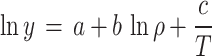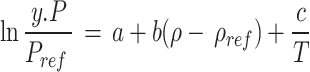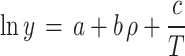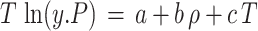Abstract
In the present study, the solubility of sulfasalazine in carbon dioxide was investigated at temperatures ranging from 313 K to 343 K and pressures ranging from 12 to 30 MPa. The experimentally determined molar solubilities of sulfasalazine in ScCO2 were found to be in the range of 4.08 × 10− 5 to 8.61 × 10− 5 at 313 K, 3.54 × 10− 5 to 11.41 × 10− 5 at 323 K, 3.04 × 10− 5 to 13.64 × 10− 5 at 333 K, and 2.66 × 10− 5 to 16.35 × 10− 5 at 343 K. The solubility values were correlated via a number of different types of equations, such as semi-empirical correlations, the Peng-Robinson, the PC-SAFT equation, and the regular solution. Furthermore, the findings demonstrate that semi-empirical, equation of state models, and the regular solution model possess the capability of precisely determining the solubility. Moreover, the solubility magnitude suggests that the gas anti-solvent method may be a viable approach for nanoparticle production.
Keywords: Sulfasalazine, Solubility, Modeling, Supercritical fluid, PC-SAFT
Subject terms: Chemical engineering, Chemistry
Introduction
Sulfasalazine, an anti-inflammatory drug utilized to treat rheumatoid arthritis and ulcerative colitis, was initially identified in the 1930s. The drug under consideration is classified as a class 4 compound in accordance with the Biopharmaceutical Classification System, primarily due to its restricted solubility (0.6 µg/ml) and permeation properties. Consequently, a recommended daily oral dose of 1–3 g/day has been established for patients with arthritis to compensate for the low level of exposure. Moreover, the low permeability and solubility of sulfasalazine would present considerable obstacles to achieving a considerable level of exposure following intravenous administration. This is due to the limited volume of doses that can be administered. It is therefore of great importance to enhance the APIs through the creation of diverse drug formulations. This objective can be met by undertaking a detailed examination of the physicochemical characteristics of sulfasalazine, with a particular focus on its solubility and distribution within the selected solvents1.
Solubility represents a pivotal parameter that significantly impacts the absorption of oral drugs from the stomach and intestines. During the initial stages of drug discovery, solubility is a valuable parameter when considered alongside other properties, including ionization, lipophilicity, and permeability. In the later stages of drug development, it is also essential in classifying biopharmaceuticals, optimizing formulations, and designing a novel drug formulation2.
Reducing the particle size of APIs is an effective method for improving solubility in poorly soluble APIs. Conventional techniques have limitations, including difficulty controlling particle size, the presence of solvent in the finished API, and potential degradation due to heating. Supercritical fluid technology is a promising approach for developing particle reduction technology for pharmaceuticals3–7. A number of effective supercritical fluid techniques have been established for reducing particle size in drugs. The key factor in choosing the right supercritical fluid technique is understanding how the drug dissolves in supercritical carbon dioxide (ScCO2). To industrialize and commercialize products derived from supercritical fluid (SCF) processes, it is essential to gain comprehensive knowledge of the quality, purity, extractability, and solubility of these materials.
Mathematical and experimental models have played a pivotal role in reducing the cost of solubility tests. By modeling solubility, we can accurately determine experimental measurements and investigate various aspects of thermodynamics and material properties. In the present era, we have advanced experimental models, equations of state, and intelligent models to achieve this objective. Accordingly, the solubility of sulfasalazine in ScCO2 was determined under a range of temperature and pressure condition. The data were then subjected to modeling using a variety of techniques, including (1) density-based models, (2) PR with vdW2, (3) PC-SAFT, and (4) the regular solution model. Subsequently, the potential of semi-empirical models for the analysis of the data was explored. Furthermore, an assessment was conducted to evaluate the capacity of the models to be correlated. This involved a comparison between the calculated data and the experimental data, with the aim of identifying any potential discrepancies. Two statistical criteria were employed to assess the models’ capabilities: the average absolute relative deviation (AARD) and the adjusted correlation coefficient (Radj). Following this analysis, the enthalpies of vaporization (ΔHvap), solvation (ΔHsol), and total enthalpy (ΔHtotal) were determined.
Materials and methods
Materials
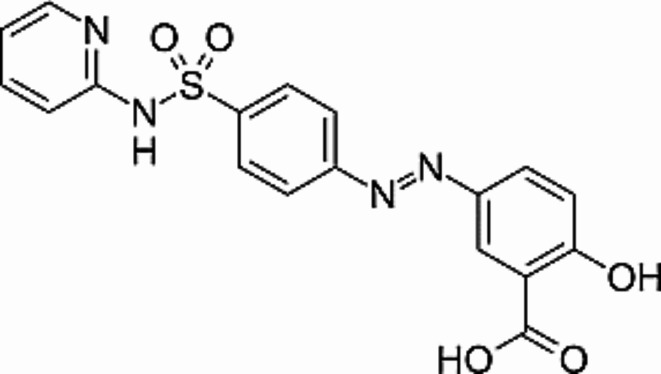
Sulfasalazine with below structure (Fig. 1) (CAS number 599-79-1) was procured from Sigma-Aldrich, while CO2 of greater than 99.5% purity was obtained from a local supplier.
Fig. 1.
Structure of sulfasalazine.
Methods
Experimental procedure
The solubility of sulfasalazine in supercritical carbon dioxide was evaluated according to the procedure reported in previous articles8,9. The initial stage of the experiment involved directing a CO2 gas with a pressure of 5.5 MPa and a temperature of 298 K to a refrigerator with a temperature of 268 K, with the objective of observing the phase transition from gas to liquid. Subsequently, the liquid is subjected to pressure through a specialized pump in order to attain the targeted pressure range (12 to 30 MPa). A high-pressure stainless steel cell with a volume of 200 ml has been constructed for the purpose of conducting solubility experiments. The fixed value of API is recorded on the sheet and positioned at the base of the cell. Subsequently, the liquid is introduced into the cell, where the drug is mixed and dissolved in the carbon dioxide using a high-speed stirrer. The temperature of the process is set as the second impact parameter on solubility using an accurate temperature-control device with a precision of ± 1 K. Based on preliminary experiments; a static time of 4 h with a stirring rate of 200 rpm was identified as sufficient to achieve equilibrium. Finally, the drug mole fraction was determined by applying the following equation using the initial and final drug masses as variables:
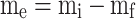 |
1 |
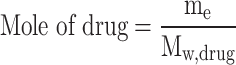 |
2 |
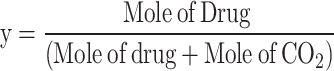 |
3 |
Semi-empirical models
A plethora of semi-empirical models have been put forth with the aim of establishing a correlation between the solubility of a solute in scCO2. The absence of a requirement for solute properties (in contrast to the EoSs), the ease of application, and the acceptable accuracy are the principal advantages of these models. The sole disadvantage of these models is the necessity for experimental solubility data. A number of equation have been formulated with varying adjustable parameters (ranging from three to eight) with the objective of enhancing the correlation with experimental data. As example, Bartle et al.10, MST11,12, Chrastil13, Kumar and Johnston14, Garlapati et al.15,16, Alwi and Garlapati17, del Valle and Aguilera18, Sung and Shim19, Adachi and Lu20, Bian et al.21,22, Sparks et al.23, Si-Moussa et al.24, Belghait et al.25 and Amooey26, Haghbakhsh et al.27, Mitra and Wilson28, Reddy et al.29,30, Gordillo et al.31, sodeifian et al.32–34 have been put forth as a means of correlating the solubility of solutes in supercritical carbon dioxide. In contrast to the EoSs, there is no necessity to consider the properties of the solute, the method of application is uncomplicated, and the accuracy is satisfactory. The only disadvantage associated with these models is that they necessitate the availability of experimental solubility data35–38. The density-based models are founded upon the tenet of straightforward error minimization. The adjustable parameters of these models can be optimized by an algorithm, such as a genetic algorithm or simulated annealing, within the MATLAB software. As shown in Table 1, five of the most popular theory-based models were used in this work to estimate data consistency and values of solvation, evaporation, and total enthalpies.
