Abstract
Background and Aim
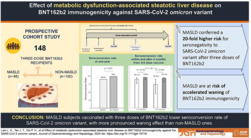
We aimed to investigate the effect of metabolic dysfunction‐associated steatotic liver disease (MASLD) on three‐dose BNT162b2 immunogenicity to the omicron variant.
Methods
Adult recipients of three doses of BNT162b2 were prospectively recruited between May and December 2021. The serology of the neutralizing antibody by live virus microneutralization (vMN) to the omicron variant was measured at baseline, day 180, and day 360 after the first dose. The primary outcome was seroconversion (vMN titer ≥ 10) at day 360. Exposure of interest was MASLD, defined as hepatic steatosis (controlled attenuation parameter ≥ 248 dB/m on transient elastography) plus at least one of five cardiometabolic risk factors. Subjects with prior COVID‐19 were excluded. A multivariable logistic regression model was used to derive the adjusted odds ratio of seroconversion with MASLD by adjusting for age, sex, antibiotic use, and proton pump inhibitor use.
Results
One hundred forty‐eight BNT162b2 recipients (male: 48 [32.4%]; median age: 51.0 years [interquartile range, IQR: 44.5–57.3]) were recruited. The median time from the first dose to the third dose was 8.5 months (IQR: 7.9–8.9). MASLD subjects had a lower seroconversion rate than non‐MASLD ones (89.6% vs 99.0%; P = 0.007). MASLD was the only independent risk factor for seroconversion (adjusted odds ratio: 0.051, 95% confidence interval: 0.002–0.440). Subgroup analysis of immunogenicity at 4 months after the third dose shows significantly lower vMN titer (13.06 [IQR: 7.69–22.20] vs 33.49 [IQR: 24.05–46.53]; P = 0.004) and seroconversion rate (76.9% vs 97.4%; P = 0.016) in MASLD than non‐MASLD subjects, but not within 4 months from the third dose (vMN titer: 46.87 [IQR: 33.12–66.02] vs 41.86 [IQR: 34.47–50.91], P = 0.240; seroconversion rate: 94.3% vs 100%, P = 0.131).
Conclusion
Metabolic dysfunction‐associated steatotic liver disease was a risk factor for poorer immunogenicity to the omicron variant, with a more pronounced waning effect compared among three‐dose BNT162b2 recipients.
Keywords: COVID‐19, MASLD, SARS‐CoV‐2, steatohepatitis, vaccination
Introduction
The ongoing coronavirus disease 2019 (COVID‐19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has emerged as a seasonal illness. COVID‐19 has affected more than 700 million people and caused nearly 7 million deaths worldwide as of October 2023. Apart from measures such as social distancing, quarantine, and isolation, vaccination is crucial in preventing infection, severe symptoms, and death. 1 Seasonal booster vaccines are recommended as an effective 2 and safe 3 preventive measure, with more than 13 billion vaccine doses having been administered so far. 4
Non‐alcoholic fatty liver disease (NAFLD) has a global prevalence of 32%. 5 As NAFLD is a multisystemic condition that presents with a wide spectrum of extrahepatic manifestations, such as obesity, diabetes mellitus, and cardiovascular diseases, the term metabolic dysfunction‐associated steatotic liver disease (MASLD) has recently been introduced to redefine this disease spectrum. 6
It has been shown that a lower proportion of patients with moderate‐to‐severe hepatic steatosis achieved a highest‐tier humoral response to wild‐type SARS‐CoV‐2 for both BNT162b2 and CoronaVac vaccines at day 56 of the first‐dose vaccine. 7 In Hong Kong, both mRNA and inactivated vaccines are available, with BNT162b2 being more commonly used. Neutralizing antibody levels are a surrogate marker of vaccine effectiveness 8 and are predictive of protection from symptomatic COVID‐19 infection. 9 , 10 Our previous study found that patients with hepatic steatosis receiving two doses of BNT162b2 had good immunogenicity to SARS‐CoV‐2 wild type and delta variant but not omicron variant. 11 However, the study only analyzed the effect of two doses of vaccine 6 months after vaccination. A recent population‐based retrospective cohort study showed that while a third dose substantially reduced infection risk, protection against omicron infection waned with time. 2 Chen et al. 12 have studied the immunogenicity of two doses of inactivated SARS‐CoV‐2 vaccine in patients with severe liver disease, in which a weakened and less durable antibody response was observed as compared to healthy controls. Although different etiologies, including chronic viral hepatitis infection and NAFLD, were evaluated, hepatitis B virus infection was the predominant cause, accounting for 88%. Also, patients with prior SARS‐CoV‐2 infections were not excluded. While there are studies investigating the effect of hepatic steatosis on two‐dose vaccine immunogenicity, 7 , 11 studies investigating the association between MASLD and longer‐term vaccine immunogenicity after booster doses are lacking.
Therefore, we aimed to conduct a 1‐year prospective cohort study to compare the immunogenicity of three doses of BNT162b2 to the SARS‐CoV‐2 omicron variant in subjects with MASLD and non‐MASLD.
