Abstract
Fibromyalgia is a musculoskeletal syndrome characterized by chronic widespread pain that is often associated with systemic manifestations. Since mitochondria are the main source of cellular energy, we hypothesized that fibromyalgia syndrome (FMS) could be linked to mitochondrial impairment. Aim was to study mitochondrial dysfunction in peripheral blood mononuclear cells isolated from 50 patients with primary FMS and 20 apparently healthy controls. Although no differences in mitochondrial basal respiration were observed between patients with primary FMS and healthy controls, a lower median bioenergetic health index (BHI; − 22.1%, p = 0.03), a proxy of mitochondrial function, was found in patients. According to fibromyalgia severity score (FSS), a composite of widespread pain index and symptom severity scale, a lower median BHI (− 18.7%) was found in patients with a FSS ≥ 20 compared to those with a FSS < 20. Negative moderate correlations were found only between BHI and FSS (r = − 0.36) and widespread pain index (r = − 0.38). We demonstrated that patients with FMS had an impaired mitochondrial function. Additionally, we found a mild correlation between the widespread pain index and the BHI, possibly indicating that the altered mitochondrial function, in these patients, narrows musculoskeletal rather than central nervous system involvement.
Subject terms: Biomarkers, Rheumatology
Introduction
Fibromyalgia, a musculoskeletal syndrome, is characterized by chronic widespread pain that is often accompanied by systemic manifestations, such as persistent fatigue, sleep disturbance and cognitive impairment, hyperalgesia and allodynia1. These symptoms significantly impact health-related quality of life. Initially, diagnostic criteria were based on tender points on the body, as defined by the American College of Rheumatology (ACR)2. However, the criteria have since shifted to include a more complex set of features including both the widespread nature of pain and associated symptoms3. Fibromyalgia syndrome (FMS) has a prevalence of 2–8% in the general population and it can develop at any age, including childhood, with higher frequency in females4–6.
Despite significantly impacting quality of life, FMS remains an under-diagnosed disease7. The pathogenic mechanisms and the underlying reason for fibromyalgia-related changes are not fully understood, leading to a lack of specific biomarkers8. Research suggests that it may be related to abnormalities in the central nervous system and immune system, as well as to genetic and environmental factors9. Initially perceived primarily as a rheumatic disorder, it is now recognized as a disorder involving pain processing and central nervous system sensitization10,11.
One theory proposed, as a potential contributor to FMS, involves the dysfunction of mitochondria, which are the energy-producing organelles within our cells and serve as the primary source of cellular energy. When mitochondria malfunction, it can lead to reduced adenosine triphosphate (ATP) production, increased oxidative stress, disrupted calcium homeostasis, and altered mitochondrial membrane potential. These impairments can affect metabolism, cell death and inflammation pathways, innate immune responses, potentially contributing to the pathogenesis of several diseases12.
In the context of FMS pathogenesis, the relevance of mitochondrial dysfunction has been highlighted in a reserpine-induced fibromyalgia rodent model. These animals exhibited reduced functionality of mitochondrial complexes I and II, decreased electron transport, impaired mitochondrial respiration, reduced mitochondrial biogenesis, and altered mitochondrial dynamic. Additionally, they experienced lower ATP synthesis in muscles and spinal cord13,14. Furthermore, individuals with FMS could show decreased ATP production in their muscle cells, contributing to muscle fatigue and overall fatigue experienced by fibromyalgia patients15. While measuring skeletal muscle mitochondrial respiration typically requires an invasive muscle biopsy, polymorphonucleate cells (PBMC) can offer a less invasive alternative for assessing muscle mitochondrial function. PBMCs are crucial for maintaining organic balance and act as “sentinel tissues” to monitor the body’s responses16. PBMC mitochondrial function is a reliable biomarker for muscle mitochondrial function in pathological populations with varying respiration capacities17. Symptoms associated with FMS, such as fatigue, exercise intolerance, and myalgia, are common in primary mitochondrial diseases, usually resulting from mitochondrial dysfunction due to nuclear or mitochondrial DNA mutations18. An analysis of PBMCs from 7 FMS patients versus 7 controls revealed morphological changes in mitochondria of FMS patients, primarily a loss of mitochondrial cristae. These findings suggest that mitochondrial dysfunction may contribute to the chronic pain and fatigue seen in FMS19.
Building upon this context, our study was aimed to study the mitochondrial function in PBMC from patients with primary FMS and to establish correlations with the clinical phenotype.