Table 1.
Semi-empirical models.
Equation of state-based models
Although the use of thermodynamic models requires a lot of information about the solvent, many authors used these types of models40–47. The solubility in scCO2 can be modeled using a variety of techniques, including semi-empirical equations, statistical models, and theoretical frameworks. Nevertheless, the conventional methodology based on the equation of state, exemplified by the Peng-Robinson (PR) and Soave-Redlich-Kwong (SRK) models, frequently yields considerable discrepancies48–50. In such cases, it is necessary to utilize the solid properties, which in some instances, particularly for complex solids, may not be readily accessible in a database. Mixing rules (e.g., van der Waals mixing rules) must be employed to ascertain the appropriate parameters by adjusting experimental solubility data in order to minimize this discrepancy. The precision of the EoS is contingent upon the specific mixing rule utilized and the number of parameters.
The use of these types of models necessitates the input of molar volume, acentric factor, melting temperature of solute, solvent vapor pressure, critical properties of the solvent, and thermodynamic properties of carbon dioxide. A plethora of experimental methodologies have been devised to identify these substances, each of which is susceptible to error. Among the most significant methods are the Joback et al.51, Marrero and Gani52, Lee Kessler53, Fedors54, and Stein et al.55 methods. In recent years, models such as the Peng Robinson, and SRK models have been employed for drugs such as lsomeprazole56, loratadine57, sunitinib malate58, and pregabalin8. The results and output of the articles indicate that, despite the numerous difficulties encountered in their use, these methods have yielded remarkable results on a consistent basis.
Moreover, the SAFT equations represent an additional class of models that have been utilized to establish correlations between the solubility of substances in supercritical carbon dioxide. These models encompass a range of variants, including SAFT, PC-SAFT, SAFT-VR, qCPA, and PCP-SAFT. In comparison to other equations of state, they are more complex in their structure. Consequently, the general or partial forms of these models have been employed in numerous published works59–66. The most important point in using these types of models is the molecular relationship between carbon dioxide and the solutes, which many works consider these parameters as adjustable parameters.
To ascertain the solubility of a particular compound in a supercritical state, it is essential to establish a solid-CO2 equilibrium. This procedure can be represented by means of the fundamental equilibrium relationship between a solid and ScCO2 in a system.
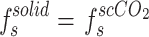 |
4 |
On the basis of certain simplifying assumptions, the mole fraction is calculated using the following Eqs67,68:
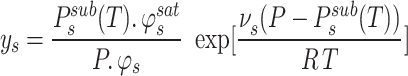 |
5 |
In this context, the solute fugacity coefficient at 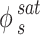 and
and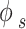 represents the sublimation pressure of the APIs, which is generally low. Therefore, it can be assumed that
represents the sublimation pressure of the APIs, which is generally low. Therefore, it can be assumed that 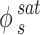 is equal to one. In order to model an equilibrium process, the value of
is equal to one. In order to model an equilibrium process, the value of 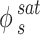 is determined using an appropriate model. To assess the EoSs, the PR and PC-SAFT models were considered.
is determined using an appropriate model. To assess the EoSs, the PR and PC-SAFT models were considered.
PC-SAFT model
The PC-SAFT model is formulated based on the residual molar Helmholtz energy (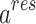 ) including the, which is the main basis of this model in the present work69:
) including the, which is the main basis of this model in the present work69:
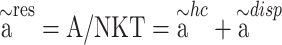 |
6 |
In accordance with the tenets of first-order thermodynamic perturbation theory, the term “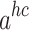 ” is defined as follows:62:
” is defined as follows:62:
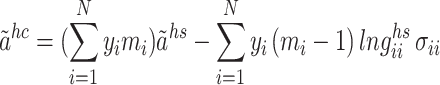 |
7 |
In addition, the term 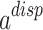 is calculated by the below relationship62:
is calculated by the below relationship62:
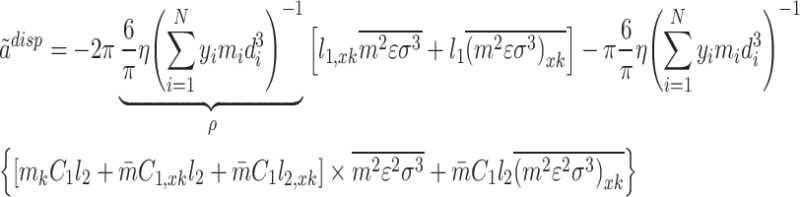 |
8 |
In order to ascertain the two cross parameters (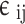 and
and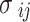 ) the conventional combination rules are employed62:
) the conventional combination rules are employed62:
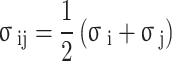 |
9 |
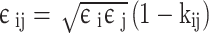 |
10 |
In the aforementioned relationship, 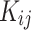 represents an adjustable parameter that is dependent on temperature. The incorporation of this parameter into the relationship is intended to account for the interactions between the two disparate chains. The correlation between the compressibility factor (Z) and
represents an adjustable parameter that is dependent on temperature. The incorporation of this parameter into the relationship is intended to account for the interactions between the two disparate chains. The correlation between the compressibility factor (Z) and 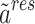 can be defined as follows62:
