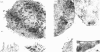Abstract
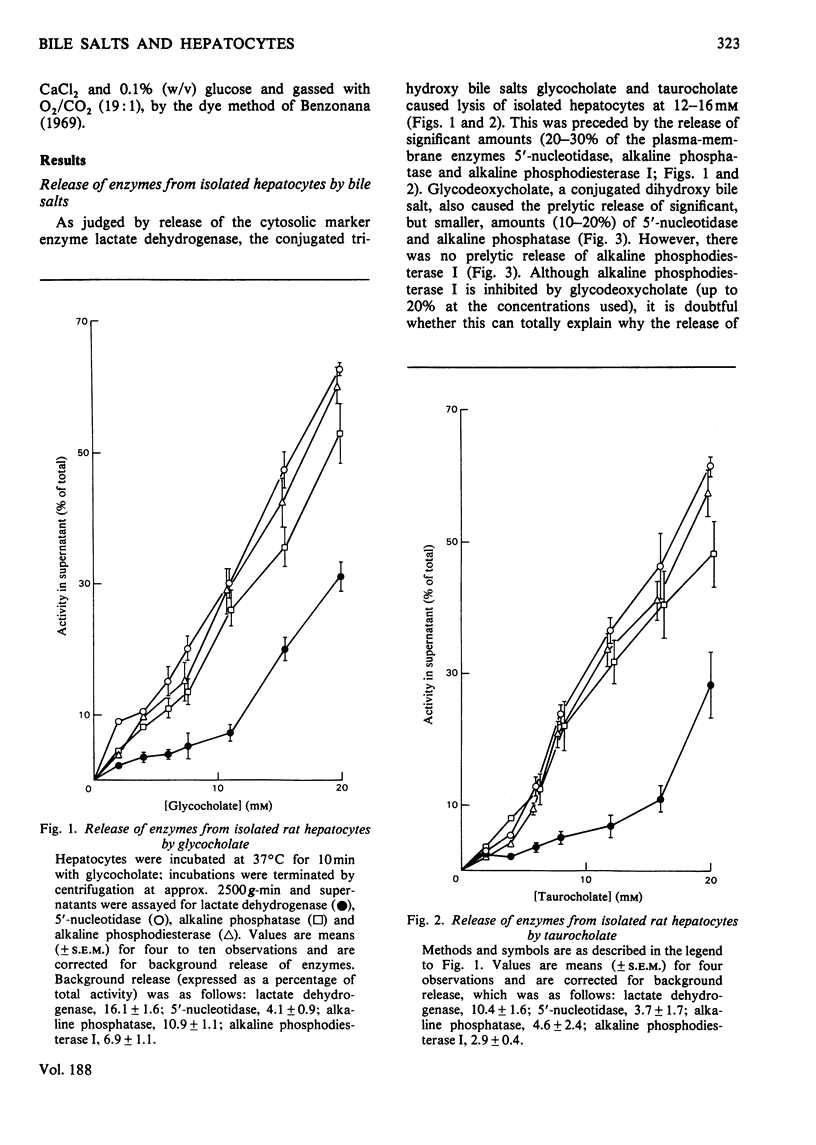
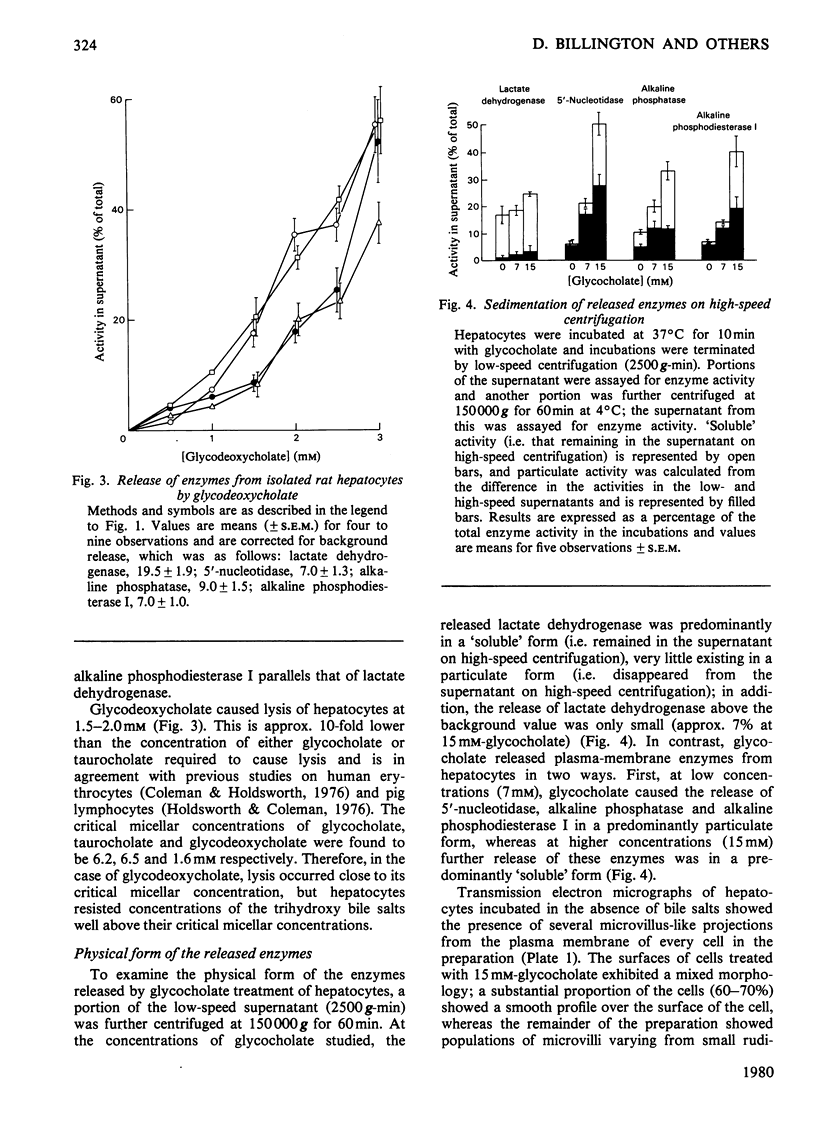
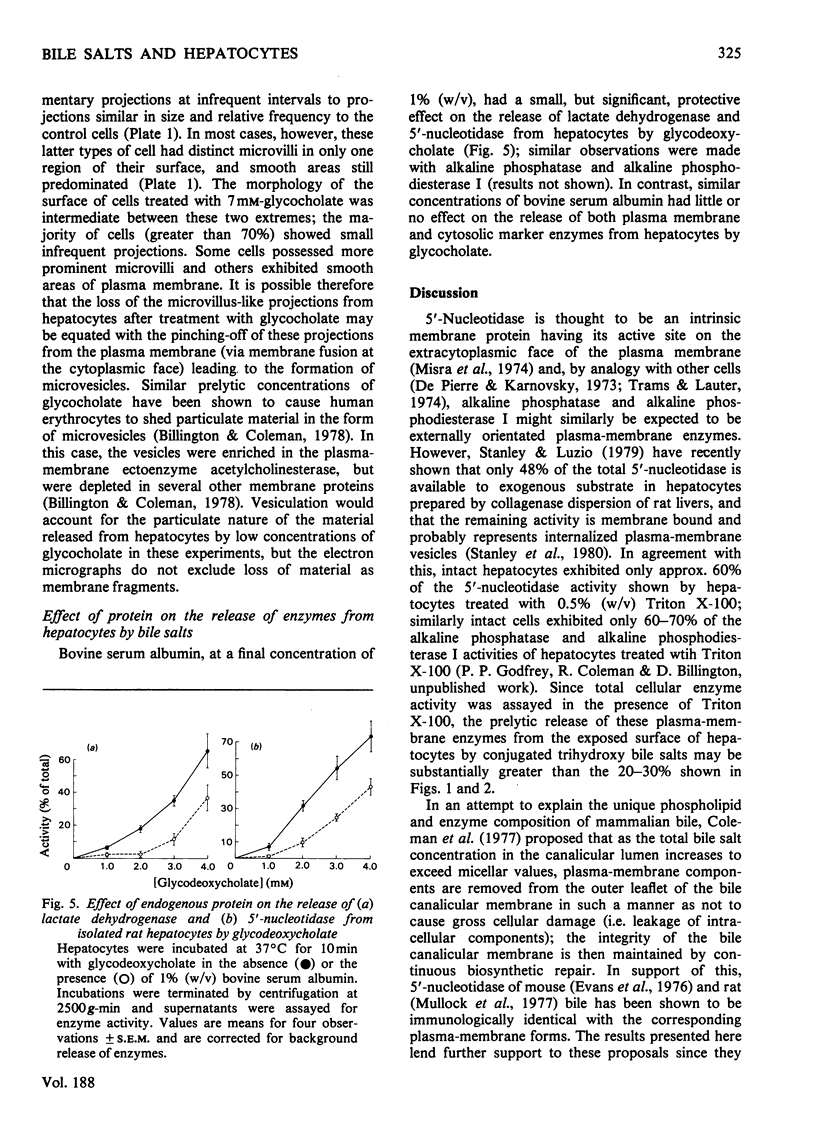
The conjugated trihydroxy bile salts glycocholate and taurocholate removed approx. 20--30% of the plasma-membrane enzymes 5'-nucleotidase, alkaline phosphatase and alkaline phosphodiesterase I from isolated hepatocytes before the onset of lysis, as judged by release of the cytosolic enzyme lactate dehydrogenase. The conjugated dihydroxy bile salt glycodeoxycholate similarly removed 10--20% of the 5'-nucleotidase and alkaline phosphatase activities, but not alkaline phosphodiesterase activity; this bile salt caused lysis of hepatocytes at approx. 10-fold lower concentrations (1.5--2.0mM) than either glycocholate or taurocholate (12--16mM). At low concentrations (7 mM), glycocholate released these enzymes in a predominantly particulate form, whereas at higher concentrations (15 mM) glycocholate further released these components in a predominantly 'soluble' form. Inclusion of 1% (w/v) bovine serum albumin in the incubations had a small protective effect on the release of enzymes from hepatocytes by glycodeoxycholate, but not by glycocholate. These observations are discussed in relation to the possible role of bile salts in the origin of some biliary proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzonana G. Study of bile salts micelles: properties of mixed oleate-deoxycholate solutions at pH 9.0. Biochim Biophys Acta. 1969 Jun 10;176(4):836–848. doi: 10.1016/0005-2760(69)90265-3. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington D., Coleman R. Effects of bile salts of human erythrocytes. Plasma membrane vesiculation, phospholipid solubilization and their possible relationships to bile secretion. Biochim Biophys Acta. 1978 May 4;509(1):33–47. doi: 10.1016/0005-2736(78)90005-6. [DOI] [PubMed] [Google Scholar]