Methods
Patients
Consecutive patients of all ages and sexes affected with primary FMS who were willing to participate in the study were included from June 2023 to January 2024 at the Fibromyalgia syndrome outpatient clinic of the S.C. Medicina—Emostasi e Trombosi at Fondazione IRCCS Ca’ Granda Ospedale Maggiore, Milano, Italy. FMS was defined based on the presence of the 2016 criteria3. Demographic data, smoke, comorbidities and current therapy were collected. Patients with a diagnosis of inflammatory or systemic autoimmune disease were excluded.
Study design and ethics committee approval
This is a cross-sectional study. Ethics committee approval was obtained by the Milan Area 2 Ethics Committee on 23rd May 2023 (approval n°310_2023). All patients provided written informed consent. The research was performed in accordance with the Declaration of Helsinki, and it adheres to relevant guidelines/regulations.
Fibromyalgia severity score
The fibromyalgia severity score (FSS) was used to classify the severity of patients3. The FSS is composed of two parts: the widespread pain index (WPI), which ranges between 0 and 19 and is a summary count of the number of 19 painful regions from the self-reported Regional Pain Scale (RPS)20; and the symptom severity scale (SSS), which ranges between 0 and 12, and is the sum of the severity scores of 3 symptoms (fatigue, waking unrefreshed, and cognitive symptoms) [0–9] plus the sum [0–3] of the score of the following symptoms occurring during the previous 6 months: (1) headaches [0–1], (2) abdominal pain or cramps [0–1] and (3) depression [0–1].
The 2016 criteria envisage that the following three conditions are met: (a) WPI ≥ 7 and SSS score ≥ 5 OR WPI of 4–6 and SSS score ≥ 9; (b) generalized pain, defined as pain in at least 4 of 5 regions, with jaw, chest, and abdominal pain not included; (c) symptoms must be present for at least three months. In addition, a diagnosis of fibromyalgia syndrome is valid irrespective of other diagnoses and does not exclude the presence of other clinically important illnesses3.
Isolation of human PBMC
PBMCs were isolated by using Ficoll-Hypaque density gradient centrifugation (Merck, Milan, Italy). Briefly, blood was collected and centrifuged (15 min. at 2500 × g) to separate the plasma. The remaining blood was then diluted with RPMI-1640 medium and layered onto the Ficoll-Hypaque media solution. The subsequent density gradient centrifugation (40 min. at 400 × g) allowed to isolate PBMC.
Mitochondrial functionality assessment
Mitochondrial functionality was measured by means of the Seahorse XF Cell Mito Stress Test (Agilent, Santa Clara, USA), which measures key parameters of mitochondrial function by directly measuring the oxygen consumption rate (OCR) of cells21–23. OCR was measured at baseline to determine basal respiration and after injection of optimized doses of specific mitochondrial activators and inhibitors to determine non-mitochondrial respiration, maximal respiration and spare respiratory capacity. This assay allows to obtain data relative to the reserve capacity, the ATP-linked mitochondrial respiration, the non-mitochondrial respiration and the proton leak. The spare respiratory capacity was calculated as a percentage, as follows: (maximal respiration/basal respiration)*100. PBMCs were seeded in a pre-coated sterile 24-well plate with a dedicated medium, at a density of 500,000 cells per well. OCR was recorded at the basal level and after the sequential injections of 1 µM Oligomycin (ATP synthase inhibitor), 1 µM Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP, uncoupling agent) and a mixture of 0.5 µM Rotenone (complex I inhibitor) and Antimycin A (complex III inhibitor)24. OCR data were then normalized per protein concentration in each well. Protein concentration was determined by the Pierce BCA protein assay (Thermo Fisher Scientific, Milan, Italy).
Mitochondrial bioenergetic health index (BHI) calculation
To evaluate the Bioenergetic Health Index (BHI)25 of each patient the OCRs of PBMCs, measured through the Seahorse XF Cell Mito Stress Test, were considered. The resulting indices of bioenergetic function were considered and the following formula was used:
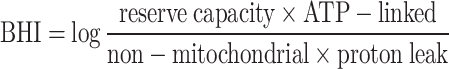 |
Sample size calculation
Based on our preliminary data, we considered the presence of an altered OCR value in healthy controls of 20% and in FMS of 70%. We decided to enroll controls vs FMS patients with a 1:2 ratio because of the difficulty in finding healthy female volunteers due to the high prevalence of FMS in the general population. To obtain a 5% alpha error and an 80% power, the minimum sample size for the group of controls should be of 10 and the minimum sample size of FMS patients should be of 20. The sample size calculation was performed using the calculation formula reported in26.
Statistical analysis
Continuous variables were described as median and interquartile range (IQR), correlation between variables was calculated via Spearman’s rho, differences between groups were calculated with Mann–Whitney U. Linear regression was employed to represent the linear model to predict the dependent variable (BHI) as a function of the independent variable (FSS and subscores). Statistical significance was set at a level of p < 0.05.