can be defined as follows62:
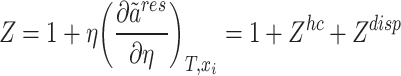 |
11 |
In this context, the dimensionless volume parameter, denoted by the symbol ɳ, is defined as follows:
The fugacity coefficient of component k (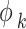 ) is ultimately established through the following methodology62:
) is ultimately established through the following methodology62:
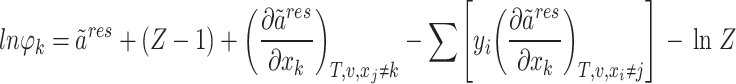 |
12 |
Regular solution models
In recent years, other models have been employed to model solubility in supercritical carbon dioxide, including those related to regular solutions. Unlike equations of state, these models do not require much information about the solvent, but some thermodynamic properties such as the melting point and enthalpy of the APIs are still required. These types of models have been used in many articles47,70–72. In the event that supercritical carbon dioxide is regarded as a solvent and the solubility is markedly low, the solubility in scCO2 is derived from the solubility coefficient in the infinitesimal dilute state (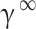 ), on the assumption that the fugacity of the API is identical in the two phases of solid and solvent47,70:
), on the assumption that the fugacity of the API is identical in the two phases of solid and solvent47,70:
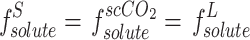 |
13 |
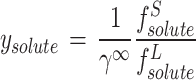 |
14 |
where,
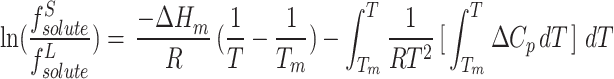 |
15 |
With Tm being melting point and ΔHm being enthalpy of fusion. If we disregard the discrepancy between the heat capacity ΔHm of the solute in the solid and supercritical phases (which is nearly constant), then Eq. 15 can be rewritten as follows:73:
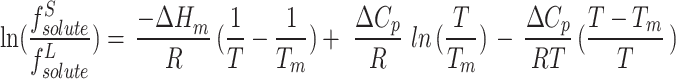 |
16 |
Therefore,
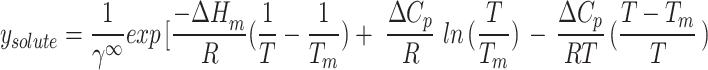 |
17 |
In this study, the regular solution is employed in conjunction with the Flory-Huggins theory74 for the purpose of determining 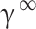 :
:
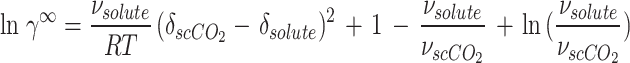 |
18 |
In the absence of consideration of the parameter of ΔCp in Eq. 17, the mole fraction in scCO2 can be determined through the following equation:
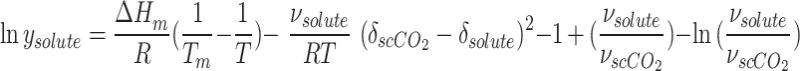 |
19 |
Where:
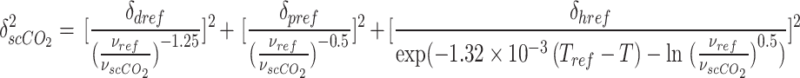 |
20 |
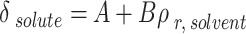 |
21 |
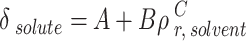 |
22 |
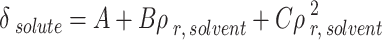 |
23 |
The A, B, and C were obtained by helping the genetic algorithm. Also, the melting point and enthalpy of fusion were 513.1 K and 29.13 J.g-1.
Results and discussions
Table 2 shows the experimental pints for the solubility of sulfasalazine in scCO2 at different pressures (12 to 30 MPa) and temperatures (313 K to 343 K). As previously stated, each data point was replicated three times to ensure the accuracy and precision of the measurements. Additionally, the standard deviation of mole fractions is presented in Table 2. The density of the CO2 was determined in accordance with the Span-Wagner equation of state. The range of the mole fraction and the solubility of the drug were 2.660 × 10− 5 to 1.635 × 10− 4 and 0.083 to 1.168 kg/m³, respectively. The lowest and highest solubilities of sulfasalazine were observed under the highest temperature (343 K) and at the lowest pressure (12 MPa) and the highest pressure (30 MPa), respectively. As illustrated in Fig. 2, the solubility for each of the isotherms was found to increase with rising pressure, and the increase was more pronounced at higher temperatures. This effect is attributed to the increase in spacing of the CO2 molecules at higher pressures due to the increase in density and performance of carbon dioxide.
Table 2.
Solubility values of sulfasalazine.
| T (K)a | P (MPa) | ρ (kg.m− 3) | y × 104 | SD (ȳ) × 104 | S (kg.m− 3) | Expanded Uncertainty of mole fraction (U×105) |
|---|---|---|---|---|---|---|
| 313 | 12 | 719.2 | 0.408 | 0.016 | 0.266 | 0.041 |
| 15 | 781.2 | 0.44 | 0.020 | 0.311 | 0.042 | |
| 18 | 820.7 | 0.499 | 0.021 | 0.371 | 0.051 | |
| 21 | 850.4 | 0.579 | 0.024 | 0.446 | 0.051 | |
| 24 | 873.9 | 0.712 | 0.031 | 0.563 | 0.074 | |
| 27 | 893.5 | 0.811 | 0.036 | 0.656 | 0.081 | |
| 30 | 910.3 | 0.861 | 0.039 | 0.710 | 0.086 | |
| 318 | 12 | 587.2 | 0.354 | 0.012 | 0.188 | 0.035 |
| 15 | 701.1 | 0.464 | 0.021 | 0.295 | 0.046 | |
| 18 | 758.8 | 0.642 | 0.029 | 0.441 | 0.064 | |
| 21 | 797.4 | 0.810 | 0.033 | 0.585 | 0.083 | |
| 24 | 826.1 | 0.974 | 0.044 | 0.729 | 0.097 | |
| 27 | 851.7 | 1.04 | 0.047 | 0.801 | 0.104 | |
| 30 | 871.5 | 1.141 | 0.050 | 0.900 | 0.114 | |
| 328 | 12 | 435.3 | 0.304 | 0.014 | 0.120 | 0.031 |
| 15 | 606.8 | 0.499 | 0.021 | 0.274 | 0.050 | |
| 18 | 688.4 | 0.739 | 0.031 | 0.460 | 0.072 | |
| 21 | 740.3 | 0.98 | 0.044 | 0.657 | 0.098 | |
| 24 | 777.5 | 1.181 | 0.053 | 0.831 | 0.119 | |
| 27 | 806.7 | 1.296 | 0.054 | 0.946 | 0.130 | |
| 30 | 830.5 | 1.364 | 0.060 | 1.025 | 0.139 | |
| 338 | 12 | 346.1 | 0.266 | 0.012 | 0.083 | 0.027 |
| 15 | 507.5 | 0.499 | 0.021 | 0.229 | 0.050 | |
| 18 | 613.5 | 0.805 | 0.033 | 0.447 | 0.081 | |
| 21 | 678.9 | 1.161 | 0.050 | 0.714 | 0.115 | |
| 24 | 724.9 | 1.319 | 0.053 | 0.866 | 0.134 | |
| 27 | 760.3 | 1.515 | 0.064 | 1.043 | 0.150 | |
| 30 | 788.9 | 1.635 | 0.070 | 1.168 | 0.164 |
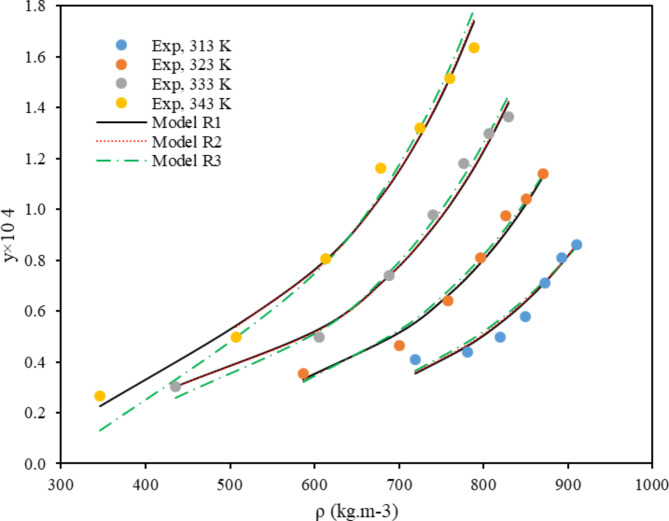
Fig. 2.