Methods
Study design
This is a prospective cohort study conducted between May 2021 and February 2023. Given Hong Kong's “dynamic covid‐zero strategy,” fewer than 4000 COVID‐19 cases were reported throughout the first four waves of the COVID‐19 outbreak. The minimal local COVID‐19 transmission prior to 2022 rationalized that community immunity to SARS‐CoV‐2 was mostly vaccine derived. In January 2022, cases driven predominantly by the omicron variant started to surge, representing the fifth wave of the COVID‐19 outbreak. The increased transmissibility and escape of vaccine‐derived immunity 13 by the SARS‐CoV‐2 omicron variant have caused more than 3 million COVID‐19 positive cases in Hong Kong up until January 2023. 14
We recruited adult BNT162b2 vaccine recipients from three local vaccination centers. Exclusion criteria included age < 18 years, patients with transplantation, taking immunosuppressives/chemotherapy, with inflammatory bowel disease, with other medical diseases (cancer, hematological, rheumatological, and autoimmune diseases), and/or with prior COVID‐19 infection. Prior COVID‐19 was identified from history taking or the presence of antibodies to SARS‐CoV‐2 nucleocapsid (N) protein from baseline blood taking.
Recruited subjects received the first two doses of intramuscular BNT162b2 (0.3 mL) 3 weeks apart, and the third dose at any time between 6 and 12 months after the first dose, according to the recipient's preference (Fig. 1). Their blood samples were collected at three time points for serology of the SARS‐CoV‐2 omicron variant: (i) baseline before vaccination (day 0), (ii) 180 days after the first dose (day 180), and (iii) 360 days after the first dose (day 360).
Figure 1.
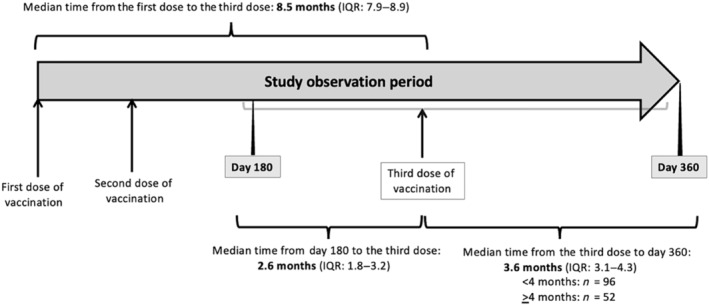
Study timeline. Recruited subjects received the first two doses of BNT162b2 3 weeks apart, and the third dose at any time between 6 and 12 months after the first dose, according to the recipient's preference. Their blood samples were collected for serology of the severe acute respiratory syndrome coronavirus 2 omicron variant at baseline before vaccination, 180 days, and 360 days after the first dose. IQR, interquartile range.
A live virus microneutralization (vMN) assay was performed in a 96‐well plate as described. 15 Serum samples were serially diluted in twofolds with minimum essential medium (Gibco, Green Island, NY, USA) starting at 1:10. Diluted sera will be mixed with 100 TCID50 of SARS‐CoV‐2 and incubated at 37°C for 1 h. The mixture was added to VeroE6 TMPRSS2 cells and incubated at 37°C and 5% CO2. The cytopathic effect was determined by examination under inversion microscopy after 72 h of incubation. The vMN antibody titer had the highest dilution with 50% inhibition of cytopathic effect, with standardization to the World Health Organization's International Standard for SARS‐CoV‐2 immunoglobulin (human). vMN positivity (seroconversion) was defined as a titer ≥ 10 (31.25 IU/mL).
The study was approved by the Institutional Review Board of the University of Hong Kong and the Hong Kong West Cluster of Hospital Authority.
Outcome of interest
The primary outcome of interest was seroconversion at 1 year after the first dose (day 360). Seroconversion was defined as a vMN titer ≥ 10 (31.25 IU/mL). The secondary outcome of interest was the vMN titer 1 year after the first dose.
Exposure of interest
The exposure of interest was MASLD, which was diagnosed in the presence of hepatic steatosis (defined by a controlled attenuation parameter ≥ 248 dB/m on transient elastography using FibroScan [Echosens, Paris, France]) 16 and one of five cardiometabolic risk factors, namely, overweight/obesity (body mass index ≥ 23 or ≥ 25 kg/m2 or waist circumference > 94 cm for male or > 80 cm for female), impaired glucose regulations (fasting serum glucose ≥ 5.6 mmol/L or hemoglobin A1c ≥ 5.7%), high blood pressure (blood pressure ≥ 130/85 mmHg), high triglyceride level (≥ 1.70 mmol/L), and low high‐density lipoprotein level (≤ 1 mmol/L for male or ≤ 1.3 mmol/L for female). 17 , 18
Covariates included age, sex, antibiotic use, and proton pump inhibitor (PPI) use. As gut microbiota are involved in the immune response to vaccination, 19 , 20 , 21 prior antibiotic use 22 , 23 and PPI use that could possibly affect gut microbiota were included in the analysis.
Sensitivity analysis was carried out by analyzing the association of hepatic steatosis and obesity with seroconversion to the SARS‐CoV‐2 omicron variant.