- Billington D., Coleman R., Lusak Y. A. Topographical dissection of sheep erythrocyte membrane phospholipids by taurocholate and glycocholate. Biochim Biophys Acta. 1977 May 2;466(3):526–530. doi: 10.1016/0005-2736(77)90348-0. [DOI] [PubMed] [Google Scholar]
- Billington D., Evans C. E., Godfrey P. P., Coleman R. Effects of bile salts on the plasma membranes of isolated hepatocytes [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):947–947. doi: 10.1042/bst0070947. [DOI] [PubMed] [Google Scholar]
- Bode J. C., Zelder O., Neuberger H. O. Effect of taurocholate, dehydrocholate and secretin on biliary output of alkaline phosphatase and GOT. Helv Med Acta. 1973 Sep;37(2):143–151. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Lipogenesis in rat hepatocytes. J Biol Chem. 1974 Apr 10;249(7):2028–2036. [PubMed] [Google Scholar]
- Coleman R., Billington D. Membrane composition affects characteristics of glycocholate-induced lysis of erythrocytes [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):948–948. doi: 10.1042/bst0070948. [DOI] [PubMed] [Google Scholar]
- Coleman R., Holdsworth G. The release of membrane components prior to haemolysis during extraction of intact eryghrocytes with bile salts. Biochim Biophys Acta. 1976 Apr 5;426(4):776–780. doi: 10.1016/0005-2736(76)90146-2. [DOI] [PubMed] [Google Scholar]
- Coleman R., Iqbal S., Godfrey P. P., Billington D. Membranes and bile formation. Composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979 Jan 15;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Kremmmer T., Culvenor J. G. Role of membranes in bile formation. Comparison of the composition of bile and a liver bile-canalicular plasma-membrane subfraction. Biochem