Results
Fifty consecutive adult patients with primary fibromyalgia syndrome (98% females; median age 54.5 years) were included in the study. Twenty healthy subjects with a median age of 41 years were enrolled as the control group. Demographic data, smoking habit, current therapy, and comorbidities of the studied population are reported in Table 1.
Table 1.
Demographic data, smoking habits, current treatments and comorbidities of the studied population.
| Cohort (n = 50) | Control (n = 20) | |
|---|---|---|
| AGE (years) | ||
| Median (IQR) | 54.5 (51.0–61.0) | 41 (31.0–54.0) |
| Sex | ||
| M, number (%) | 1 (2.0) | 1 (5.0) |
| F, number (%) | 49 (98.0) | 19 (95.0) |
| Smoker | ||
| Yes, number (%) | 3 (6.0) | 5 (25.0) |
| No, number (%) | 46 (92.0) | 15 (75.0) |
| Fibromyalgia score | ||
| Total fibromyalgia score, median (IQR) | 21 (18–24) | |
| Widespread Pain Index score, median (IQR) | 13 (10–16) | |
| Symptom Severity Score, median (IQR) | 9 (7–10) | |
| Clinical characteristics | ||
| History of Hashimoto’s thyroiditis, number (%) | 9 (18) | |
| Laboratory characteristics | ||
| ANA positivity*, number (%) | 11 (22) | |
| ACPA positivity, number (%) | 0 (0) | |
| RF positivity, number (%) | 1 (2) | |
| ESR, mm/h, median (IQR) | 13 (5–21) | |
ACPA, anti-citrullinated protein antibodies; ANA, antinuclear antibodies; ESR, Erythrocyte sedimentation rate; F, females; IQR, interquartile range; M, males; RF, rheumatoid factor. *no anti-extractable nuclear antigen (ENA) antibody positivity and no clinical signs and symptoms of connective tissue diseases were present.
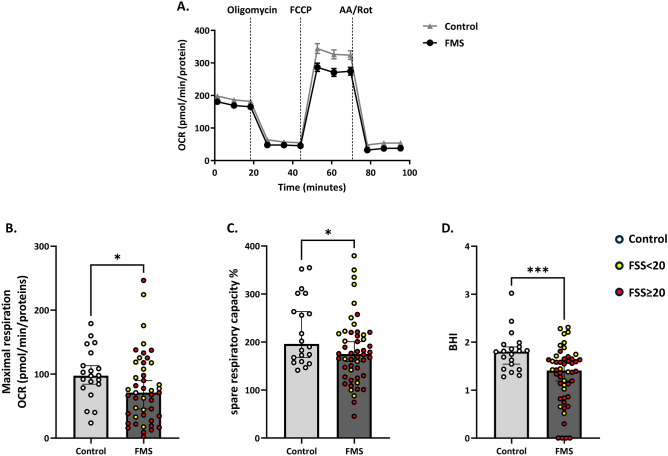
To pursue the hypothesis that FMS was related with an impairment of mitochondrial functionality, we isolated PBMC from the selected population (Table 1) and we evaluated the mitochondrial functionality through the Mito Stress Test. As shown in Fig. 1A, cellular OCR was reduced in patients with FMS following injection of FCCP, an uncoupling agent used to stimulate the respiratory chain at maximum capacity. Correspondingly, maximal respiration was reduced by 27.3% (p = 0.025; Fig. 1B) and spare respiratory capacity, being a proxy of cellular flexibility, was significantly lower in patients with primary FMS, i.e., 174.7% (129.2%, 218.0%) compared to controls [195.8% (161.2%, 291.8%); p = 0.047; Fig. 1C]. Analyzing key parameters of mitochondrial function by measuring cellular OCR enabled the calculation of the BHI, a dynamic index of bioenergetic health. This parameter was also significantly different between controls (median 1.81; 1.35–1.91; p = 0.033) and patients with FMS (median 1.41; 0.85–1.65) (Fig. 1D). No significant differences in mitochondrial parameters were found in patients with Hashimoto’s disease. In patients with positive ANA (n = 11), a significantly lower BHI was observed (p = 0.02).
Fig. 1.
Evaluation of mitochondrial functionality in PBMC of controls and patients with fibromyalgia syndrome (FMS). Panel A) shows the oxygen consumption rate (OCR) measured through the Seahorse XF Cell Mito Stress Test. Panel B) shows maximal respiration. Panel C) shows the spare respiratory capacity (as expressed in %) and panel D) the distribution of the BHI. Light grey line and bars represent the control group (n = 20), and dark grey line and bars represent patients with FMS (n = 50). Yellow dots represent patients with FSS score < 20, while red dots represent patients with FSS score ≥ 20. Results are expressed as median (Q1, Q3). Differences between groups were assessed by Mann–Whitney U test, *p < 0.05. AA, Antimycin A; BHI, Bioenergetic Health Index; FCCP Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; FMS, Fibromyalgia patients; FSS, Fibromyalgia Severity Score; OCR, Oxygen Consumption Rate; PBMC, peripheral blood mononuclear cells; Rot, rotenone.
