Abstract
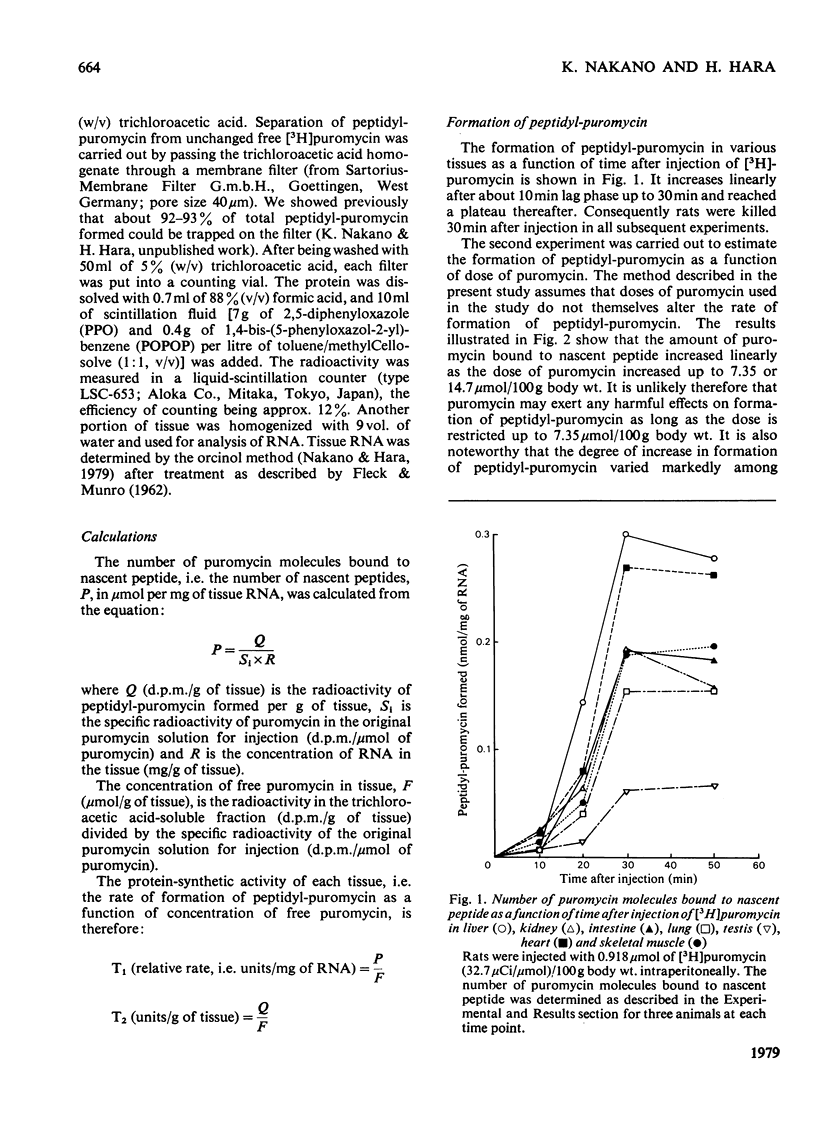
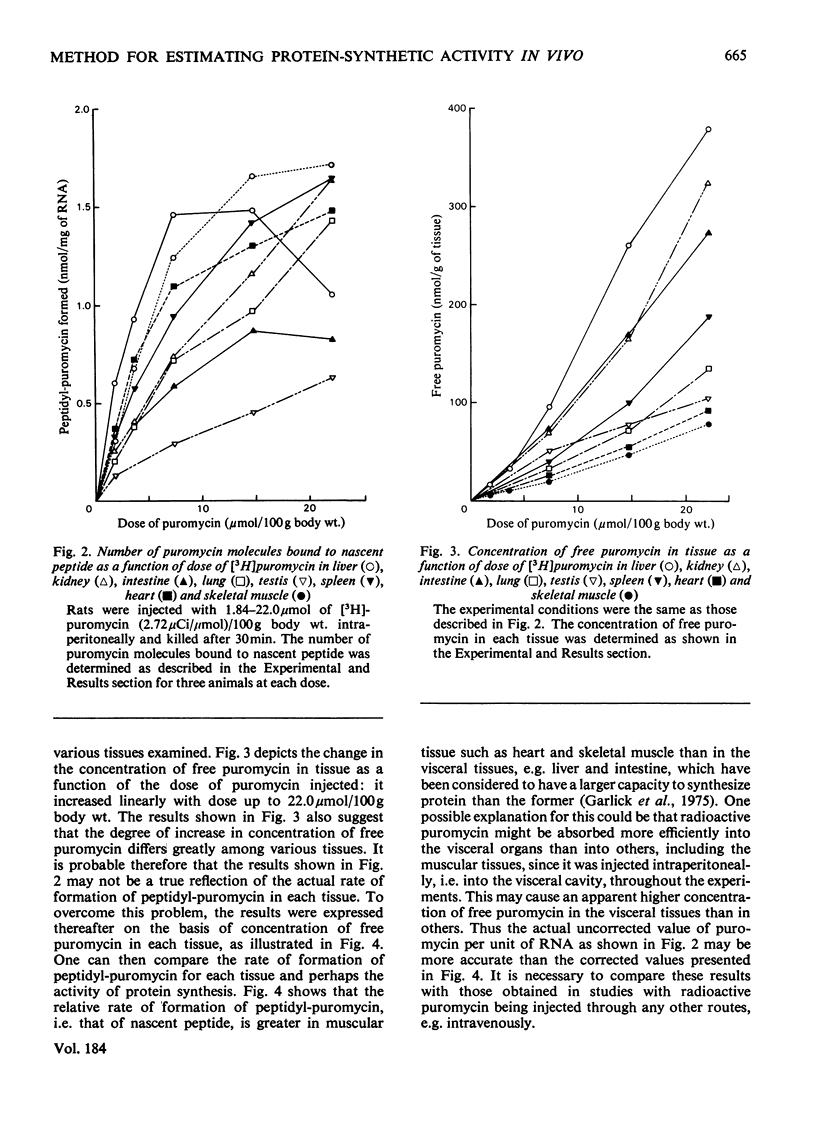
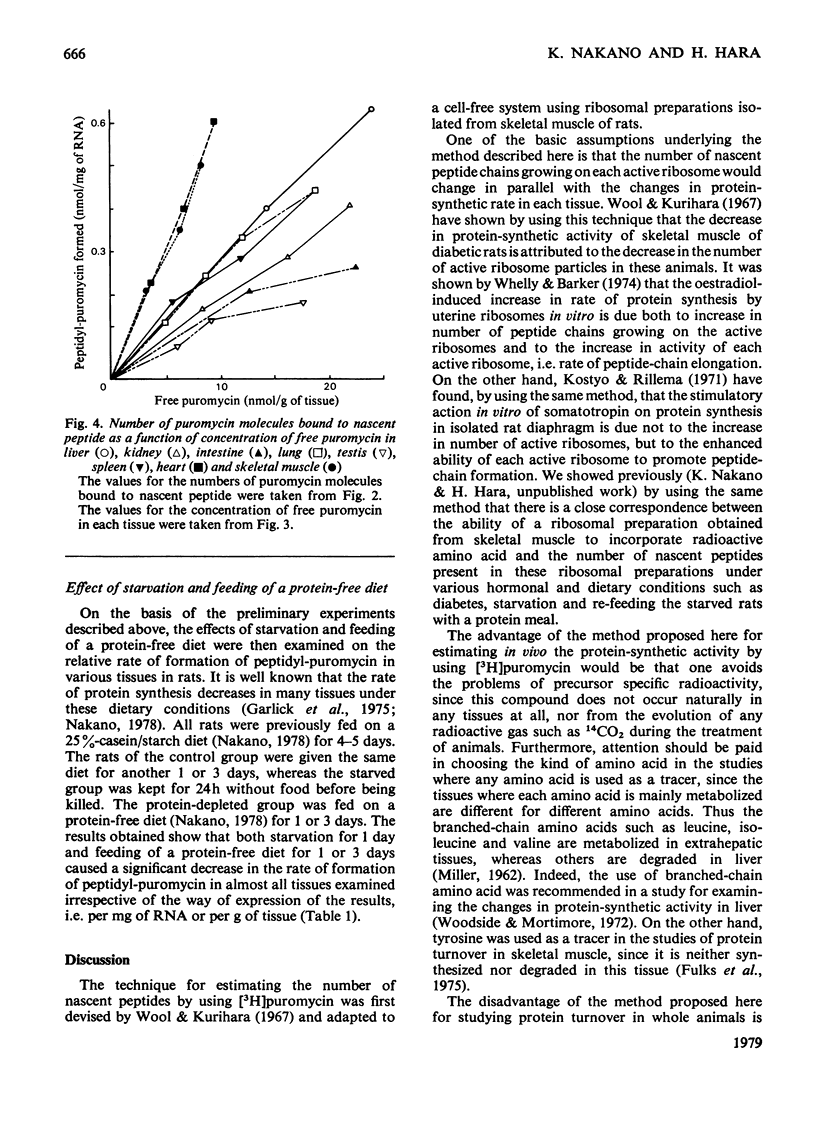
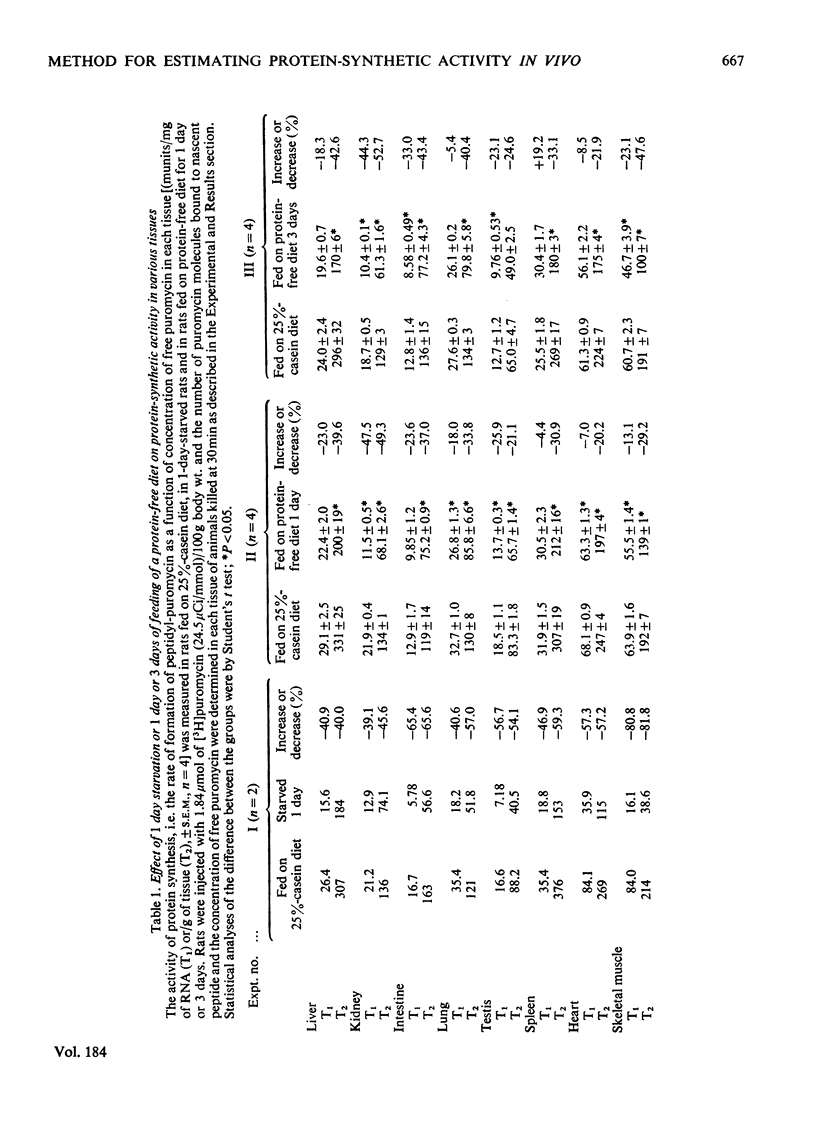
The validity of a new technique was examined for estimating the protein-synthetic activity of various tissues in vivo. The basic assumption underlying the method is that the number of peptide chains growing on each active ribosome would increase as the protein-synthetic activity of each tissue increases. The principle of the procedure, which was devised originally by Wool & Kurihara [(1967) Proc. Natl. Acad. Sci. U.S.A. 58, 2401-2407] to determine in vitro the number of functional ribosomes in skeletal muscle, is as follows. Puromycin is known to bind easily to the C-terminal end of the growing peptide on ribosomes and thus stop further chain elongation. Hence, if the number of puromycin molecules attached to the nascent peptide is determined by using radioactive puromycin as a tracer, one can estimate the number of growing peptides, i.e. the activity of tissue protein synthesis. By using this