017) TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol 18:1172–1181. 10.1016/S1470-2045(17)30425-4 [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer P, Yilmaz M, Möller S et al (2020) TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol 21:412–420. 10.1016/S1470-2045(19)30827-7 [DOI] [PubMed] [Google Scholar]
- 8.Kotani D, Kuboki Y, Horasawa S et al (2019) Retrospective cohort study of trifluridine/tipiracil (TAS-102) plus bevacizumab versus trifluridine/tipiracil monotherapy for metastatic colorectal cancer. BMC Cancer 19:1253. 10.1186/s12885-019-6475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satake H, Kato T, Oba K et al (2020) Phase Ib/II study of biweekly TAS-102 in combination with bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (BiTS study). Oncologist 25:e1855–e1863. 10.1634/theoncologist.2020-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Lago N, Chucla TC, De Castro BA et al (2022) Efficacy, safety and prognostic factors in patients with refractory metastatic colorectal cancer treated with trifluridine/tipiracil plus bevacizumab in a real-world setting. Sci Rep 12:14612. 10.1038/s41598-022-18871-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa-Senda H, Hori M, Matsuda T et al (2019) Prognostic impact of tumor location in colon cancer: the monitoring of Cancer incidence in Japan (MCIJ) project. BMC Cancer 19:431. 10.1186/s12885-019-5644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mekenkamp LJ, Heesterbeek KJ, Koopman M et al (2012) Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer 48:501–509. 10.1016/j.ejca.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 13.Roth AD, Tejpar S, Delorenzi M et al (2010) Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol 28:466–474. 10.1200/JCO.2009.23.3452 [DOI] [PubMed] [Google Scholar]
- 14.Di Nicolantonio F, Martini M, Molinari F et al (2008) Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 26:5705–5712. 10.1200/JCO.2008.18.0786 [DOI] [PubMed] [Google Scholar]
- 15.Karagkounis G, Torbenson MS, Daniel HD et al (2013) Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer 119:4137–4144. 10.1002/cncr.28347 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.