Solubility data based on pressure (a) and density (b) at different temperatures.
Figure 2 illustrates the experimental solubility profile for sulfasalazine at varying temperatures as a function of ScCO2 density and pressure. At a fixed pressure, it becomes evident that two distinct opposing effects of temperature on solubility can be observed: firstly, the CO2 density, and secondly, the sublimation pressure of the API. The most effective method for elucidating and contrasting these two parameters with regard to solubility is to introduce a parameter designated as the “crossover.” This point or range can serve as a valuable reference for comprehending the influence of these two parameters on solubility. A crossover point was identified at 15 MPa, which marked a notable shift in the solubility of the compound under investigation. Moreover, multiple researchers have identified consistent values for the SC-CO2 crossover region across a range of compounds. These include decitabine (C8H12N4O4)75, aripiprazole (C23H27Cl2N3O2)76, and oxzepam (C23H23NO5S)77. Additionally, the same findings were observed for other compounds including hydroxybenzaldehyde (C7H6O2)78, probenecid (C13H19NO4S)79, gemifloxacin (C18H20FN5O4)80 and anthraquinone (C14H8O2)81. At pressures below the crossover point, the value of the solubility exhibited a decline with increasing temperature. Conversely, at pressures above this point, the solubility of the drug demonstrated an increase with rising temperature. A comparable trend was observed for the solubility of other compounds in ScCO217,38,59,82,83. As the temperature increases, the sublimation vapor pressure increases, leading to an increase in solubility. However, it is observed that below the crossover point, as the temperature increases, the density also decreases, resulting in a decrease in solubility. In the other words, at lower pressures, the effect of density becomes more pronounced than that of sublimation pressure, resulting in a decrease in solubility with increasing temperature. At higher pressures (above cross over point), the solubility increases with temperature, indicating that the sublimation pressure effect is the dominant factor. This is to be expected, given that the sublimation pressure increases exponentially with temperature.
Comparison of the correlation results
In this scientific work, an investigation was conducted into a number of different types of models. These models of solubility were created using a variety of approaches, such as, EoS, semi-empirical, and regular solution. As previously stated, each of these models requires a number of input parameters, which were discussed in detail in the preceding sections. In order to facilitate comparison between the models, two criteria were identified and considered: criteria AARD, Radj8,9.
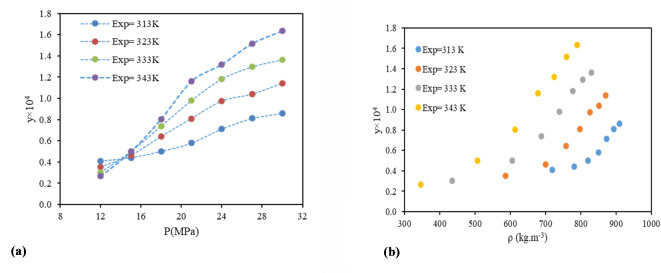
This work considers a number of density based models, namely Chrastil, Bartle et al., MST, and KJ. Each of the models is underpinned by a comprehensive theoretical framework. The findings from modelling these models are presented in Table 3, and it is evident from this that all of the models demonstrate satisfactory performance. The most precise results were produced by the KJ model with an error rate of 6.12% and a Radj value of 0.994.
Table 3.
The outcome of the density based models.
| Equation | Parameters | Criteria | ||||
|---|---|---|---|---|---|---|
| a | b | c | d | AARD (%) | R adj | |
| Chrastil | 3.71 | -12.92 | -4020.3 | - | 9.22 | 0.989 |
| Bartle | 6497.1 | 10.55 | -6447.3 | - | 11.92 | 0.971 |
| KJ | 0.445 | 0.0042 | -4203.4 | - | 6.12 | 0.994 |
| MST | 2.53 | 13.6 | -8464.3 | - | 7.15 | 0.991 |
Furthermore, ensuring the precise portrayal of data represents a substantial obstacle in the domain of scientific investigation. Nevertheless, it is feasible to determine whether these data align with specific thermodynamic principles, thereby substantiating their thermodynamic coherence or incoherence. In order to assess the self-consistency of the data, the MST is a frequently employed instrument for the examination of experimental data, with the objective of determining its consistency. In addition to its capacity to correlate data, the ability to extrapolate is a crucial attribute of any model or correlation. Accordingly, the MST (self-consistency test) results were utilized to assess the extrapolation capabilities of the examined models (Fig. 3). As illustrated in Fig. 3, the examined samples exhibited linear trends under all pressures and temperatures, thereby enabling the estimation of data outcomes beyond the present range. Moreover, in accordance with the constants documented in Table 4 and the theoretical framework of the models, the enthalpy values of vaporization, solvation, and total were calculated and presented in Table 4.
Fig. 3.
Consistency solubility data of sulfasalazine.
Table 4.
The enthalpies for sulfasalazine.
| Compound | ΔHtotal (kJ mol− 1) a | ΔHvap. (kJ mol− 1)b | ΔHsol. (kJ mol− 1)c |
|---|---|---|---|
| sulfasalazine | 33.42 | 53.60 | -20.18 |
a Taken from Chrastil model. b Taken from Bartle et al., model. c The difference between Chrastil and Bartle et al., model.
In this study, Peng Robinson was employed alongside the Van der Waals mixing rule to analyze the data.
In addition, the sublimation vapor pressure associated with each temperature was determined to ensure that accurate comparisons could be made. Prior to using EoS to model solubility, it is of utmost importance to obtain the properties of the solute in question using an appropriate methodology. These properties include, but are not limited to, sublimation pressure, molar volume, critical temperature and pressure, acentric factor, and boiling point. Solubility is subject to several different influences that must be taken into account when modeling solubility. The value of the sublimation pressure depends on the acentric factor, which in turn is significantly influenced by the boiling temperature84. Solubility is subject to considerable influence from a number of interdependent factors, the consideration of which is essential when attempting to ascertain the solubility of a given drug. In order to ascertain the critical temperature and pressure, the Marrero-Gani method is employed, which utilises group contributions. Subsequently, the Ambrose-Walton is employed for the calculation of the acentric factor by mentioned information (the critical temperature and pressure obtained). In conclusion, the aforementioned values are employed to ascertain the sublimation pressure via Grain-Watson method at four discrete temperatures (313, 323, 33, and 343 K). The boiling temperatures were determined using the Marrero-Gani while molar volume were calculated using the Immirzi–Perini method, as illustrated in Table 5.
Table 5.