Statistical analysis
All statistical analyses were conducted in R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) statistical software. Data are displayed as the median (interquartile range [IQR]) for continuous variables and as the number of patients (percentage) for categorical variables. The Mann–Whitney U‐test was used for two continuous variables, and the χ 2 test or Fisher's exact test was used for categorical variables to assess the statistical significance between groups. A geometric mean titer (GMT) with a 95% confidence interval (95% CI) was used to express the average vMN titer. A multivariable logistic regression model was used to estimate the adjusted odds ratio (aOR) of seroconversion with MASLD and all the aforementioned covariates. To investigate the interplay between hepatic steatosis and obesity, a sensitivity analysis was performed by adjusting for hepatic steatosis and obesity in addition to age, sex, PPI use, and antibiotic use. For the purpose of statistical analysis, an MN titer of < 10 was considered a 5.
As antibody titers decreased with time, we performed subgroup analysis by dividing the cohort according to a 4‐month cut‐off after the third dose (Fig. 1). A prior study showed that vaccine effectiveness against hospital admission decreased from 96% to 68% by using this cut‐off. 24
A two‐sided P‐value ≤ 0.05 was considered statistically significant.
Results
Between May 2021 and February 2022, 752 vaccine recipients were screened. In the end, 148 three‐dose BioNTech recipients were included in this study (Fig. S1). The median age was 51.0 years (IQR: 44.5–57.3), and there were 48 (32.4%) males. There were 48 (32.4%) and 100 (67.6%) MASLD and non‐MASLD subjects, respectively. Nine subjects had a chronic hepatitis B infection. The median alanine transaminase and aspartate aminotransferase levels were 19 and 23 U/L, respectively. The demographics of subjects are shown in Table 1.
Table 1.
Baseline characteristics of three‐dose BNT162b2 recipients
| Whole cohort (n = 148) | MASLD (n = 48) | Non‐MASLD (n = 100) | P‐value | |
|---|---|---|---|---|
| Age (years) | 50.95 (44.50–57.30) | 51.80 (49.25–55.42) | 49.90 (39.77–57.35) | 0.057 |
| Male sex (n, %) | 48/148 (32.4%) | 18/48 (37.5%) | 30/100 (30%) | 0.362 |
| Antibiotic use (n, %) | 13/148 (8.8%) | 4/48 (8.3%) | 9/100 (9%) | 0.999 |
| Proton pump inhibitor use (n, %) | 17/148 (11.5%) | 9/48 (18.8%) | 8/100 (8%) | 0.055 |
| BMI (kg/m2) | 23.00 (21.10–25.40) | 25.85 (23.98–28.13) | 22.30 (20.17–23.72) | < 0.001 |
| Waist circumference (cm) | 81.70 (74.75–88.40) † | 89.25 (84.88–95.85) | 76.50 (72.00–83.10) † | < 0.001 |
| Fasting glucose (mmol/L) | 5.10 (4.70–5.40) | 5.40 (5.00–5.80) | 4.90 (4.60–5.23) | < 0.001 |
| HbA1c level (%) | 5.50 (5.27–5.70) | 5.70 (5.47–6.00) | 5.40 (5.20–5.60) | < 0.001 |
| Systemic blood pressure (mmHg) | 122 (112.00–129.00) ‡ | 127.00 (121.00–139.25) | 117.50 (108.50–127.00) ‡ | < 0.001 |
| Triglyceride level (mmol/L) | 1.00 (0.70–1.30) | 0.30 (1.00–1.55) | 0.80 (0.68–1.10) | < 0.001 |
| High‐density lipoprotein cholesterol level (mmol/L) | 1.60 (1.40–1.90) | 1.30 (1.10–1.63) | 1.80 (1.50–2.10) | < 0.001 |
| ALT (U/L) | 19.00 (14.00–26.00) | 25.00 (19.00–34.50) | 16.50 (14.00–24.00) | < 0.001 |
| AST (U/L) | 23.00 (19.00–26.00) | 23.50 (20.75–29.50) | 21.50 (18.00–26.00) | 0.018 |
| HBsAg positive (n, %) | 9/148 (6.1%) | 2/48 (4.2%) | 7/100 (7.0%) | 0.719 |
| MASLD criteria | ||||
| Overweight/obesity (BMI ≥ 23 kg/m2) (n, %) | 77/148 (52.0%) | 43/48 (89.6%) | 34/100 (34.0%) | < 0.001 |
| Criteria on weight (BMI ≥ 23 kg/m2 or WC > 94 cm [M] and 80 cm [F]) (n, %) | 86/148 (58.1%) | 45/48 (93.8%) | 41/100 (41%) | < 0.001 |
| Criteria on glucose regulation (FG ≥ 5.6 mmol/L or A1c ≥ 5.7%) (n, %) | 57/148 (38.5%) | 32/48 (66.7%) | 25/100 (25%) | < 0.001 |
| Systolic blood pressure ≥ 130/85 mmHg (n, %) | 15/148 (10.1%) | 11/48 (22.9%) | 4/100 (4%) | < 0.001 |
| TG ≥ 1.7 mmol/L | 19/148 (12.8%) | 12/48 (25%) | 7/100 (7%) | 0.002 |
| HDL ≤ 1.0 mmol/L (M) or ≤ 1.3 mmol/L (F) (n, %) | 22/148 (14.9%) | 18/48 (37.5%) | 4/100 (4%) | < 0.001 |
| Transient elastography | ||||
| Liver stiffness (kPa) | 4.20 (3.58–5.23) | 4.80 (4.07–5.93) | 4.00 (3.38–4.70) | < 0.001 |
| CAP score (dB/m) | 226.50 (205.75–260.50) | 276.00 (262.75–305.25) | 215.00 (198.00–227.00) | < 0.001 |
| Hepatic steatosis (CAP ≥ 248) (n, %) | 49/148 (33.1%) | 48/48 (100%) | 1/100 (1%) | < 0.001 |
One subject has no available data.
