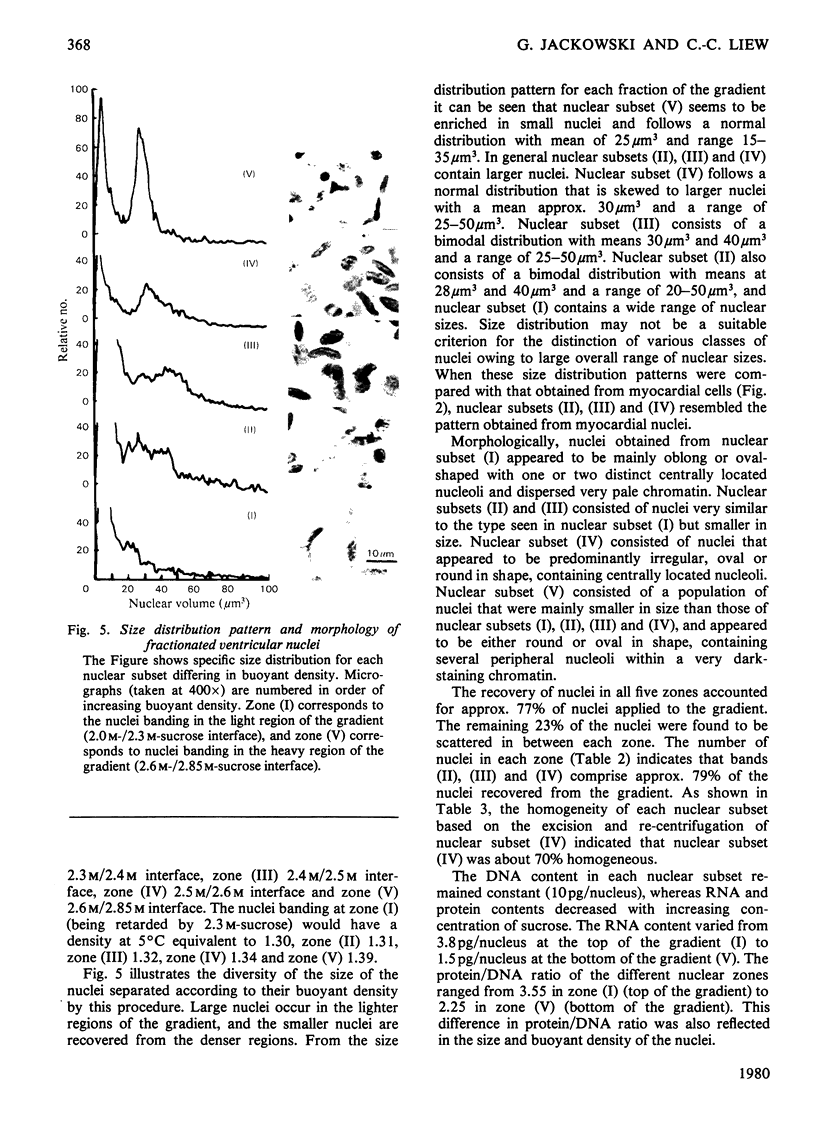
Abstract
Myocardial cells were isolated after treatment with collagenase (0.05%) and hyaluronidase (0.1%) by discontinuous-gradient centrifugation on 3% Ficoll. Nuclei derived from these myocardial cells were then fractionated on a discontinuous sucrose density gradient with the following steps: (I) 2.0M/2.3M, (II) 2.3M/2.4M, (III) 2.4M/2.5M, (IV) 2.5M/2.6M, and (V) 2.6M/2.85M. The myocardial nuclei were sedimented in the interfaces of gradient fractions (II) and (III). Nuclei from whole ventricles that had been treated with the enzymes before isolation sedimented into five major subsets of nuclei. These findings suggest that nuclei sedimented in the isopycnic gradient at fractions (II) and (III) are most probably derived from myocardial cells. However, this procedure is laborious and lengthy, and the recovery of myocardial-cell nuclei is low. An alternative method was developed to isolate an enriched fraction of myocardial-cell nuclei from whole ventricular tissue without exposing the tissues to enzyme digestion. These ventricular nuclei could be fractionated into five nuclear subsets by using the same discontinuous sucrose density gradient as that described above. The content of DNA, RNA and protein per nucleus for each band was determined. Although the DNA content per nucleus was constant (10pg), that of RNA varied from 1.5 to 4.5pg and that of protein from 16 to 24pg. Nuclei from each band were examined by light-microscopy: large nuclei occurred in the ligher regions whereas smaller nuclei were found in the denser regions of the gradient. From the size distribution pattern of myocardial-cell nuclei compared with that of total ventricular nuclei, it was found that nuclear subsets (II), (III), and (IV) were similar to myocardial nuclei. Electrophoretic analyses of the proteins solubilized in sodium dodecyl sulphate/phenol or Tris/EDTA/2-mercaptoethanol/phenol obtained from each nuclear subset indicate that these fractions are similar, with limited qualitative differences. These findings indicate that isolation of an enriched fraction of myocardial-cell nuclei could be achieved by discontinuous-sucrose-density-gradient centrifugation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austoker J., Cox D., Mathias A. P. Fractionation of nuclei from brain by zonal centrifugation and a study of the ribonucleic acid polymerase activity in the various classes of nuclei. Biochem J. 1972 Oct;129(5):1139–1155. doi: 10.1042/bj1291139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S., Scheuer J. Morphology and metabolism of intact muscle cells isolated from adult rat heart. Circ Res. 1970 Jun;26(6):679–687. doi: 10.1161/01.res.26.6.679. [DOI] [PubMed] [Google Scholar]
- Beznak M., French I., Garg V., Rajhathy J., Kako K. J. Myocardial nucleic acid synthesis following constriction of the aorta in rats. Basic Res Cardiol. 1974 Sep-Oct;69(5):499–508. doi: 10.1007/BF01906982. [DOI] [PubMed] [Google Scholar]