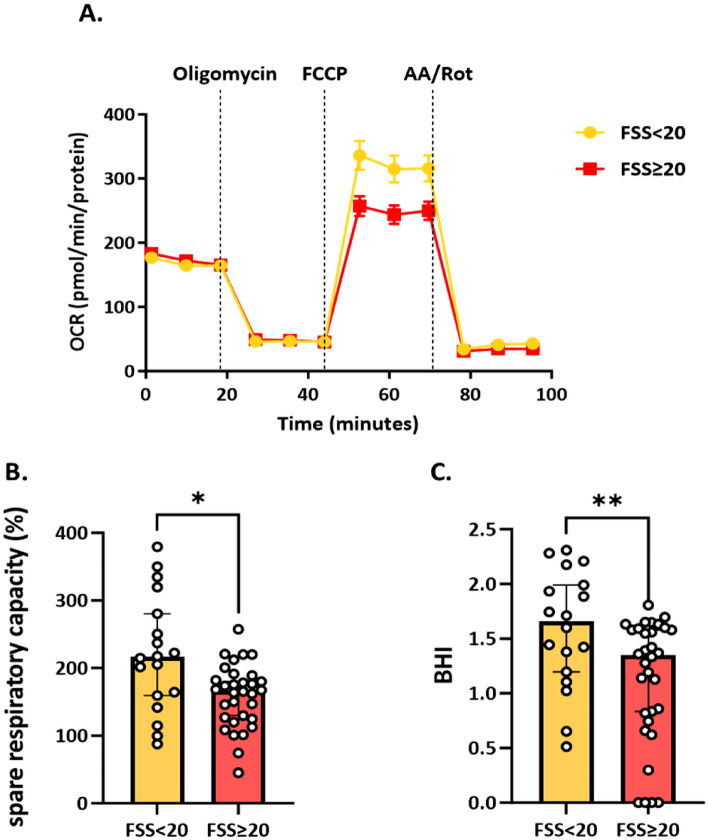
As FSS ≥ 20 indicates a more severe clinical phenotype27, we stratified fibromyalgia patients in two groups with FSS ≥ 20 and < 20. As shown in Fig. 2, the lower the BHI and spare respiratory capacity were, the more severe the clinical symptoms. In particular, in the group with FSS ≥ 20, the spare respiratory capacity was reduced by 23% and the BHI by 19% compared with patients with FSS < 20. These differences were statistically different for both variables, respectively, p = 0.013 and p = 0.009.
Fig. 2.
Evaluation of mitochondrial functionality in peripheral blood mononuclear cells (PBMC) of patients with fibromyalgia syndrome and a FSS < 20 (n = 18) and in the group with FSS ≥ 20 (n = 32). Panel A) shows the oxygen consumption rate (OCR) measured through the Seahorse XF Cell Mito Stress Test. Panel B) indicates the spare respiratory capacity (as expressed in %) and Panel C) the distribution of BHI. Orange bars represent the group with FSS < 20 and red bars represent the FSS ≥ 20 group. Results are expressed as median (Q1, Q3). Differences between groups were by Mann–Whitney U test, *p < 0.05; **p < 0.01. AA Antimycin A; BHI, Bioenergetic Health Index; FCCP Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; FSS, Fibromyalgia Severity Score; OCR, Oxygen Consumption Rate; PBMCS, peripheral blood mononuclear cells; Rot, rotenone.
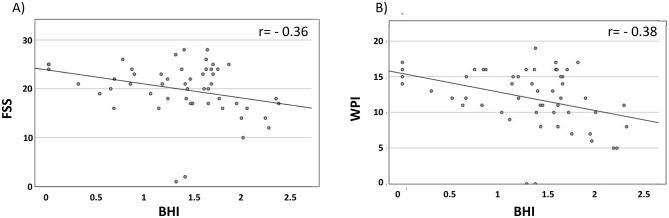
Based on these findings, we sought to evaluate the correlation among BHI, spare capacity and the fibromyalgia syndrome, assessed by Spearman correlations. As shown in Fig. 3A–B, we found negative moderate correlations between BHI and FSS (r = − 0.36) and WPI (r = − 0.38). A poor negative correlation was instead found between BHI and SSS (r = − 0.1). The correlations between spare respiratory capacity and FSS and WPI were moderate, r = − 0.31 and r = − 0.37, respectively. A poor negative correlation was instead found between spare capacity and SSS (r = − 0.12).