Two subjects have no available data.
Data are displayed as the median (interquartile range) and number (%).
ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; CAP, controlled attenuation parameter; F, female; FG, fasting glucose; HbA1c, hemoglobin A1c; HBsAg, hepatitis B surface antigen; HDL, high‐density lipoprotein; M, male; MASLD, metabolic dysfunction‐associated steatotic liver disease; TG, triglyceride; WC, waist circumference.
The median age was similar between the MASLD and non‐MASLD groups (51.8 vs 49.9 years; P = 0.057). There was a preponderance of females in both groups (62.5% vs 70%; P = 0.362). Only one subject in the non‐MASLD group had hepatic steatosis. The median controlled attenuation parameter score of the MASLD group was significantly higher than that of the non‐MASLD group (276 vs 215 dB/m; P < 0.001).
No subject had a history of high‐risk drinking (defined as 14 units of alcohol per week, according to the National Health Service). The proportion of chronic hepatitis B infection was similar between MASLD and non‐MASLD groups (4.2% vs 7.0%; P = 0.719). MASLD subjects had significantly higher median alanine transaminase (25 vs 17 U/L; P ≤ 0.001) and aspartate aminotransferase levels (23 vs 22 U/L; P = 0.018) than the non‐MASLD group.
Subjects with MASLD had a significantly higher median body mass index than the non‐MASLD group (25.85 vs 22.30 kg/m2; P < 0.001). Of each MASLD criteria, the difference in proportion of patients between the MASLD and non‐MASLD groups was as follows: overweight or obese (89.6% vs 34%; P < 0.001), impaired glucose regulation (66.7% vs 25.0%; P < 0.001), high blood pressure (22.9% vs 4.0%; P < 0.001), high triglyceride (25% vs 7.0%; P = 0.002), and low high‐density lipoprotein (37.5% vs 4.0%; P < 0.001).
The median time from the first dose to the third dose of the vaccine was 8.5 months (IQR: 7.9–8.9). The median time from day 180 to the third dose was 2.6 months (IQR: 1.8–3.2), and from the third dose to day 360, it was 3.6 months (IQR: 3.1–4.4) (Fig. 1).
Comparison of vaccine immunogenicity between the metabolic dysfunction‐associated steatotic liver disease and non‐metabolic dysfunction‐associated steatotic liver disease groups among three‐dose BNT162b2 recipients
Table 2 shows the humoral immune response among 148 three‐dose BNT162b2 recipients. At day 180 prior to the third vaccine dose, only four (2.7%) subjects were seropositive. At day 360 after the third vaccine dose, 142 (95.9%) recipients were seropositive, with a lower seroconversion rate (43 [89.6%] vs 99 [99.0%]; P = 0.007) in the MASLD group than the non‐MASLD group (Table 2). The vMN GMT of the MASLD and non‐MASLD groups was 33.15 (IQR: 23.81–46.06) and 38.37 (IQR: 32.14–45.60), respectively (P = 0.696).
Table 2.
Seroconversion rate and vMN GMT between MASLD and non‐MASLD three‐dose BNT162b2 recipients for the SARS‐CoV‐2 omicron variant
| MASLD | Non‐MASLD | P‐value | |
|---|---|---|---|
| Half year after the 1st dose (n = 148) | n = 48 | n = 100 | |
| Seroconversion rate | 2/48 (4.17%) | 2/100 (98%) | 0.447 |
| vMN GMT | 5.00 (5.00–5.00) | 5.00 (5.00–5.00) | 0.826 |
| One year after the 1st dose (n = 148) | n = 48 | n = 100 | |
| Seroconversion rate | 43/48 (89.6%) | 99/100 (99%) | 0.007 |
| vMN GMT | 33.15 (23.81–46.06) | 38.37 (32.14–45.60) | 0.696 |
| Within 4 months from the 3rd dose (n = 96) | n = 35 | n = 61 | |
| Seroconversion rate | 33/35 (94.3%) | 61/61 (100%) | 0.131 |
| vMN GMT | 46.87 (33.12–66.02) | 41.86 (34.47–50.91) | 0.240 |
| After 4 months from the 3rd dose (n = 52) | n = 13 | n = 39 | |
| Seroconversion rate | 10/13 (76.9%) | 38/39 (97.4%) | 0.016 |
| vMN GMT | 13.06 (7.69–22.20) | 33.49 (24.05–46.53) | 0.004 |
GMT, geometric mean titer; MASLD, metabolic dysfunction‐associated steatotic liver disease; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; vMN, live virus microneutralization.
