Abstract
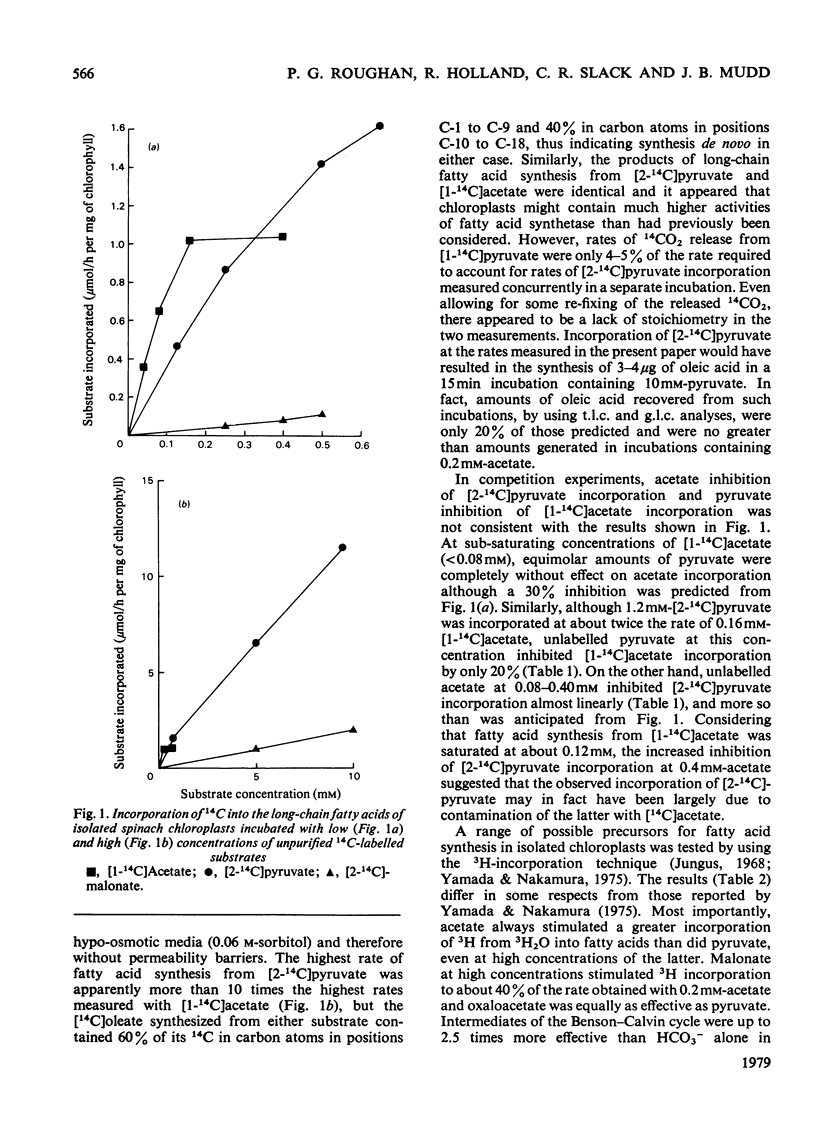
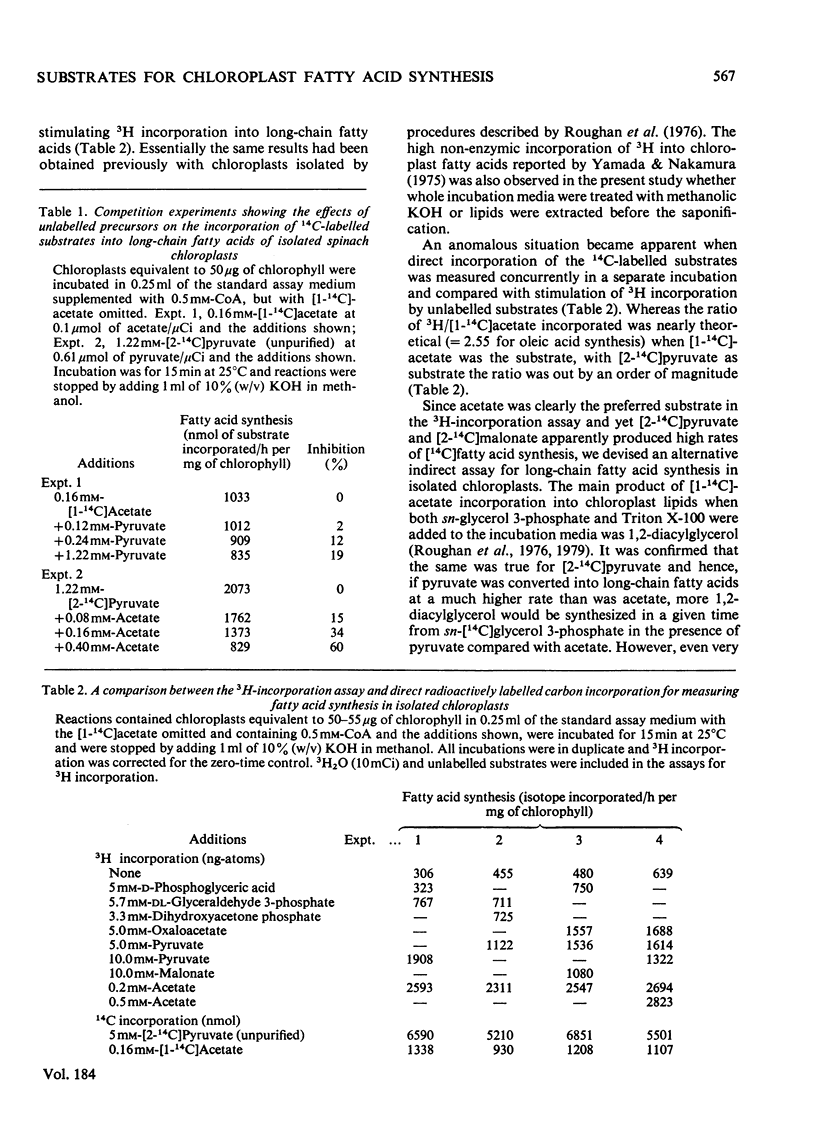
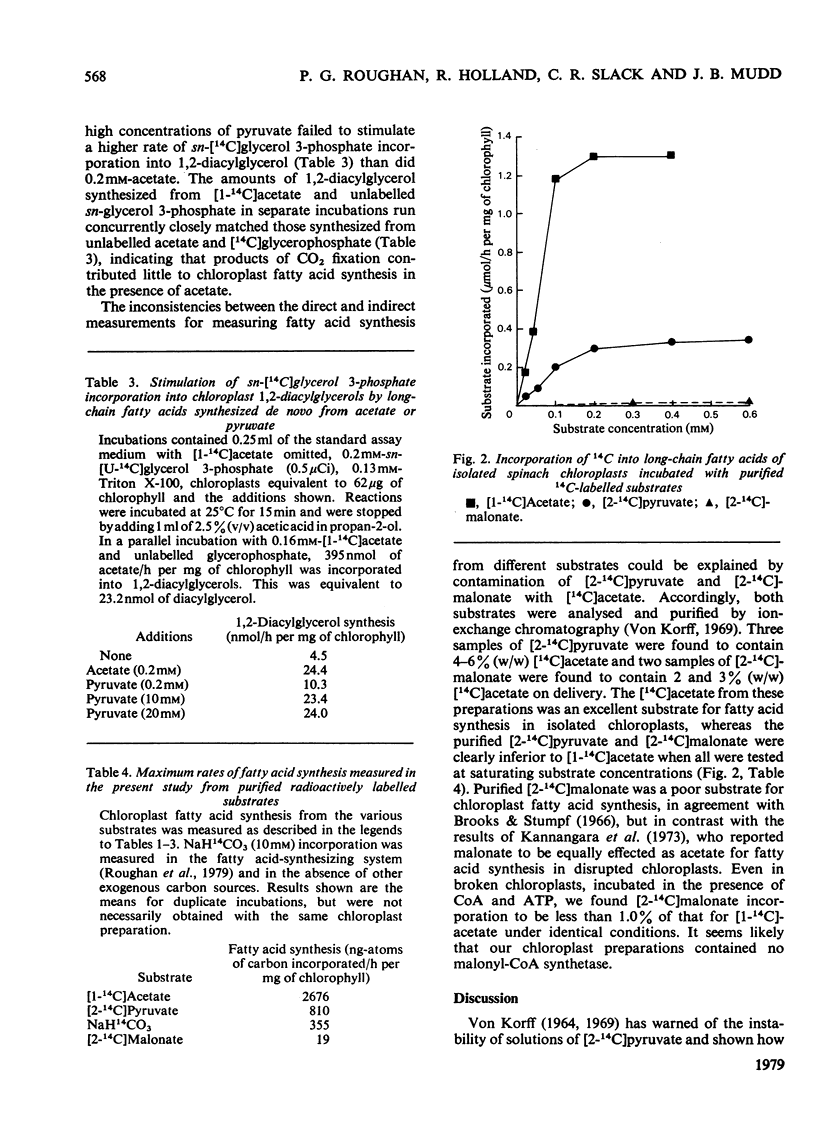
1. Commercially available [2-14C]pyruvate and [2-14C]malonate were found to contain 3-6% (w/w) of [14C]acetate. 2. The contaminating [14C]acetate was efficiently utilized for fatty acid synthesis by isolated chloroplasts, whereas the parent materials were poorer substrates. 3. Maximum incorporation rates of the different substrates examined were (ng-atoms of C/h per mg of chlorophyll): [1-14C]acetate, 2676; [2-14C]pyruvate, 810; H14CO3-, 355; [2-14C]malonate, 19. 4. Products of CO2 fixation were probably not a significant carbon source for fatty acid synthesis in the presence of exogenous acetate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks J. L., Stumpf P. K. Fat metabolism in higher plants. XXXIX. Properties of a soluble fatty acid synthesizing system from lettuce chloroplasts. Arch Biochem Biophys. 