Properties of Sulfasalazine.
| Component | Boiling point (K) | Critical temperature (K) | Critical pressure (MPa) | Acentric factor | Molar volume (cm3/mol) |
|---|---|---|---|---|---|
| Sulfasalazine | 844.58a | 1177.3a | 1.76a | 0.495b | 421.4c |
| Psub (Pa) d | |||||
|
8.33 × 10− 5 (313 K) |
3.52 × 10− 4 (323 K) |
1.36 × 10− 3 (333 K) |
4.81 × 10− 3 (343 K) |
||
As mentioned above, the Vander Waals mixing rule was used to evaluated the data by PR. The adjustable parameters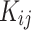 and
and 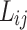 as temperature dependent parameters were obtained by AARD. In addition,
as temperature dependent parameters were obtained by AARD. In addition, 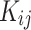 was considered as a temperature dependent adjustable parameter for PC-SAFT. The intrinsic properties of the API were treated as variables in the PC-SAFT model. The segment diameter (σ), the segment number (m), and the segment energy parameter (ε/k) were estimated to be 4.13, 7.35, and 309 k, respectively, through a process of data fitting. The parameters show an opposite trend to temperature as shown in Table 6. The values of
was considered as a temperature dependent adjustable parameter for PC-SAFT. The intrinsic properties of the API were treated as variables in the PC-SAFT model. The segment diameter (σ), the segment number (m), and the segment energy parameter (ε/k) were estimated to be 4.13, 7.35, and 309 k, respectively, through a process of data fitting. The parameters show an opposite trend to temperature as shown in Table 6. The values of 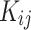 and
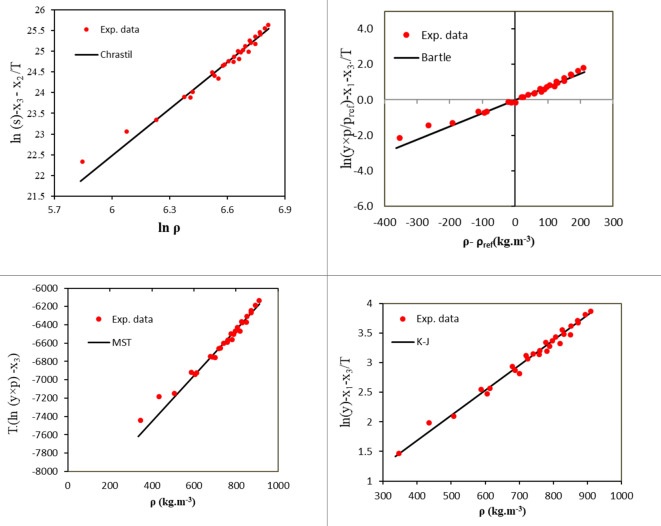
and 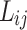 were presented in Table 6 according to the minimum of AARD. The AARDs were 5.55, 8.50, 11.37 and 11.69 for temperatures of 313, 323, 333, and 343 K, respectively. The ARRD of 4.55, 6.95, 7.90, and 9.84 also reported for PC-SAFT at 313, 323, 333, and 343 K respectively. In addition, Radj for each temperature was 0.9586, 0.9402, 0.9670, and 0.9805 for the PR model and 0.9625, 0.9477, 0.9761, and 0.9708 for the PC-SAFT model. The findings indicated that PC-SAFT and PR were effective in correlating the solubility with a high degree of accuracy, Fig. 4.
were presented in Table 6 according to the minimum of AARD. The AARDs were 5.55, 8.50, 11.37 and 11.69 for temperatures of 313, 323, 333, and 343 K, respectively. The ARRD of 4.55, 6.95, 7.90, and 9.84 also reported for PC-SAFT at 313, 323, 333, and 343 K respectively. In addition, Radj for each temperature was 0.9586, 0.9402, 0.9670, and 0.9805 for the PR model and 0.9625, 0.9477, 0.9761, and 0.9708 for the PC-SAFT model. The findings indicated that PC-SAFT and PR were effective in correlating the solubility with a high degree of accuracy, Fig. 4.
Table 6.
Correlation results for solubility of sulfasalazine in ScCO2, by PR and PC-SAFT.
| Model | Parameter | T = 313 K | T = 323 K | T = 333 K | T = 343 K | Overall |
|---|---|---|---|---|---|---|
| PR- vdW2 |
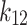
|
0.3961 | 0.3732 | 0.367 | 0.3597 | |
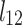
|
0.2803 | 0.2241 | 0.2003 | 0.1842 | ||
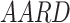
|
5.55 | 8.50 | 11.37 | 11.69 | 9.27 | |
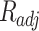
|
0.9586 | 0.9402 | 0.9670 | 0.9805 | 0.9615 | |
| PC-SAFT |
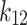
|
0.095 | 0.063 | 0.049 | 0.025 | |
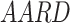
|
4.55 | 6.95 | 7.90 | 9.84 | 7.31 | |
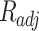
|
0.9625 | 0.9477 | 0.9761 | 0.9708 | 0.9642 |
Fig. 4.
Modeling by PR and PC-SAFT models.
The outcomes of the regular solution with three-category solubility parameter calculation are presented in Table 7. The solute solubility parameter (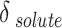 ) is a function of the reduced CO2 density (
) is a function of the reduced CO2 density (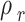 ), which can be expressed using the parameters of A, B, and C. Table 7 shows the values of adjustable parameters for different definitions of
), which can be expressed using the parameters of A, B, and C. Table 7 shows the values of adjustable parameters for different definitions of 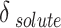 with the statistical parameters of these models. Figure 5 also compares the experimental and the data obtained with these models for different descriptions of
with the statistical parameters of these models. Figure 5 also compares the experimental and the data obtained with these models for different descriptions of 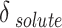 . It is evident that the regular solution, using all given definitions of
. It is evident that the regular solution, using all given definitions of 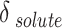 , demonstrates an ability to align with the semi-empirical evidence. In consideration of the acquired AARDs, all temperature-independent models exhibit a satisfactory alignment with the data.
, demonstrates an ability to align with the semi-empirical evidence. In consideration of the acquired AARDs, all temperature-independent models exhibit a satisfactory alignment with the data.
Table 7.
The outcomes of the regular solution method.
Fig. 5.
Modeling by regular solution model.
Conclusion
The solubility of sulfasalazine (a compound with poor solubility in water) in scCO2 was investigated at seven pressures (12, 15, 18, 21, 24, 27, and 30 MPa) and four temperatures (313, 223, 333, and 343 K). To the best of the authors’ knowledge, no previous experimental solubility data exist for this system. In light of the aforementioned considerations, the data presented herein constitute a notable addition to the existing body of literature on the subject. As previously demonstrated, the solubility outcomes exhibited a considerable range, spanning from 0.083 to 1.168 kg/m³. The minimum and maximum mole fractions were obtained at the highest of the four temperatures utilized in the experiments, namely, 343 K. The maximum measured mole fraction was 2.660 × 10− 5 at 12 MPa, and the minimum was 1.635 × 10− 4 at 30 MPa. The experimental data were modeled with five density-based models (Chrastil et al., Bartle et al., MST, Kumar-Johnston and, two EoS (PR, and PC-SAFT) and regular solution. The semi-empirical model that yielded the most precise results was that proposed by KJ model, with an absolute relative deviation (AARD) of 6.12% and Radj, 0.994. In comparison, the model with the highest AARD (11.92%) was that proposed by Bartle et al. The results are of significant value in the context of an expanding pharmaceutical industry, particularly in light of the ongoing advancement of supercritical technology in this field. It is clear that this and similar research will contribute to the continued growth and development of this field.
Acknowledgements
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Research Group. Under the grant number RGP2/150/45.