- Bloom S., Cancilla P. A. Conformational changes in myocardial nuclei of rats. Circ Res. 1969 Feb;24(2):189–196. doi: 10.1161/01.res.24.2.189. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Vidali G., Allfrey V. G. Changes in nuclear non-histone protein composition during normal differentiation and carcinogenesis of intestinal epithelial cells. Exp Cell Res. 1976 Mar 15;98(2):396–410. doi: 10.1016/0014-4827(76)90449-3. [DOI] [PubMed] [Google Scholar]
- Brown J. W., Cristian D., Paradise R. R. Histological effects of procedural and environmental factors on isolated rat heart preparations. Proc Soc Exp Biol Med. 1968 Nov;129(2):455–462. doi: 10.3181/00379727-129-33343. [DOI] [PubMed] [Google Scholar]
- Chan P. K., Liew C. C. Identification of specific phosphoproteins in nuclease-digested chromatin subunits. Can J Biochem. 1977 Aug;55(8):847–855. doi: 10.1139/o77-125. [DOI] [PubMed] [Google Scholar]
- Cutilletta A. F., Aumont M., Nag A., Zak R. Separation of muscle and non-muscle cells from adult rat myocardium: an application to the study of RNA polymerase. J Mol Cell Cardiol. 1977 May;9(5):399–407. doi: 10.1016/s0022-2828(77)80006-0. [DOI] [PubMed] [Google Scholar]
- DeHann R. L. Regulation of spontaneous activity and growth of embryonic chick heart cells in tissue culture. Dev Biol. 1967 Sep;16(3):216–249. doi: 10.1016/0012-1606(67)90025-5. [DOI] [PubMed] [Google Scholar]
- ENESCO M., PUDDY D. INCREASE IN THE NUMBER OF NUCLEI AND WEIGHT IN SKELETAL MUSCLE OF RATS OF VARIOUS AGES. Am J Anat. 1964 Mar;114:235–244. doi: 10.1002/aja.1001140204. [DOI] [PubMed] [Google Scholar]
- Farmer B. B., Harris R. A., Jolly W. W., Hathaway D. R., Katzberg A., Watanabe A. M., Whitlow A. L., Besch H. R., Jr Isolation and characterization of adult rat hearts cells. Arch Biochem Biophys. 1977 Mar;179(2):545–558. doi: 10.1016/0003-9861(77)90143-6. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., McNutt N. S. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969 Jul;42(1):1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick M. R., Burns A. H., Reddy W. J. Dispersion and isolation of beating cells from adult rat heart. Anal Biochem. 1974 Sep;61(1):32–42. doi: 10.1016/0003-2697(74)90329-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mujica F., Mathias A. P. Studies of nuclei separated by zonal centrifugation from liver of rats treated with thioacetamide. Biochem J. 1973 Feb;132(2):163–183. doi: 10.1042/bj1320163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso D. S., Frangakis C. J., Carlson E. C., Bressler R. Isolation and characterization of myocytes from the adult rat heart. Prep Biochem. 1977;7(5):383–401. doi: 10.1080/00327487708061656. [DOI] [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies on single beating rat heart cells. I. Growth and organization. Exp Cell Res. 1963 Feb;29:451–465. doi: 10.1016/s0014-4827(63)80008-7. [DOI] [PubMed] [Google Scholar]