1966 Sep 26;116(1):108–116. doi: 10.1016/0003-9861(66)90019-1. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Fatty acid synthesis in adipose tissue incubated in tritiated water. Biochemistry. 1968 Oct;7(10):3708–3717. doi: 10.1021/bi00850a050. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Jacobson B. S., Stumpf P. K. Fat Metabolism in Higher Plants: LVII. A Comparison of Fatty Acid-Synthesizing Enzymes in Chloroplasts Isolated from Mature and Immature Leaves of Spinach. Plant Physiol. 1973 Aug;52(2):156–161. doi: 10.1104/pp.52.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. I. The biosynthesis of polyunsaturated fatty acids by isolated spinach chloroplasts. Arch Biochem Biophys. 1972 Feb;148(2):414–424. doi: 10.1016/0003-9861(72)90159-2. [DOI] [PubMed] [Google Scholar]
- MUDD J. B., McMANUS T. T. Metabolism of acetate by cellfree preparations from spinach leaves. J Biol Chem. 1962 Jul;237:2057–2063. [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Nothelfer H. G., Barckhaus R. H., Spener F. Localisation and characterization of the fatty acid synthesizing system in cells of Glycine max (soubean) suspension cultures. Biochim Biophys Acta. 1977 Dec 21;489(3):370–380. doi: 10.1016/0005-2760(77)90157-6. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R., Holland R. High rates of [1-14C]acetate incorporation into the lipid of isolated spinach chloroplasts. Biochem J. 1976 Sep 15;158(3):593–601. doi: 10.1042/bj1580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. INSTABILITY OF PYRUVATE-C-14 IN AQUEOUS SOLUTION AS DETECTED BY ENZYMIC ASSAY. Anal Biochem. 1964 Aug;8:470–476. doi: 10.1016/0003-2697(64)90244-1. [DOI] [PubMed] [Google Scholar]
- STUMPF P. K., JAMES A. T. The biosynthesis of long-chain fatty acids by lettuce chloroplast preparations. Biochim Biophys Acta. 1963 Feb 19;70:20–32. doi: 10.1016/0006-3002(63)90715-7. [DOI] [PubMed] [Google Scholar]