List of symbols

The model adjustable parameters
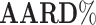
Average absolute relative deviation
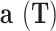
Parameter of the EoS (Nm4 mol− 2)
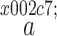
Helmholtz free energy
Parameter of the EoS (m3 mol− 1)
- k
Boltzman constant, J K− 1
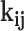
Binary interaction parameter in the mixing rules
Binary interaction parameter in the mixing rules
Number of data points, dimensionless
- Nf
Number of fitted parameters
Pressure
Critical pressure
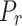
Reduced pressure
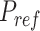
Reference pressure
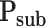
Sublimation pressure (Pa)
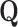
Number of independent variables
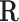
Gas constant, Jmol− 1 K− 1
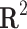
Correlation coefficient
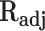
Adjusted correlation coefficient
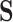
Equilibrium solubility
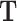
Temperature, K
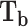
Boling point
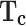
Critical temperature
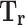
Reduced temperature
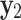
Mole fraction solubility
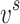
Solid molar volume
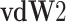
Van der Waals mixing rule with two adjustable parameters
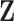
Compressibility factor
Greek symbols
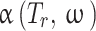
parameter of the EoS, Temperature-dependent
- ε
Depth of pair potential, J
- η
Packing fraction
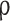
Density, kg m− 3
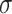
Segment diameter, Å
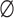
Fugacity coefficient
Acentric factor
Superscripts
- cal
Calculated
- disp
Contribution due to dispersive attraction
- exp
Experimental
- hc
Residual contribution of hard-chain system
- hs
Residual contribution of hard-sphere system
- i, j
Component
- l
Liquid
- s
Solid
- scf
Supercritical fluid
Subscripts
- i, j
Component
- c
Critical property
- 2
Solute
Author contributions
Y.A. : Writing, drafting. editing, formal analysis, validation, conceptualization, supervisionM.G.: Writing, drafting. editing, modeling, resourcesS.T.: Writing, drafting. editing, investigation, analysisA.W.: Writing, drafting. editing, resources, analysis, modelingS.B.S.: Writing, drafting. editing, investigation, formal analysisF.F.: Writing, drafting. editing, resources, analysis, modelingU.H.: Writing, supervision, editing, validation, analysis, modeling.
Data availability
The datasets used and analysed during the current study are available from the corresponding author (Umme Hani) on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ol’khovich, M. V., Sharapova, A. V., Blokhina, S. V. & Perlovich, G. L. Sulfasalazine: dissolution and distribution in pharmaceutically relevant mediums. J. Chem. Eng. Data. 62, 123–128 (2017). [Google Scholar]
- 2.Shadid, M. et al. Sulfasalazine in ionic liquid form with improved solubility and exposure. MedChemComm6, 1837–1841 (2015). [Google Scholar]
- 3.Esfandiari, N. & Sajadian, S. A. CO2 utilization as Gas Antisolvent for the Pharmaceutical Micro and Nanoparticle production: a review. Arab. J. Chem. 104164. (2022).
- 4.Padrela, L. et al. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals–a comprehensive review. Adv. Drug Deliv. Rev.131, 22–78 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Franco P. & De Marco I. Supercritical antisolvent process for pharmaceutical applications: a review. Processes8, 938 (2020). [Google Scholar]
- 6.Gurikov, P. & Smirnova, I. Amorphization of drugs by adsorptive precipitation from supercritical solutions: a review. J. Supercrit. Fluids. 132, 105–125 (2018). [Google Scholar]
- 7.Xiang, S. T., Chen, B. Q., Kankala, R. K., Wang, S. B. & Chen, A. Z. Solubility measurement and RESOLV-assisted nanonization of gambogic acid in supercritical carbon dioxide for cancer therapy. J. Supercrit. Fluids. 150, 147–155 (2019). [Google Scholar]
- 8.Gahtani, R. M., Talath, S., Hani, U., Rahmathulla, M. & Khalid, A. Pregabalin solubility in supercritical green solvent: a comprehensive experimental and theoretical-intelligent assessment. J. Mol. Liq. 125339. (2024).
- 9.Hani, U. et al. Mathematical optimization and prediction of Febuxostat xanthine oxidase inhibitor solubility through supercritical CO2 system using machine-learning approach. J. Mol. Liq.387, 122486 (2023). [Google Scholar]
- 10.Bartle, K. D., Clifford, A. A., Jafar, S. A. & Shilstone, G. F. Solubilities of solids and liquids of low volatility in supercritical Carbon Dioxide. J. Phys. Chem. Ref. Data. 20, 713–756 (1991). [Google Scholar]
- 11.Méndez-Santiago, J. & Teja, A. S. The solubility of solids in supercritical fluids. Fluid. Phase. Equilibria. 158, 501–510 (1999). [Google Scholar]
- 12.Mendez-Santiago, J. & Teja, A. S. Solubility of solids in supercritical fluids: consistency of data and a new model for cosolvent systems. Ind. Eng. Chem. Res.39, 4767–4771 (2000). [Google Scholar]
- 13.Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem.86, 3016–3021 (1982). [Google Scholar]
- 14.Kumar, S. K. & Johnston, K. P. Modelling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids. 1, 15–22 (1988). [Google Scholar]
- 15.Garlapati, C. & Madras, G. Solubilities of solids in supercritical fluids using dimensionally consistent modified solvate complex models. Fluid. Phase. Equilibria. 283, 97–101 (2009). [Google Scholar]
- 16.Garlapati, C. & Madras, G. New empirical expressions to correlate solubilities of solids in supercritical carbon dioxide. Thermochim. Acta. 500, 123–127 (2010). [Google Scholar]
- 17.Alwi, R. S., Garlapati, C. & Tamura, K. Solubility of anthraquinone derivatives in supercritical carbon dioxide: new correlations. Molecules26, 460 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Valle, J. M. & Aguilera, J. M. An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide. Ind. Eng. Chem. Res.27, 1551–1553 (1988). [Google Scholar]
- 19.Sung, H. D. & Shim, J. J. Solubility of C. I. disperse Red 60 and C. I. disperse Blue 60 in supercritical Carbon Dioxide. J. Chem. Eng. Data. 44, 985–989 (1999). [Google Scholar]
- 20.Adachi, Y. & Lu, B. C. Y. Supercritical fluid extraction with carbon dioxide and ethylene. Fluid. Phase. Equilibria. 14, 147–156 (1983). [Google Scholar]
- 21.Bian, X. Q., Zhang, Q., Du, Z. M., Chen, J. & Jaubert, J. N. A five-parameter empirical model for correlating the solubility of solid compounds in supercritical carbon dioxide. Fluid. Phase. Equilibria. 411, 74–80 (2016). [Google Scholar]
- 22.Bian, X., Du, Z. & Tang, Y. An improved density-based model for the solubility of some compounds in supercritical carbon dioxide. Thermochim. Acta. 519, 16–21 (2011). [Google Scholar]
- 23.Sparks, D. L., Hernandez, R. & Estévez, L. A. Evaluation of density-based models for the solubility of solids in supercritical carbon dioxide and formulation of a new model. Chem. Eng. Sci.63, 4292–4301 (2008). [Google Scholar]
- 24.Si-Moussa, C., Belghait, A., Khaouane, L., Hanini, S. & Halilali, A. Novel density-based model for the correlation of solid drugs solubility in supercritical carbon dioxide. C. R. Chim. (2016).