Fig. 3.
Correlation analysis between the bioenergetic health index (BHI) vs total fibromyalgia severity score (FSS) (panel A); and vs widespread pain index (panel B). BHI, bioenergetic health index; WPI, widespread pain index.
A subsequent step involved assessing the linear regression between BHI and the FSS, revealing a beta coefficient of − 0.39 (p = 0.005). Similarly, the linear regression between BHI and WPI yielded a beta of − 0.43 (p = 0.002). Conversely, the linear regression between BHI and SSS did not show statistical significance (beta − 0.07, ns).
Discussion
The main findings of the present study were twofold. First, we observed that patients suffering from FM exhibited impaired mitochondrial functionality compared to apparent healthy individuals. Among FM patients, those with a severe clinical presentation (FSS ≥ 20) demonstrated even worse mitochondrial functionality (lower BHI). Second, we identified a negative correlation only between BHI and WPI and not between BHI and SSS. The FSS, the score we used to measures FMS severity, is composed by the WPI, reflecting musculoskeletal pain, and SSS, regarding the functional domains (e.g., fatigue, sleep disturbance, cognitive symptoms, headache, abdominal pain or cramps, and depression) that are more linked to the psychological sphere than to the physical sphere. Together with the knowledge that a greater trafficking exists between PBMCs in the circulating blood and mononuclear cells resident in muscles than between PBMCs and mononuclear cells resident in the CNS, we assumed that this finding suggests that mitochondrial activity in PBMCs reflects more specifically the mitochondrial activity of the peripheral tissues than that of CNS.
The choice of evaluating mitochondrial function in PBMCs and using them as a surrogate biomarker to assess fibromyalgia severity can be justified by the following evidence: (a) PBMC mitochondrial respiration correlates with walking speed in older human adults28 and with heart and skeletal muscle mitochondrial function in nonhuman primates29; (b) markers of PBMC mitochondrial content (i.e., PGC1α, and cytochrome c oxidase subunit IV) appeared to reflect training status in humans30; (c) high levels of mitochondrial reactive oxygen species and low levels of citrate synthase, a mitochondrial matrix protein whose activity correlates with mitochondrial mass, have been described in PBMCs of patients with FMS31; (d) when the analysis of mitochondrial function was tested in skin biopsies of fibromyalgia syndrome patients, this was similar to the one detected in PBMCs32; (e) lipid peroxidation levels, one of the consequences of reactive oxygen species overproduction, are better associated to clinical symptoms in FMS when measured in PBMCs than in plasma33.
Regarding the hypothesis that assessing mitochondrial function in PBMCs could serve as a surrogate marker of fibromyalgia syndrome severity, our findings align with the literature for similar clinical conditions. Deficits in mitochondrial function have been linked to chronic pain, and approximately 70% of patients with heritable mitochondrial diseases suffer from chronic pain34. Impairment in mitochondrial basal respiration, extracellular acidification rate and ATP-linked OCR have been reported in patients suffering from chronic pain35. Mitochondria play a role in pain resolution. For instance, macrophages can control the resolution of inflammatory pain by transferring mitochondria to sensory neurons36. Proper regulation of mitochondrial activity appears crucial to prevent the transition from acute to chronic pain. Notably, compared to age- and sex-matched controls, PBMCs from fibromyalgia syndrome patients showed significantly lower mitochondrial coupling efficiency. Large-scale proteomics studies revealed altered mitochondrial metabolism, centered on pyruvate dehydrogenase and coenzyme A metabolism, leading to a decreased capacity to provide adequate intracellular ATP levels20.
The importance of mitochondria dysfunctionality in fatigue onset has been further highlighted by the ability to use mitochondrial electron transport chain gene expression to distinguish between individuals with systemic sclerosis and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) from non-fatigued patients. In this study, the presence of fatigue was associated with a specific gene expression pattern (i.e., reduced expression of mitochondrially encoded genes ND4 and CyB, and increased expression of nuclear gene Cox7C and ND4 and CyB expression). These expression changes were also correlated with disease severity, providing a distinct mitochondrial signature for systemic sclerosis patients with ME/CFS37.
Mitochondrial dysfunction has also been implicated in neuropathic pain. In a murine model induced by chronic constriction injury, various mitochondrial genes and mtDNA copy number were decreased, emphasizing the importance of impaired mitochondrial biogenesis and function in neuropathic pain38.
Furthermore, mitochondrial function may be linked to alterations in neurotransmitter release, including those involved in pain perception. This connection could contribute to the hypersensitivity to pain experienced by patients with fibromyalgia39,40.