Metabolic dysfunction‐associated steatotic liver disease was a significant risk factor for lower odds of seroconversion to the SARS‐CoV‐2 omicron variant on both univariable (aOR: 0.087, 95% CI: 0.004–0.559; P = 0.028) and multivariable analyses (aOR: 0.051, 95% CI: 0.002–0.440; P = 0.022) (Table 3).
Table 3.
Factors associated with seroconversion to the SARS‐CoV‐2 omicron variant at 1 year among three‐dose BNT162b2 recipients
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Odds ratio | P‐value | Adjusted odds ratio | P‐value | |
| Sex | 0.464 (0.083–2.591) | 0.358 | 0.537 (0.090–3.201) | 0.476 |
| Age | 1.014 (0.940–1.087) | 0.699 | 1.067 (0.966–1.198) | 0.218 |
| MASLD | 0.087 (0.004–0.559) | 0.028 | 0.051 (0.002–0.440) | 0.022 |
| PPI use | 0.635 (0.094–12.569) | 0.687 | 0.906 (0.091–22.952) | 0.939 |
| Antibiotic use | 0.462 (0.067–9.221) | 0.496 | 0.425 (0.044–10.106) | 0.500 |
MASLD, metabolic dysfunction‐associated steatotic liver disease; PPI, proton pump inhibitor; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Table S1 shows the sensitivity analysis by adjusting for hepatic steatosis and obesity in addition to age, sex, PPI use, and antibiotic use. On univariable analysis, hepatic steatosis was associated with lower odds of seroconversion (OR: 0.090, 95% CI: 0.005–0.57; P = 0.030), while obesity was of borderline significance (OR: 0.183, 95% CI: 0.025–0.975; P = 0.055). On multivariable analysis, hepatic steatosis was of borderline significance (aOR: 0.078, 95% CI: 0.002–0.847; P = 0.071), while obesity was no longer a significant risk factor (P = 0.487).
Comparison of virus microneutralization geometric mean titer within and after 4 months from the third‐dose vaccine between metabolic dysfunction‐associated steatotic liver disease and non‐metabolic dysfunction‐associated steatotic liver disease BNT162b2 recipients
Within 4 months from the third dose, no difference was observed between the MASLD and non‐MASLD groups in vMN GMT (46.87 [IQR: 33.12–66.02] vs 41.86 [IQR: 34.47–50.91]; P = 0.240) or seroconversion rate (94.3% vs 100%; P = 0.131) (Table 2 and Fig. 2).
Figure 2.
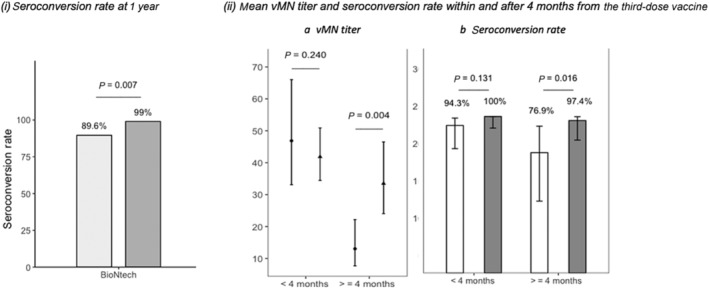
Seroconversion rate and virus microneutralization (vMN) geometric mean titer between metabolic dysfunction‐associated steatotic liver disease (MASLD) and non‐MASLD three‐dose BNT162b2 recipients for the severe acute respiratory syndrome coronavirus 2 omicron variant. (i) Comparison of vaccine immunogenicity between MASLD and non‐MASLD groups at 1 year after the first‐dose vaccine. (ii) Comparison of (a) vMN geometric mean titer and (b) seroconversion rate within and after 4 months from the third‐dose vaccine between MASLD and non‐MASLD groups. (a)  , MASLD;
, MASLD;  , non‐MASLD. (b)
, non‐MASLD. (b)  , MASLD;
, MASLD;  , non‐MASLD.
, non‐MASLD.
On the other hand, after 4 months from the third dose, MASLD subjects had lower vMN GMT than non‐MASLD subjects (13.06 [IQR: 7.69–22.20] vs 33.49 [IQR: 24.05–46.53]; P = 0.004) and seroconversion rate (76.9% vs 97.4%; P = 0.016) (Table 2 and Fig. 2).
Discussion
This prospective cohort study demonstrates that MASLD conferred a 20‐fold higher risk for seronegativity to the SARS‐CoV‐2 omicron variant after three doses of BNT162b2. There was a more pronounced waning effect of antibody level among MASLD subjects than non‐MASLD ones at 4 months after the third dose.