- 25.Belghait, A., Si-Moussa, C., Laidi, M. & Hanini, S. Semi-empirical correlation of solid solute solubility in supercritical carbon dioxide: comparative study and proposition of a novel density-based model. C. R. Chim.21, 494–513 (2018). [Google Scholar]
- 26.Amooey, A. A. A simple correlation to predict drug solubility in supercritical carbon dioxide. Fluid. Phase. Equilibria. 375, 332–339 (2014). [Google Scholar]
- 27.Haghbakhsh, R., Hayer, H., Saidi, M., Keshtkari, S. & Esmaeilzadeh, F. Density estimation of pure carbon dioxide at supercritical region and estimation solubility of solid compounds in supercritical carbon dioxide: correlation approach based on sensitivity analysis. Fluid. Phase. Equilibria. 342, 31–41 (2013). [Google Scholar]
- 28.Mitra, S. & Wilson, N. K. An empirical method to predict solubility in supercritical fluids. J. Chromatogr. Sci.29, 305–309 (1991). [Google Scholar]
- 29.Reddy, T. A. & Garlapati, C. Dimensionless empirical model to correlate pharmaceutical compound solubility in supercritical carbon dioxide. Chem. Eng. Technol.42, 2621–2630 (2019). [Google Scholar]
- 30.Tippana Ashok, R. S., Reddy, C. & Garlapati A new empirical model to correlate solubility of pharmaceutical compounds in supercritical carbon dioxide. J. Appl. Sci. Eng. Methodologies. 4, 575–590 (2018). [Google Scholar]
- 31.Gordillo, M., Blanco, M., Molero, A. & De La Ossa, E. M. Solubility of the antibiotic penicillin G in supercritical carbon dioxide. J. Supercrit. Fluids. 15, 183–190 (1999). [Google Scholar]
- 32.Sodeifian, G., Sajadian, S. A., Razmimanesh, F. & Hazaveie, S. M. Solubility of Ketoconazole (antifungal drug) in SC-CO2 for binary and ternary systems: measurements and empirical correlations. Sci. Rep.11, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: Assessment of new empirical model. J. Supercrit. Fluids. 146, 89–99 (2019). [Google Scholar]
- 34.Sodeifian, G., Nateghi, H. & Razmimanesh, F. Measurement and modeling of dapagliflozin propanediol monohydrate (an anti-diabetes medicine) solubility in supercritical CO2: evaluation of new model. J. CO2 Utilization. 80, 102687 (2024). [Google Scholar]
- 35.Pishnamazi, M. et al. Using static method to measure tolmetin solubility at different pressures and temperatures in supercritical carbon dioxide. Sci. Rep.10, 1–7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pishnamazi, M. et al. Measuring solubility of a chemotherapy-anti cancer drug (busulfan) in supercritical carbon dioxide. J. Mol. Liq.317, 113954 (2020). [Google Scholar]
- 37.Zabihi, S. et al. Thermodynamic study on solubility of brain tumor drug in supercritical solvent: Temozolomide case study. J. Mol. Liq.321, 114926 (2021). [Google Scholar]
- 38.Pishnamazi, M. et al. Chloroquine (antimalaria medication with anti SARS-CoV activity) solubility in supercritical carbon dioxide. J. Mol. Liq.322, 114539 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartle, K., Clifford, A., Jafar, S. & Shilstone, G. Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data. 20, 713–756 (1991). [Google Scholar]
- 40.Ardestani, N. S., Sajadian, S. A., Esfandiari, N., Rojas, A. & Garlapati, C. Experimental and modeling of solubility of sitagliptin phosphate, in supercritical carbon dioxide: proposing a new association model. Sci. Rep.13, 17506 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sajadian, S. A., Ardestani, N. S., Esfandiari, N., Askarizadeh, M. & Jouyban, A. Solubility of favipiravir (as an anti-COVID-19) in supercritical carbon dioxide: an experimental analysis and thermodynamic modeling. J. Supercrit. Fluids 105539. (2022). [DOI] [PMC free article] [PubMed]
- 42.Esfandiari, N. & Sajadian, S. A. Experimental and modeling investigation of Glibenclamide Solubility in Supercritical Carbon dioxide. Fluid. Phase. Equilibria 113408. (2022).
- 43.Sajadian, S. A., Ardestani, N. S. & Jouyban, A. Solubility of montelukast (as a potential treatment of COVID-19) in supercritical carbon dioxide: experimental data and modelling. J. Mol. Liq.349, 118219 (2022). [Google Scholar]
- 44.Anitha, N. & Chandrasekhar, G. A simple model to correlate solubility of thermolabile solids in supercritical fluids, in: AIP Conference Proceedings, AIP Publishing, (2022).
- 45.Yan, J. et al. Comparison of Four Density-Based Semi-Empirical Models for the Solubility of Azo Disperse Dyes in Supercritical Carbon Dioxide, Processes. 10 1960. (2022).
- 46.Nguyen, H. C. et al. Computational prediction of drug solubility in supercritical carbon dioxide: thermodynamic and artificial intelligence modeling. J. Mol. Liq.354, 118888 (2022). [Google Scholar]
- 47.Abourehab, M. A. S. et al. Laboratory determination and thermodynamic analysis of Alendronate Solubility in Supercritical Carbon Dioxide. J. Mol. Liq. 120242. (2022).
- 48.Razmimanesh, F., Sodeifian, G. & Sajadian, S. A. An investigation into Sunitinib malate nanoparticle production by US-RESOLV method: Effect of type of polymer on dissolution rate and particle size distribution. J. Supercrit. Fluids. 170, 105163 (2021). [Google Scholar]
- 49.Sodeifian, G., Sajadian, S. A. & Derakhsheshpour, R. Experimental measurement and thermodynamic modeling of Lansoprazole solubility in supercritical carbon dioxide: application of SAFT-VR EoS. Fluid. Phase. Equilibria. 507, 112422 (2020). [Google Scholar]
- 50.Sodeifian, G., Saadati Ardestani, N., Sajadian, S. A., Golmohammadi, M. R. & Fazlali, A. Prediction of solubility of Sodium Valproate in Supercritical Carbon Dioxide: experimental study and thermodynamic modeling. J. Chem. Eng. Data. 65, 1747–1760 (2020). [Google Scholar]
- 51.Joback, K. G., Reid, R. C. & ESTIMATION OF PURE-COMPONENT PROPERTIES FROM GROUP-CONTRIBUTIONS. Chem. Eng. Commun.57 233–243. (1987). [Google Scholar]
- 52.Marrero, J. & Gani, R. Group-contribution based estimation of pure component properties. Fluid. Phase. Equilibria. 183, 183–208 (2001). [Google Scholar]
- 53.Lee, B. I. & Kesler, M. G. A generalized thermodynamic correlation based on three-parameter corresponding states. AIChE J.21, 510–527 (1975). [Google Scholar]
- 54.Fedors, R. F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci.14, 147–154 (1974). [Google Scholar]
- 55.Stein, S. E. & Brown, R. L. Estimation of normal boiling points from group contributions. J. Chem. Inform. Comput. Sci.34, 581–587 (1994). [Google Scholar]
- 56.Sodeifian, G., Drakhsheshpoor, R. & Sajadian, S. A. Experimental study and thermodynamic modeling of Esomeprazole (proton-pump inhibitor drug for stomach acid reduction) solubility in supercritical carbon dioxide. J. Supercrit. Fluids 104606. (2019).