Although the relationship between FMS and mitochondrial dysfunction remains an active area of research, accumulating evidence suggests that mitochondrial dysfunction may play a role in the development and maintenance of fibromyalgia symptoms. A recent study showed that a murine model of FMS, characterized by reduced spontaneous locomotor activity and heightened pain sensitivity, showed diminished activity of electron transport chain complexes and citrate synthase, together with decreased mitochondrial biogenesis at the gastrocnemius muscle. These findings support the hypothesis that mitochondrial dysfunction in central nervous system and in skeletal muscles could be an important feature of FMS41,42. Notably, a study investigating muscle energetics performance in a large cohort of older adults found that lower skeletal muscle energetics were associated with more severe performance fatigability43. Interestingly, mitochondria ultrastructural alterations may underlie muscle fatigue. An analysis of murine fibers, particularly the fast-twitch type IIB/IIX fibers prone to fatigue, revealed elongated mitochondrial constrictions in response to fatigue. This effect was accompanied by elevated phosphorylation of Drp1, a key regulator of the fission44.
We propose mitochondrial dysfunction, and BHI in particular, as a biomarker of fibromyalgia syndrome severity that may support clinicians in the diagnosis of a disease that is still today underestimated and underdiagnosed also due to the absence of validated biomarkers. The BHI is a metric used to evaluate the overall bioenergetic capacity and efficiency of cells, with a particular focus on mitochondrial function. It is generally calculated using data from Seahorse assays or similar platforms that measure OCR and extracellular acidification rate (ECAR), providing insights into mitochondrial respiration and glycolytic function25. A high BHI indicates that mitochondria are functioning efficiently and can robustly meet energy demands, while a low BHI may signal mitochondrial dysfunction or stress. This index can be particularly useful in studying diseases linked to mitochondrial dysfunction, such as neurodegenerative diseases, metabolic disorders, and primary mitochondrial diseases45. As an additional measure of mitochondrial function in FMS, we showed that the spare respiratory capacity was significantly reduced in patients with FMS.
Mitochondrial dysfunction could serve as a therapeutic target in a disease where pathogenic mechanisms are still unclear, leading to unsatisfactory treatment outcomes. Lastly, since mitochondria are maternally inherited46, this aspect warrants further examination to determine a possible correlation with the higher prevalence of the FMS in women.
These results should be interpreted whilst keeping in mind potential limitations. First, the finding of a lower BHI in patients with positive ANA may suggest a role of these antibodies in mitochondrial function impairment. However, this study was not specifically designed to address this point. Therefore, a future study in a dedicated cohort will be designed to respond to this research question. Second, we assumed that FMS patients showed reduced physical activity levels than controls. However, whether the deconditioning in these patients is a cause or a consequence of the mitochondrial dysfunction is still to be elucidated47. Finally, as this was a spontaneous clinical research study, the time allocated for each visit, as well as for sample collection and clinical and laboratory assessments, was limited. The collection of data on health-related quality of life, physical activity and sports in a study considering also these aspects, will help clarify this important point.
Conclusions
In our study, we observed impaired mitochondrial function in patients affected with primary fibromyalgia syndrome. Notably, the degree of impairment was more significant in patients with a more severe clinical phenotype and a more evident correlation with musculoskeletal pain was observed. Our findings support the possibility to use the measurement of this imbalance as a biomarker in the diagnosis and follow-up of patients with fibromyalgia syndrome. Our results underscore the need for further investigations in a larger cohort to identify potential biomarkers and treatment targets for this debilitating condition, which significantly impacts quality of life and currently lacks specific etiopathogenic treatments.
Acknowledgements
S. P. was supported by Fondazione Umberto Veronesi. We thank the patients for participating in this study.
Author contributions
CM, MR and RG conceived the study, analyzed the data and wrote the manuscript. CM, AG, IF, SL, SP performed the experiments and the analyses. NM and AC contributed to data interpretation and revised the manuscript. All authors provided critical feedback on drafts and approved the final manuscript.
Fundings
This study was partially supported by the Italian Ministry of Health—Bando Ricerca Corrente (2024 to RG), by Banca d’Italia (2023 to MR), by Associazione Italiana Sindrome Fibromialgica (AISF ODV to RG and MR), by the European Union MSCA Doctoral Networks Grant Number 101167421 (to MR and CM), by Progetto Eccellenza (2023–2027) to the Department of Pharmacological and Biomolecular Sciences “Rodolfo Paoletti,” Università degli Studi di Milano, and by the Italian Ministry of Health with Ricerca Corrente and “5xmille” funds to N.M.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors have contributed equally: Massimiliano Ruscica and Roberta Gualtierotti.
Change history
1/23/2025
The original online version of this Article was revised: In the original version of this Article, the author name P. Sarzi-Puttini was incorrectly indexed. The original Article has been corrected.