Patients with chronic liver disease display immune dysfunction, which predisposes them to infection, organ inflammatory damage, and a poor response to vaccination. 21 Recent studies have described the impact of SARS‐CoV‐2 on patients with chronic liver disease, including chronic viral hepatitis infection, NAFLD, and hepatocellular carcinoma. 25 , 26 There are only a few studies that have assessed the efficacy of the SARS‐CoV‐2 vaccine in patients with hepatic steatosis. Wang et al. reported that 95.5% of patients with hepatic steatosis had detectable neutralizing antibody levels after two doses of the inactivated vaccine. 27 Our previous study found that while there was no difference in the seroconversion rate after either the first or second dose between moderate‐to‐severe hepatic steatosis and control groups, a lower proportion of hepatic steatosis patients achieved the highest‐tier response. 7 Nonetheless, no studies are currently available evaluating the effect of three doses of COVID‐19 vaccination on patients with MASLD, a disease entity that has only recently been coined.
To our understanding, our study is the first to compare the longer‐term immunogenicity of booster dose BNT162b2 among patients with and without MASLD. We found that the seroconversion rate of BNT162b2 in the MASLD group was significantly lower than in the non‐MASLD group (89.6% vs 99.0%) 1 year after the first dose of the vaccine. Hepatic steatosis elevates cytokine levels, 28 and excessive hepatic and systemic baseline inflammation alters cytokine production and immune responses. 29 Various other mechanisms affecting immunogenicity have been proposed, including links through hyperglycemia, insulin resistance, 30 obesity, gut microbiota imbalance, 31 and alterations in innate immunity. 32 The CAVEAT study has observed that the immunity induced by COVID‐19 vaccines is weaker in diabetic patients with poor glycemic control compared to those with good glycemic control and normoglycemic individuals. 33 A meta‐analysis found that antibody titers generated after COVID‐19 vaccines were significantly reduced in individuals with obesity as compared to those with normal weight. 34 A recent prospective study further demonstrated an accelerated waning effect of COVID‐19 vaccine‐induced humoral immunity in individuals with severe obesity, where 55% of severe obese individuals had unquantifiable neutralizing antibody titers against SARS‐CoV‐2 compared to 12% of normal individuals at 6 months after the second vaccine dose. 35 The connection between reduced immunogenicity via modeling the effects of metabolic disease on COVID‐19 vaccine immunogenicity was established by a murine study, where chronic low‐grade inflammation associated with metabolic dysfunction (“metaflammation”) and the aging process (“inflammaging”) were proposed to be the key pathogenesis. 36 Our sensitivity analysis by adjusting for hepatic steatosis and obesity showed that hepatic steatosis was of borderline significance while obesity was not a significant risk factor for seroconversion. This observation could be explained by a relatively small sample size and the fact that vaccine immunogenicity was contributed by the interplay of hepatic steatosis and metabolic risk factors. These effects coincide with the pathophysiology of MASLD, which is part of the metabolic syndrome characterized by overweight or obesity and dysregulation of glucose, blood pressure, and lipids, which could explain the association between MASLD and lowered vaccine immunogenicity.
The waning effect of antibody levels is an important consideration when the timing and frequency of booster vaccines are being considered. It is well reported that vaccine effectiveness against infection progressively diminishes with time, 37 which was also observed in both the MASLD and non‐MASLD groups in our study. A cut‐off of 4 months was used to evaluate the waning effect, as a prior study showed that the vaccine immunogenicity started to wane by 4 months after the third dose, with vaccine effectiveness decreasing from 96% to 68% against hospital admission. 24 Subgroup analysis revealed that both the MASLD and non‐MASLD groups had comparable immunogenicity within 4 months after the third dose of vaccination (vMN GMT: 46.87 vs 41.86; P = 0.240), but the difference in immunogenicity started to merge 4 months after the third dose (vMN GMT: 13.06 vs 33.49; P = 0.004; seroconversion rate: 76.9% vs 97.4%; P = 0.016). This finding supports that MASLD contributes to a greater waning effect of the BNT162b2 booster dose. Given that a higher level of antibodies is associated with a higher level of protection against infection, 9 , 38 MASLD should be identified to identify individuals at risk of accelerated waning of BNT162b2 immunogenicity who may require a shortened interval of administration of booster doses to maintain an adequate immunogenicity profile.
Our study is unique in a few different ways. First, we assessed the vaccine immunogenicity after three doses of vaccination with a follow‐up period of 1 year that would allow evaluation of the longer‐term effect. Second, our study is the first to report the effect of MASLD on vaccine immunogenicity. Third, we used a live virus assay, which is the gold standard for analyzing vaccine humoral responses. 39 Fourth, we excluded patients with prior SARS‐CoV‐2 infection to ensure immunogenicity is not mounted by antibodies generated from natural infection. Fifth, we addressed the waning effect by analyzing the time interval between the third dose and the collection of the blood sample, which is of valuable importance in determining the optimal timing for subsequent vaccine doses.
Several limitations of our study should be noted. First, vaccine‐induced cellular immunity against SARS‐CoV‐2 was not investigated. It is proposed that vaccine‐induced T‐cell responses may protect against severe infection despite seronegativity 9 via suppressing viral replication and producing long‐term memory of the immune system. 40 Second, only mRNA (BNT162b2) but not an inactivated vaccine platform was evaluated. Third, data on chronic hepatitis C infection were unavailable. Fourth, the sample size was relatively small, which may have led to the non‐significant numerical difference in the vMN GMT between the MASLD and non‐MASLD groups. Future studies with a larger sample size and evaluation of other vaccine platforms, such as CoronaVac and mixed vaccines, will allow for better demonstration of the effect of MASLD on vaccine immunogenicity.
