Abstract
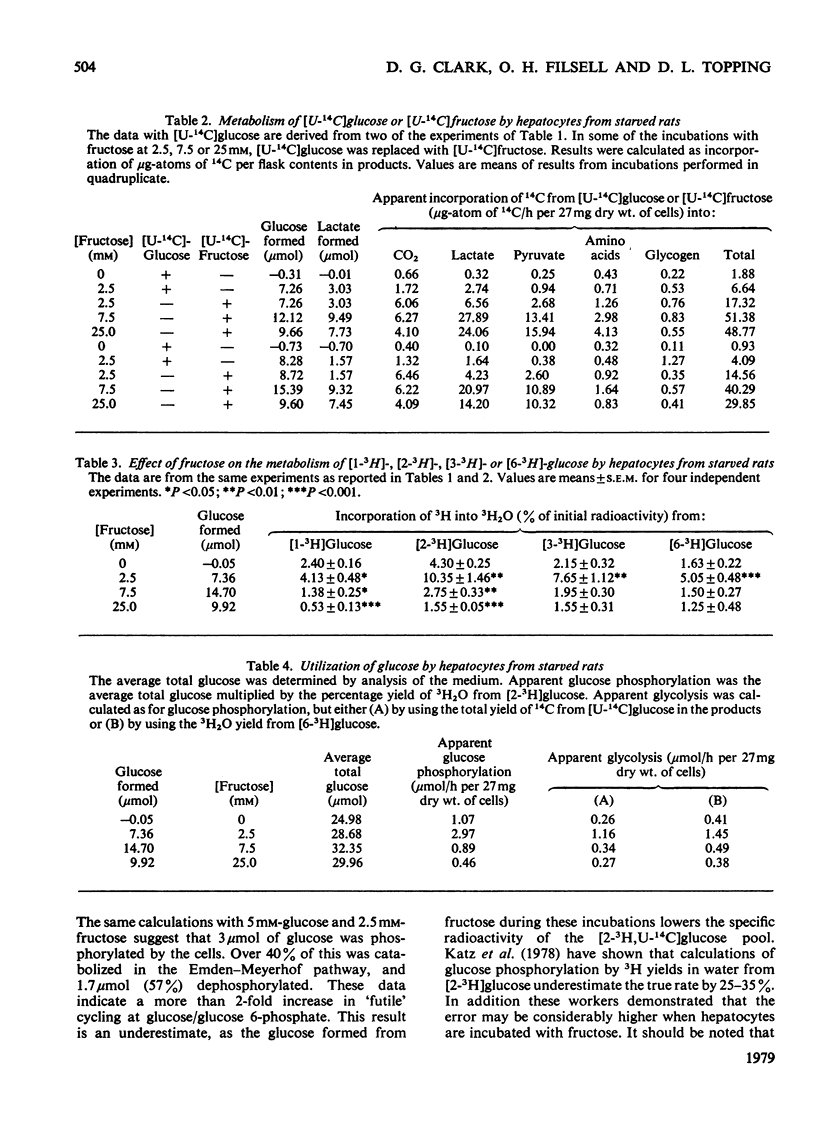
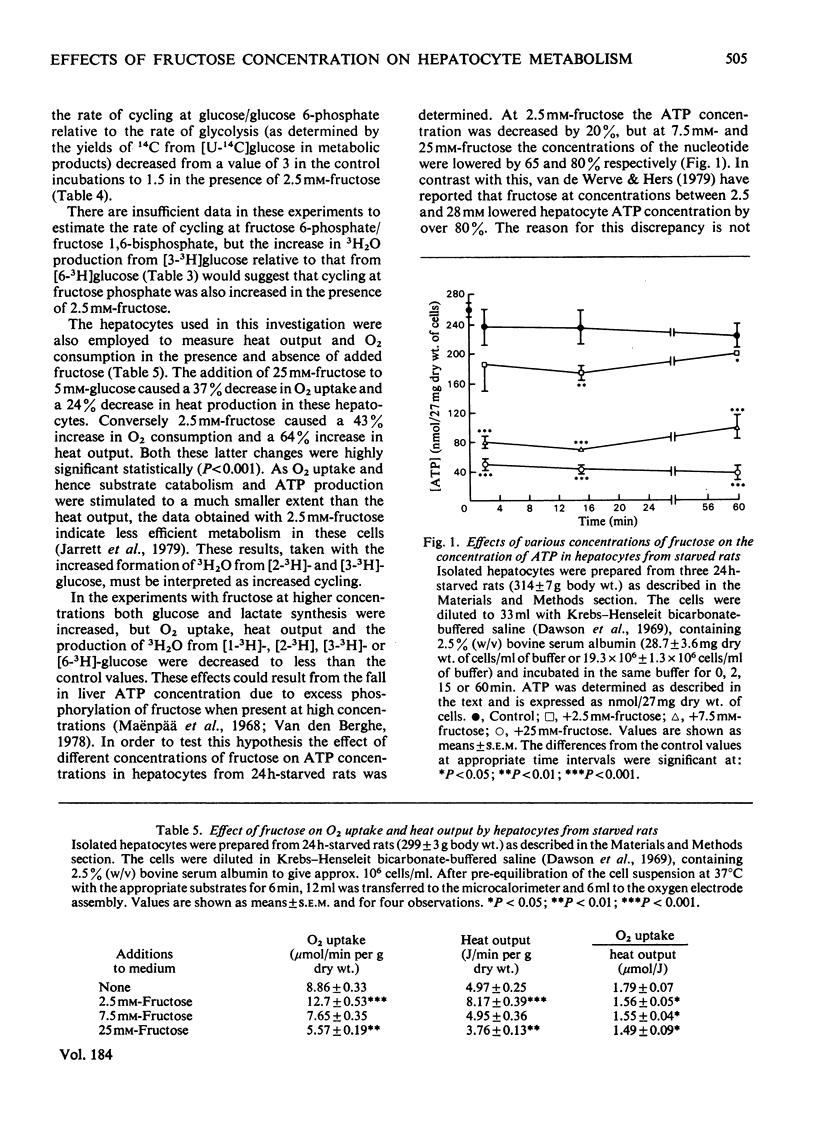
1. Hepatocytes from starved rats were incubated with 5mm-glucose, labelled uniformly with 14C and specifically with 3H at positions 1, 2, 3 or 6, and with fructose at concentrations of 2.5, 7.5 or 25mm. 2. In the absence of other substrates only 1% of the radioactivity initially present in [U-14C]glucose appeared in the metabolic products, CO2, lactate, pyruvate, amino acids and glycogen. 3. Fructose at 2.5mm caused a 30% increase in the glucose concentration and a 4-fold increase in the apparent oxidation of [U-14C]-glucose. 4. The formation of 3H2O from [1-3H]-, [2-3H]-, [3-3H]- or [6-3H]-glucose was 2.4, 4.3, 2.15 or 1.6% respectively in the control incubations and 4.1, 10.4, 7.7 or 5.1% with 2.5mm-fructose. 5. Fructose at 7.5 and 25mm decreased the 3H2O yields to less than the control values, but had no apparent effect on the amount of [U-14C]glucose metabolized. 6. In the incubations with 5mm-glucose and 25mm-fructose there were significant decreases in heat production, O2 consumption and in the ratio of O2 uptake to heat output. 7. Fructose at 2.5mm caused a 64% increase in heat output, but only a 43% increase in O2 uptake. 8. The radioisotopic and calorimetric data demonstrate that physiological concentrations of fructose greatly increase metabolism in hepatocytes from starved rats. These data also indicate increased cycling at glucose/glucose 6-phosphate and at fructose 6-phosphate/fructose 1,6-bisphosphate in the presence of 2.5mm-fructose, although the rates of cycling were actually decreased relative to the amount of glucose catabolized. 