Contributor Information
Massimiliano Ruscica, Email: massimiliano.ruscica@unimi.it.
Roberta Gualtierotti, Email: roberta.gualtierotti@unimi.it.
References
- 1.Clauw, D. J. Fibromyalgia: A clinical review. JAMA.311(15), 1547–1555 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Wolfe, F. et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum.33(2), 160–172 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Wolfe, F. et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum.46(3), 319–329 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Vincent, A. et al. Prevalence of fibromyalgia: A population-based study in Olmsted county, Minnesota, utilizing the rochester epidemiology project. Arthritis Care Res. (Hoboken).65(5), 786–792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBeth, J. & Jones, K. Epidemiology of chronic musculoskeletal pain. Best Pract. Res. Clin. Rheumatol.21(3), 403–425 (2007). [DOI] [PubMed] [Google Scholar]
- 6.White, K. P., Speechley, M., Harth, M. & Ostbye, T. The London fibromyalgia epidemiology study: the prevalence of fibromyalgia syndrome in London. Ontario. J. Rheumatol.26(7), 1570–1576 (1999). [PubMed] [Google Scholar]
- 7.Choy, E. et al. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv. Res.10, 102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarzi-Puttini, P., Giorgi, V., Marotto, D. & Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol.16(11), 645–660 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Ghavidel-Parsa, B. & Bidari, A. The crosstalk of the pathophysiologic models in fibromyalgia. Clin. Rheumatol.42(12), 3177–3187 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Woolf, C. J. Central sensitization: implications for the diagnosis and treatment of pain. Pain152(3 Suppl), S2-s15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe, F. & Rasker, J. J. The evolution of fibromyalgia, its concepts, and criteria. Cureus.13(11), e20010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington, J. S., Ryter, S. W., Plataki, M., Price, D. R. & Choi, A. M. K. Mitochondria in health, disease, and aging. Physiol. Rev.103(4), 2349–2422 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brum, E. D. S. et al. Relevance of mitochondrial dysfunction in the reserpine-induced experimental fibromyalgia model. Mol. Neurobiol.57(10), 4202–4217 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Favero, G., Bonomini, F., Franco, C., Rezzani, R. Mitochondrial dysfunction in skeletal muscle of a fibromyalgia model: The potential benefits of melatonin. Int. J. Mol. Sci. 20(3), (2019). [DOI] [PMC free article] [PubMed]
- 15.Gerdle, B. et al. Decreased muscle concentrations of ATP and PCR in the quadriceps muscle of fibromyalgia patients–a 31P-MRS study. Eur. J. Pain.17(8), 1205–1215 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Da Rosa, P. C., Bertomeu, J. B., Royes, L. F. F. & Osiecki, R. The physical exercise-induced oxidative/inflammatory response in peripheral blood mononuclear cells: Signaling cellular energetic stress situations. Life Sci.321, 121440 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Hedges, C. P. et al. Peripheral blood mononuclear cells do not reflect skeletal muscle mitochondrial function or adaptation to high-intensity interval training in healthy young men. J. Appl. Physiol.126(2), 454–461 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Gorman, G. S. et al. Perceived fatigue is highly prevalent and debilitating in patients with mitochondrial disease. Neuromuscul. Disord.25(7), 563–566 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Israel, L. et al. Mitochondrial structural alterations in fibromyalgia: A pilot electron microscopy study. Clin. Exp. Rheumatol.42(6), 1215–1223 (2024). [DOI] [PubMed] [Google Scholar]
- 20.Wolfe, F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J. Rheumatol.30(2), 369–378 (2003). [PubMed] [Google Scholar]
- 21.Audano, M., Pedretti, S., Cermenati, G., Brioschi, E., Diaferia, G. R., Ghisletti, S., et al. Zc3h10 is a novel mitochondrial regulator. EMBO Rep. 19(4), (2018). [DOI] [PMC free article] [PubMed]
- 22.Macchi, C. et al. Monocarboxylate transporter 1 deficiency impacts CD8(+) T lymphocytes proliferation and recruitment to adipose tissue during obesity. iScience25(6), 104435 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehinger, J. K. et al. Mitochondrial function in peripheral blood cells across the human lifespan. NPJ Aging.10(1), 10 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greco, M. F., Rizzuto, A. S., Zarà, M., Cafora, M., Favero, C., Solazzo, G., et al. PCSK9 confers inflammatory properties to extracellular vesicles released by vascular smooth muscle cells. Int J Mol Sci. 23(21), (2022). [DOI] [PMC free article] [PubMed]
- 25.Chacko, B. K. et al. The bioenergetic health index: A new concept in mitochondrial translational research. Clin Sci (Lond).127(6), 367–373 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosner, B. Fundamentals of biostatistics. 7th ed. Boston: Brooks/Cole, Cengage Learning; (2011).