Conclusion
Subjects with MASLD vaccinated with three doses of BNT162b2 had a lower seroconversion rate of the SARS‐CoV‐2 omicron variant, with a more pronounced waning effect than non‐MASLD ones. MASLD subjects may need to have more frequent administrations of booster doses to maintain an adequate immunogenicity profile.
Supporting information
Figure S1. Patient selection flow diagram.
Table S1. Factors associated with seroconversion to SARS‐CoV‐2 omicron variant at one year among three‐dose BNT162b2 recipients.
Lam, L. K. , Tan, J. T. , Ooi, P. H. , Zhang, R. , Chan, K. H. , Mao, X. , Hung, I. F. N. , Seto, W. K. , Yuen, M. F. , and Cheung, K. S. (2024) Effect of metabolic dysfunction‐associated steatotic liver disease on BNT162b2 immunogenicity against the severe acute respiratory syndrome coronavirus 2 omicron variant. Journal of Gastroenterology and Hepatology, 39: 2386–2393. 10.1111/jgh.16716.
Declaration of conflict of interest: The authors declare no conflicts of interest.
Author contribution: Lok Ka Lam was involved with the study concept and design; the analysis and interpretation of the data; and the drafting of the manuscript. Jing Tong Tan, Xianhua Mao, Ruiqi Zhang, and Kwok Hung Chan were involved with the analysis and interpretation of the data and the critical revision of the manuscript for important intellectual content. Poh Hwa Ooi and Ivan F N Hung were involved with the acquisition of data. Ka Shing Cheung, Wai Kay Seto, and Man Fung Yuen were involved with the study concept and design; the critical revision of the manuscript for important intellectual content; and the study supervision. The corresponding authors had full access to all data and were fully responsible for the data integrity and statistical analysis. All authors revised the manuscript and approved the final version of this article.
Ethical approval: This study did not involve animal research. This is a retrospective cohort study and has been approved by the Institutional Review Board of the University of Hong Kong and the West Cluster of Hospital Authority, Hong Kong.
Informed consent: Participant identities were anonymized in this study.
Financial support: The research was funded by the Health and Medical Research Fund, Food and Health Bureau, The Government of HKSAR (COVID1903010, Project 16).
Guarantor of the article: Professor Ka Shing Cheung and Professor Man Fung Yuen are the guarantors of the article.
Clinical trials registration: This is not a clinical trial.
Contributor Information
Man Fung Yuen, Email: mfyuen@hkucc.hku.hk.
Ka Shing Cheung, Email: cks634@hku.hk.
Data availability statement
Data will not be shared due to confidentiality.
References
- 1. Haas EJ, Angulo FJ, McLaughlin JM et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397: 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chemaitelly H, Ayoub HH, Tang P et al. Long‐term COVID‐19 booster effectiveness by infection history and clinical vulnerability and immune imprinting: a retrospective population‐based cohort study. Lancet Infect. Dis. 2023; 23: 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shimabukuro TT. COVID‐19 mRNA Bivalent Booster Vaccine Safety. Presented at the Advisory Committee on Immunization Practices Meeting. Centers for Disease Control and Prevention (CDC) February 24, 2023. [Google Scholar]
- 4. Organization WH . Coronavirus Disease (COVID‐19). 2023. [Google Scholar]
- 5. Teng ML, Ng CH, Huang DQ et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023; 29: S32–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eskridge W, Cryer DR, Schattenberg JM et al. Metabolic dysfunction‐associated steatotic liver disease and metabolic dysfunction‐associated steatohepatitis: the patient and physician perspective. J. Clin. Med. 2023: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung KS, Lam LK, Hui RWH et al. Effect of moderate‐to‐severe hepatic steatosis on neutralising antibody response among BNT162b2 and CoronaVac recipients. Clin. Mol. Hepatol. 2022; 28: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meschi S, Matusali G, Colavita F et al. Predicting the protective humoral response to a SARS‐CoV‐2 mRNA vaccine. Clin. Chem. Lab. Med. 2021; 59: 2010–2018. [DOI] [PubMed] [Google Scholar]
- 9. Khoury DS, Cromer D, Reynaldi A et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat. Med. 2021; 27: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 10. Bergwerk M, Gonen T, Lustig Y et al. Covid‐19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021; 385: 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheung KS, Lam LK, Mao X et al. Effect of moderate to severe hepatic steatosis on vaccine immunogenicity against wild‐type and mutant virus and COVID‐19 infection among BNT162b2 recipients. Vaccines (Basel) 2023; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Z, Zhang Y, Song R et al. Waning humoral immune responses to inactivated SARS‐CoV‐2 vaccines in patients with severe liver disease. Signal Transduct. Target. Ther. 2022; 7: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lau JJ, Cheng SMS, Leung K et al. Real‐world COVID‐19 vaccine effectiveness against the Omicron BA.2 variant in a SARS‐CoV‐2 infection‐naive population. Nat. Med. 2023; 29: 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burki T. Hong Kong's fifth COVID‐19 wave—the worst yet. Lancet Infect. Dis. 2022; 22: 455–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan KH, Leung KY, Zhang RR et al. Performance of a surrogate SARS‐CoV‐2‐neutralizing antibody assay in natural infection and vaccination samples. Diagnostics (Basel) 2021; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karlas T, Petroff D, Sasso M et al. Individual patient data meta‐analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017; 66: 1022–1030. [DOI] [PubMed] [Google Scholar]