9. At concentrations of 2.5, 7.5 and 25mm, fructose depressed hepatocyte ATP concentrations by 20, 65 and 80% respectively. Although fructose at 7.5 and 25mm increased glucose and lactate release, O2 consumption, production of heat and formation of3H2O from [1-3H]-, [2-3H]-, [3-3H]- or [6-3H]-glucose were lowered to values equal to, or less than, controls. These effects probably reflect a severe derangement of hepatic metabolism due to excess phosphorylation of fructose when present at high concentrations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Isotopic evidence for futile cycles in liver cells. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1141–1148. doi: 10.1016/0006-291x(73)90811-5. [DOI] [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Lipogenesis in rat hepatocytes. J Biol Chem. 1974 Apr 10;249(7):2028–2036. [PubMed] [Google Scholar]
- Clark D., Lee D., Rognstad R., Katz J. Futile cycles in isolated perfused rat liver and in isolated rat liver parenchymal cells. Biochem Biophys Res Commun. 1975 Nov 3;67(1):212–219. doi: 10.1016/0006-291x(75)90304-6. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Bloxham D. P., Holland P. C., Lardy H. A. Estimation of the fructose 1,6-diphosphatase-phosphofructokinase substrate cycle and its relationship to gluconeogenesis in rat liver in vivo. J Biol Chem. 1974 Jan 10;249(1):279–290. [PubMed] [Google Scholar]
- Clark M. G., Filsell O. H., Jarrett I. G. An effect of extracellular redox state on the glucagon-stimulated glucose release by rat hepatocytes and perfused liver. Horm Metab Res. 1977 May;9(3):213–217. doi: 10.1055/s-0028-1093539. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Kneer N. M., Bosch A. L., Lardy H. A. The fructose 1,6-diphosphatase-phosphofructokinase substrate cycle. A site of regulation of hepatic gluconeogenesis by glucagon. J Biol Chem. 1974 Sep 25;249(18):5695–5703. [PubMed] [Google Scholar]