- 27.Wolfe, F., Walitt, B. T., Rasker, J. J., Katz, R. S. & Häuser, W. The use of polysymptomatic distress categories in the evaluation of fibromyalgia (FM) and FM severity. J. Rheumatol.42(8), 1494–1501 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyrrell, D. J. et al. Respirometric profiling of muscle mitochondria and blood cells are associated with differences in gait speed among community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci.70(11), 1394–1399 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyrrell, D. J., Bharadwaj, M. S., Jorgensen, M. J., Register, T. C. & Molina, A. J. Blood cell respirometry is associated with skeletal and cardiac muscle bioenergetics: Implications for a minimally invasive biomarker of mitochondrial health. Redox. Biol.10, 65–77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Busquets-Cortés, C. et al. Training and acute exercise modulates mitochondrial dynamics in football players’ blood mononuclear cells. Eur. J. Appl. Physiol.117(10), 1977–1987 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Cordero, M. D. et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: Implications in the pathogenesis of the disease. Arthritis. Res. Ther.12(1), R17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordero, M. D. et al. Mitochondrial dysfunction in skin biopsies and blood mononuclear cells from two cases of fibromyalgia patients. Clin. Biochem.43(13–14), 1174–1176 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Cordero, M. D. et al. Clinical symptoms in fibromyalgia are better associated to lipid peroxidation levels in blood mononuclear cells rather than in plasma. PLoS One.6(10), e26915 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Ameele, J. et al. Chronic pain is common in mitochondrial disease. Neuromuscul. Disord.30(5), 413–419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chelimsky, G. et al. Impaired mitochondrial bioenergetics function in pediatric chronic overlapping pain conditions with functional gastrointestinal disorders. Pain Res. Manag.2021, 6627864 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Vlist, M. et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron.110(4), 613–26.e9 (2022). [DOI] [PubMed] [Google Scholar]
- 37.van Eeden, C., Redmond, D., Mohazab, N., Larché, M. J., Mason, A. L., Cohen Tervaert, J. W., et al. Evidence of a Novel Mitochondrial Signature in Systemic Sclerosis Patients with Chronic Fatigue Syndrome. Int. J. Mol. Sci. 24(15), (2023). [DOI] [PMC free article] [PubMed]
- 38.Sun, J. et al. Nrf2 activation attenuates chronic constriction injury-induced neuropathic pain via Induction of PGC-1α-mediated mitochondrial biogenesis in the spinal cord. Oxid. Med. Cell Longev.2021, 9577874 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivannikov, M. V., Sugimori, M. & Llinás, R. R. Synaptic vesicle exocytosis in hippocampal synaptosomes correlates directly with total mitochondrial volume. J. Mol. Neurosci.49(1), 223–230 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duarte, F. V., Ciampi, D. & Duarte, C. B. Mitochondria as central hubs in synaptic modulation. Cell Mol. Life Sci.80(6), 173 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inferrera, F. et al. Impaired mitochondrial quality control in fibromyalgia: Mechanisms involved in skeletal muscle alteration. Arch Biochem Biophys.758, 110083 (2024). [DOI] [PubMed] [Google Scholar]
- 42.Marino, Y. et al. Role of mitochondrial dysfunction and biogenesis in fibromyalgia syndrome: Molecular mechanism in central nervous system. Biochim. Biophys. Acta Mol. Basis. Dis.1870(7), 167301 (2024). [DOI] [PubMed] [Google Scholar]
- 43.Qiao, Y. S., Santanasto, A. J., Coen, P. M., Cawthon, P. M., Cummings, S. R., Forman, D. E., et al. Associations between skeletal muscle energetics and accelerometry-based performance fatigability: Study of muscle, mobility and aging. Aging Cell. e14015, (2023). [DOI] [PMC free article] [PubMed]
- 44.Lavorato, M., Loro, E., Debattisti, V., Khurana, T. S., Franzini-Armstrong, C. Elongated mitochondrial constrictions and fission in muscle fatigue. J. Cell Sci. 131(23), (2018). [DOI] [PMC free article] [PubMed]
- 45.Koklesova, L. et al. Mitochondrial health quality control: Measurements and interpretation in the framework of predictive, preventive, and personalized medicine. EPMA J.13(2), 177–193 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, W. et al. Molecular basis for maternal inheritance of human mitochondrial DNA. Nat Genet.55(10), 1632–1639 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martínez-Lara, A. et al. Mitochondrial imbalance as a new approach to the study of fibromyalgia. Open Access Rheumatol.12, 175–185 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on request.