J. 1976 Mar 15;154(3):589–595. doi: 10.1042/bj1540589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley S. J., Al-Bassam S. S., Taylor J. R., Sherratt H. S. Inhibition by diphenyleneiodonium and by some of its substituted derivatives of gluconeogenesis in isolated rat hepatocytes. Biochem Soc Trans. 1975;3(2):333–335. doi: 10.1042/bst0030333. [DOI] [PubMed] [Google Scholar]
- Holdsworth G., Coleman R. Enzyme profiles of mammalian bile. Biochim Biophys Acta. 1975 Apr 21;389(1):47–50. doi: 10.1016/0005-2736(75)90384-3. [DOI] [PubMed] [Google Scholar]
- Holdsworth G., Coleman R. Plasma-membrane components can be removed from isolated lymphocytes by the bile salts glycocholate and taurocholate without cell lysis. Biochem J. 1976 Aug 15;158(2):493–495. doi: 10.1042/bj1580493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth G., Coleman R. Presence of plasma-membrane enzymes in bile and their partial purification by affinity chromatography with concanavalin A. Biochem Soc Trans. 1975;3(5):746–747. doi: 10.1042/bst0030746. [DOI] [PubMed] [Google Scholar]
- Jackson G. D., Lemaître-Coelho I., Vaerman J. P., Bazin H., Beckers A. Rapid disappearance from serum of intravenously injected rat myeloma IgA and its secretion into bile. Eur J Immunol. 1978 Feb;8(2):123–126. doi: 10.1002/eji.1830080210. [DOI] [PubMed] [Google Scholar]
- Kakis G., Yousef I. M. Protein composition of rat bile. Can J Biochem. 1978 May;56(5):287–290. doi: 10.1139/o78-044. [DOI] [PubMed] [Google Scholar]
- Kremmer T., Wisher M. H., Evans W. H. The lipid composition of plasma membrane subfractions originating from the three major functional domains of the rat hepatocyte cell surface. Biochim Biophys Acta. 1976 Dec 14;455(3):655–664. doi: 10.1016/0005-2736(76)90039-0. [DOI] [PubMed] [Google Scholar]