- 17. New MASLD Nonmenclature. American Association for the Study of Liver Diseases, 2023. [Google Scholar]
- 18. Shaikh A. Steatotic—What? Changes in Fatty Liver Nomenclature. American Association for the Study of Liver Diseases, 2023. [Google Scholar]
- 19. Ng HY, Leung WK, Cheung KS. Association between gut microbiota and SARS‐CoV‐2 infection and vaccine immunogenicity. Microorganisms 2023; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lynn DJ, Benson SC, Lynn MA, Pulendran B. Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nat. Rev. Immunol. 2022; 22: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zimmermann P, Curtis N. The influence of the intestinal microbiome on vaccine responses. Vaccine 2018; 36: 4433–4439. [DOI] [PubMed] [Google Scholar]
- 22. Cheung KS, Lam LK, Zhang R et al. Association between recent usage of antibiotics and immunogenicity within six months after COVID‐19 vaccination. Vaccines (Basel) 2022; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheung KS, Yan VKC, Lam LK, Ye X, Hung IFN, Chan EW, Leung WK. Antibiotic use prior to COVID‐19 vaccine is associated with higher risk of COVID‐19 and adverse outcomes: a propensity‐scored matched territory‐wide cohort. Vaccines (Basel) 2023; 11: 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferdinands JM, Rao S, Dixon BE et al. Waning of vaccine effectiveness against moderate and severe covid‐19 among adults in the US from the VISION network: test negative, case‐control study. BMJ 2022; 379: e072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheung KS, Mok CH, Mao X, Zhang R, Hung IFN, Seto WK, Yuen MF. COVID‐19 vaccine immunogenicity among chronic liver disease patients and liver transplant recipients: a meta‐analysis. Clin. Mol. Hepatol. 2022; 28: 890–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ali FEM, Mohammedsaleh ZM, Ali MM, Ghogar OM. Impact of cytokine storm and systemic inflammation on liver impairment patients infected by SARS‐CoV‐2: prospective therapeutic challenges. World J. Gastroenterol. 2021; 27: 1531–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang J, Hou Z, Liu J et al. Safety and immunogenicity of COVID‐19 vaccination in patients with non‐alcoholic fatty liver disease (CHESS2101): a multicenter study. J. Hepatol. 2021; 75: 439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prins GH, Olinga P. Potential implications of COVID‐19 in non‐alcoholic fatty liver disease. Liver Int. 2020; 40: 2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alter G, Sekaly RP. Beyond adjuvants: antagonizing inflammation to enhance vaccine immunity. Vaccine 2015; 33: B55–B59. [DOI] [PubMed] [Google Scholar]
- 30. Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014; 510: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu H, Lin A, Kong M et al. Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J. Gastroenterol. 2020; 55: 142–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Albillos A, Lario M, Álvarez‐Mon M. Cirrhosis‐associated immune dysfunction: distinctive features and clinical relevance. J. Hepatol. 2014; 61: 1385–1396. [DOI] [PubMed] [Google Scholar]
- 33. Marfella R, D'Onofrio N, Sardu C et al. Does poor glycaemic control affect the immunogenicity of the COVID‐19 vaccination in patients with type 2 diabetes: the CAVEAT study. Diabetes Obes. Metab. 2022; 24: 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ou X, Jiang J, Lin B, Liu Q, Lin W, Chen G, Wen J. Antibody responses to COVID‐19 vaccination in people with obesity: a systematic review and meta‐analysis. Influenza Other Respi. Viruses 2023; 17: e13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Klaauw AA, Horner EC, Pereyra‐Gerber P et al. Accelerated waning of the humoral response to COVID‐19 vaccines in obesity. Nat. Med. 2023; 29: 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Meara TR, Nanishi E, McGrath ME et al. Reduced SARS‐CoV‐2 mRNA vaccine immunogenicity and protection in mice with diet‐induced obesity and insulin resistance. J. Allergy Clin. Immunol. 2023; 152: 1107–1120.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nordström P, Ballin M, Nordström A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID‐19 vaccine: a retrospective, total population cohort study in Sweden. Lancet 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feng S, Phillips DJ, White T et al. Correlates of protection against symptomatic and asymptomatic SARS‐CoV‐2 infection. Nat. Med. 2021; 27: 2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matusali G, Colavita F, Lapa D et al. SARS‐CoV‐2 serum neutralization assay: a traditional tool for a brand‐new virus. Viruses 2021; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Le Bert N, Tan AT, Kunasegaran K et al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature 2020; 584: 457–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient selection flow diagram.
Table S1. Factors associated with seroconversion to SARS‐CoV‐2 omicron variant at one year among three‐dose BNT162b2 recipients.
Data Availability Statement
Data will not be shared due to confidentiality.