- 57.Sodeifian, G., Razmimanesh, F., Sajadian, S. A., Soltani, H. & Panah Solubility measurement of an antihistamine drug (loratadine) in supercritical carbon dioxide: Assessment of qCPA and PCP-SAFT equations of state. Fluid. Phase. Equilibria. 472, 147–159 (2018). [Google Scholar]
- 58.Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Prediction of solubility of sunitinib malate (an anti-cancer drug) in supercritical carbon dioxide (SC–CO2): experimental correlations and thermodynamic modeling. J. Mol. Liq. 111740. (2019).
- 59.Morales-Díaz, C., Cabrera, A. L., Juan, C. & Mejía, A. Modelling of solubility of vitamin K3 derivatives in supercritical carbon dioxide using cubic and SAFT equations of state. J. Supercrit. Fluids. 167, 105040 (2021). [Google Scholar]
- 60.Sodeifian, G., Razmimanesh, F., Sajadian, S. A. & Panah, H. S. Solubility measurement of an antihistamine drug (loratadine) in supercritical carbon dioxide: Assessment of qCPA and PCP-SAFT equations of state. Fluid. Phase. Equilibria. 472, 147–159 (2018). [Google Scholar]
- 61.Hosseini Anvari, M. & Pazuki, G. A study on the predictive capability of the SAFT-VR equation of state for solubility of solids in supercritical CO2. J. Supercrit. Fluids. 90, 73–83 (2014). [Google Scholar]
- 62.Abdallah, A. E., Si-Moussa, C., Hanini, S. & Laidi, M. Application of PC-SAFT and cubic equations of state for the correlation of solubility of some pharmaceutical and statin drugs in SC-CO2. Chem. Ind. Chem. Eng. Q.19, 449–460 (2013). [Google Scholar]
- 63.McCabe, C. & Galindo, A. SAFT associating fluids and fluid mixtures. Appl. Thermodyn. Fluids 215–279. (2010).
- 64.Gross, J. & Sadowski, G. Perturbed-chain SAFT: an equation of state based on a perturbation theory for chain molecules. Ind. Eng. Chem. Res.40, 1244–1260 (2001). [Google Scholar]
- 65.McCabe, C., Galindo, A., García-Lisbona, M. N. & Jackson, G. Examining the adsorption (vapor – liquid equilibria) of short-chain hydrocarbons in low-density polyethylene with the SAFT-VR approach. Ind. Eng. Chem. Res.40, 3835–3842 (2001). [Google Scholar]
- 66.McCabe, C. & Jackson, G. SAFT-VR modelling of the phase equilibrium of long-chain n-alkanes. Phys. Chem. Chem. Phys.1, 2057–2064 (1999). [Google Scholar]
- 67.Smith, J. M. Introduction to chemical engineering thermodynamics. in, ACS Publications, (1950).
- 68.Ali Sajadian, S., Amani, M., Saadati Ardestani, N. & Shirazian, S. Experimental analysis and thermodynamic modelling of Lenalidomide Solubility in Supercritical Carbon Dioxide. Arab. J. Chem. 103821. (2022). [DOI] [PMC free article] [PubMed]
- 69.Gross, J. & Sadowski, G. Application of perturbation theory to a hard-chain reference fluid: an equation of state for square-well chains. Fluid. Phase. Equilibria. 168, 183–199 (2000). [Google Scholar]
- 70.Esfandiari, N. et al. Solubility measurement of verapamil for the preparation of developed nanomedicines using supercritical fluid. Sci. Rep.13, 17089 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Abadian, M. Solubility of Dasatinib monohydrate (anticancer drug) in supercritical CO2: experimental and thermodynamic modeling. J. Mol. Liq.346, 117899 (2022). [Google Scholar]
- 72.Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Roshanghias, A. Solubility of pazopanib hydrochloride (PZH, anticancer drug) in supercritical CO2: experimental and thermodynamic modeling. J. Supercrit. Fluids. 190, 105759 (2022). [Google Scholar]
- 73.Nasri, L. Modified Wilson’s model for correlating solubilities in supercritical fluids of some polycyclic aromatic solutes. Polycycl. Aromat. Compd.38, 244–256 (2018). [Google Scholar]
- 74.Cheng, J. S., Tang, M. & Chen, Y. P. Correlation of solid solubility for biological compounds in supercritical carbon dioxide: comparative study using solution model and other approaches. Fluid. Phase. Equilibria. 194, 483–491 (2002). [Google Scholar]
- 75.Pishnamazi, M. et al. Experimental and thermodynamic modeling decitabine anti cancer drug solubility in supercritical carbon dioxide. Sci. Rep.11, 1075 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ansari, E., Honarvar, B., Sajadian, S. A., Aboosadi, Z. A. & Azizi, M. Solubility of Aripiprazole in Supercritical Carbon Dioxide (Experimental and modeling evaluations, 2023). [DOI] [PMC free article] [PubMed]
- 77.Rojas, A. et al. Solubility of oxazepam in supercritical carbon dioxide: experimental and modeling. Fluid. Phase. Equilibria 114165. (2024).
- 78.Jin, J., Wang, Y., Zhang, H., Fan, X. & Wu, H. Solubility of 4-hydroxybenzaldehyde in supercritical carbon dioxide with and without cosolvents. J. Chem. Eng. Data. 59, 1521–1527 (2014). [Google Scholar]
- 79.Khudaida, S. H. et al. Solubility of probenecid in supercritical carbon dioxide and composite particles prepared using supercritical antisolvent process. J. Supercrit. Fluids. 194, 105851 (2023). [Google Scholar]
- 80.Arabgol, F., Amani, M., Ardestani, N. S. & Sajadian, S. A. Experimental and thermodynamic investigation of gemifloxacin solubility in supercritical CO2 for the production of nanoparticles. J. Supercrit. Fluids. 206, 106165 (2024). [Google Scholar]
- 81.Alwi, R. S. & Tamura, K. Measurement and correlation of derivatized anthraquinone solubility in supercritical carbon dioxide. J. Chem. Eng. Data. 60, 3046–3052 (2015). [Google Scholar]
- 82.Sodeifian, G., Garlapati, C., Razmimanesh, F. & Sodeifian, F. Solubility of amlodipine besylate (calcium channel blocker drug) in supercritical carbon dioxide: measurement and correlations. J. Chem. Eng. Data. 66, 1119–1131 (2021). [Google Scholar]
- 83.Sodeifian, G., Garlapati, C., Razmimanesh, F. & Sodeifian, F. The solubility of Sulfabenzamide (an antibacterial drug) in supercritical carbon dioxide: evaluation of a new thermodynamic model. J. Mol. Liq.335, 116446 (2021). [Google Scholar]
- 84.Poling, B. E. & Prausnitz, J. M. J.P. O’connell, The properties of gases and liquids, Mcgraw-hill New York, (2001).
- 85.Poling, B. E., Prausnitz, J. M., Paul, J. & Reid, O. C. R.C., the Properties of Gases and Liquids (McGraw-Hill New York, 2001).
- 86.Immirzi, A. & Perini, B. Prediction of density in organic crystals. Acta Crystallogr. Sect. A. 33, 216–218 (1977). [Google Scholar]
- 87.Morales-Díaz, C., Cabrera, A. L., de la Fuente, J. C. & Mejía, A. Modelling of solubility of vitamin K3 derivatives in supercritical carbon dioxide using cubic and SAFT equations of state. J. Supercrit. Fluids. 167, 105040 (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author (Umme Hani) on reasonable request.