- Lemaître-Coelho I., Jackson G. D., Vaerman J. P. Rat bile as a convenient source of secretory IgA and free secretory component. Eur J Immunol. 1977 Aug;7(8):588–590. doi: 10.1002/eji.1830070818. [DOI] [PubMed] [Google Scholar]
- Makino S., Reynolds J. A., Tanford C. The binding of deoxycholate and Triton X-100 to proteins. J Biol Chem. 1973 Jul 25;248(14):4926–4932. [PubMed] [Google Scholar]
- Misra D. N., Gill T. J., 3rd, Estes L. W. Lymphocyte plasma membranes. II. Cytochemical localization of 5'-nucleotidase in rat lymphocytes. Biochim Biophys Acta. 1974 Jun 29;352(3):455–461. [PubMed] [Google Scholar]
- Mullock B. M., Dobrota M., Hinton R. H. Sources of the proteins of rat bile. Biochim Biophys Acta. 1978 Nov 1;543(4):497–507. doi: 10.1016/0304-4165(78)90304-5. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Issa F. S., Hinton R. H. Bile 5'-nucleotidase in the serum of jaundiced rats. Clin Chim Acta. 1977 Aug 15;79(1):129–140. doi: 10.1016/0009-8981(77)90470-3. [DOI] [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Edwards M. R., Luzio J. P. Subcellular distribution and movement of 5'-nucleotidase in rat cells. Biochem J. 1980 Jan 15;186(1):59–69. doi: 10.1042/bj1860059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. The subcellular distribution of 5'-nucleotidase in isolated fat-cells and liver cells from rat [proceedings]. Biochem Soc Trans. 1979 Apr;7(2):361–362. doi: 10.1042/bst0070361. [DOI] [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974 Apr 29;345(2):180–197. doi: 10.1016/0005-2736(74)90257-0. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef I. M., Bloxam D. L., Phillips M. J., Fisher M. M. Liver cell plasma membrane lipids and the origin of biliary phospholipid. Can J Biochem. 1975 Sep;53(9):989–997. doi: 10.1139/o75-135. [DOI] [PubMed] [Google Scholar]