technique, it is shown that both starvation and the feeding of a protein-free diet caused marked decreases in the relative rate of formation of peptidyl-puromycin, i.e. activity of protein synthesis in liver, skeletal muscle, heart, spleen, testis, lung, kidney and intestine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Harney M. E., Swick R. W., Benevenga N. J. Estimation of tissue protein synthesis in rats fed diets labeled with (U-14C)tyrosine. Am J Physiol. 1976 Oct;231(4):1018–1023. doi: 10.1152/ajplegacy.1976.231.4.1018. [DOI] [PubMed] [Google Scholar]
- Henshaw E. C., Hirsch C. A., Morton B. E., Hiatt H. H. Control of protein synthesis in mammalian tissues through changes in ribosome activity. J Biol Chem. 1971 Jan 25;246(2):436–446. [PubMed] [Google Scholar]
- Kostyo J. L., Rillema J. A. In vitro effects of growth hormone on the number and activity of ribosomes engaged in protein synthesis in the isolated rat diaphragm. Endocrinology. 1971 Apr;88(4):1054–1062. doi: 10.1210/endo-88-4-1054. [DOI] [PubMed] [Google Scholar]
- Millward D. J. Protein turnover in skeletal muscle. I. The measurement of rates of synthesis and catabolism of skeletal muscle protein using (14C)Na2CO3 to label protein. Clin Sci. 1970 Nov;39(5):577–590. doi: 10.1042/cs0390577. [DOI] [PubMed] [Google Scholar]
- Nakano K., Hara H. Insulin dependent and independent actions of dietary protein on in vitro protein synthesis in skeletal muscle of rats. J Nutr. 1979 Aug;109(8):1390–1398. doi: 10.1093/jn/109.8.1390. [DOI] [PubMed] [Google Scholar]
- Picou D., Taylor-Roberts T. The measurement of total protein synthesis and catabolism and nitrogen turnover in infants in different nutritional states and receiving different amounts of dietary protein. Clin Sci. 1969 Apr;36(2):283–296. [PubMed] [Google Scholar]
- SWICK R. W. Measurement of protein turnover in rat liver. J Biol Chem. 1958 Apr;231(2):751–764. [PubMed] [Google Scholar]
- Scornik O. A. In vivo rate of translation by ribosomes of normal and regenerating liver. J Biol Chem. 1974 Jun 25;249(12):3876–3883. [PubMed] [Google Scholar]
- Waterlow J. C., Stephen J. M. The effect of low protein diets on the turn-over rates of serums, liver and muscle proteins in the rat, measured by continuous infusion of L-[14C]lysine. Clin Sci. 1968 Oct;35(2):287–305. [PubMed] [Google Scholar]
- Whelly S. M., Barker K. L. Early effect of estradiol on the peptide elongation rate by uterine ribosomes. Biochemistry. 1974 Jan 15;13(2):341–346. doi: 10.1021/bi00699a019. [DOI] [PubMed] [Google Scholar]
- Woodside K. H., Mortimore G. E. Suppression of protein turnover by amino acids in the perfused rat liver. J Biol Chem. 1972 Oct 25;247(20):6474–6481. [PubMed] [Google Scholar]
- Wool I. G., Kurihara K. Determination of the number of active muscle ribosomes: effect of diabetes and insulin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2401–2407. doi: 10.1073/pnas.58.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. R., Stothers S. C., Vilaire G. Synthesis and degradation of mixed proteins, and composition changes in skeletal muscle of malnourished and refed rats. J Nutr. 1971 Oct;101(10):1379–1390. doi: 10.1093/jn/101.10.1379. [DOI] [PubMed] [Google Scholar]