- Jarrett I. G., Clark D. G., Filsell O. H., Harvey J. W., Clark M. G. The application of microcalorimetry to the assessment of metabolic efficiency in isolated rat hepatocytes. Biochem J. 1979 Jun 15;180(3):631–638. doi: 10.1042/bj1800631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Golden S., Rognstad R. Recycling of glucose by rat hepatocytes. Eur J Biochem. 1975 Dec 1;60(1):91–101. doi: 10.1111/j.1432-1033.1975.tb20979.x. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A. Pentose cycle and reducing equivalents in rat mammary-gland slices. Biochem J. 1972 Jul;128(4):879–899. doi: 10.1042/bj1280879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Rognstad R. Glucose phosphorylation, glucose-6-phosphatase, and recycling in rat hepatocytes. J Biol Chem. 1978 Jul 10;253(13):4530–4536. [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Wals P. The metabolism of l-[3-3h]lactate by isolated hamster liver cells. Biochim Biophys Acta. 1976 Jun 23;437(1):16–21. doi: 10.1016/0304-4165(76)90343-3. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. Effects of fructose concentration on adenine nucleotide concentrations and pyruvate dehydrogenase activity of perfused rat liver [proceedings]. Biochem Soc Trans. 1977;5(4):1001–1002. doi: 10.1042/bst0051001. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. The concentration of fructose, glucose and lactate in the splanchnic blood vessels of rats absorbing fructose. Nutr Metab. 1971;13(6):331–338. doi: 10.1159/000175352. [DOI] [PubMed] [Google Scholar]
- Van de Werve G., Hers H. G. Mechanism of activation of glycogen phosphorylase by fructose in the liver. Stimulation of phosphorylase kinase related to the consumption of adenosine triphosphate. Biochem J. 1979 Jan 15;178(1):119–126. doi: 10.1042/bj1780119. [DOI] [PMC free article] [PubMed] [Google Scholar]