- Haines M. E., Johnston I. R., Mathias A. P., Ridge D. The synthesis of nicotinamide-adenine dinucleotide and poly (adenosine diphosphate ribose) in various classes of rat liver nuclei. Biochem J. 1969 Dec;115(5):881–887. doi: 10.1042/bj1150881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I. R., Mathias A. P., Pennington F., Ridge D. Distribution of RNA polymerase activity among the various classes of liver nuclei. Nature. 1968 Nov 16;220(5168):668–672. doi: 10.1038/220668a0. [DOI] [PubMed] [Google Scholar]
- Johnston I. R., Mathias A. P., Pennington F., Ridge D. The fractionation of nuclei from mammalian cells by zonal centrifugation. Biochem J. 1968 Aug;109(1):127–135. doi: 10.1042/bj1090127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeStourgeon W. M., Beyer A. L. The rapid isolation, high-resolution electrophoretic characterization, and purification of nuclear proteins. Methods Cell Biol. 1977;16:387–406. [PubMed] [Google Scholar]
- Liew C. C., Liu D. K., Gornall A. G. Effects of aldosterone on RNA polymerase in rat heart and kidney nuclei. Endocrinology. 1972 Feb;90(2):488–495. doi: 10.1210/endo-90-2-488. [DOI] [PubMed] [Google Scholar]
- Liew C. C., Sole M. J. Nuclear proteins in the heart of the cardiomyopathic Syrian hamster. Fractionation of phenol-soluble nonhistone proteins by two-dimensional polyacrylamide gel electrophoresis. Circ Res. 1978 May;42(5):628–636. doi: 10.1161/01.res.42.5.628. [DOI] [PubMed] [Google Scholar]
- Liew C. C., Sole M. J. Nuclear proteins in the heart of the cardiomyopathic Syrian hamster. Phosphorylation of phenol-soluble nonhistone proteins. Circ Res. 1978 May;42(5):637–643. doi: 10.1161/01.res.42.5.637. [DOI] [PubMed] [Google Scholar]
- Lovtrup-Rein H., McEwen B. S. Isolation and fractionation of rat brain nuclei. J Cell Biol. 1966 Aug;30(2):405–415. doi: 10.1083/jcb.30.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUIR A. R. FURTHER OBSERVATIONS ON THE CELLULAR STRUCTURE OF CARDIAC MUSCLE. J Anat. 1965 Jan;99:27–46. [PMC free article] [PubMed] [Google Scholar]
- Mathias A. P., Wynter C. V. The uses of metrizamide in the fractionation of nuclei from brain and liver tissue by zonal centrifugation. FEBS Lett. 1973 Jun 15;33(1):18–22. doi: 10.1016/0014-5793(73)80149-8. [DOI] [PubMed] [Google Scholar]
- Meerson F. Z., Alekhina G. M., Aleksandrov P. N., Bazardjan A. G. Dynamics of nucleic acid and protein synthesis of the myocardium in compensatory hyperfunction and hypertrophy of the heart. Am J Cardiol. 1968 Sep;22(3):337–348. doi: 10.1016/0002-9149(68)90118-5. [DOI] [PubMed] [Google Scholar]
- Morkin E., Ashford T. P. Myocardial DNA synthesis in experimental cardiac hypertrophy. Am J Physiol. 1968 Dec;215(6):1409–1413. doi: 10.1152/ajplegacy.1968.215.6.1409. [DOI] [PubMed] [Google Scholar]
- Moustafa E., Skomedal T., Osnes J. B., Oye I. Cyclic AMP formation and morphology of myocardial cells isolated from adult heart: effect of Ca2+ and Mg2+. Biochim Biophys Acta. 1976 Feb 24;421(2):411–415. doi: 10.1016/0304-4165(76)90308-1. [DOI] [PubMed] [Google Scholar]
- Neal G. E., Judah D. J., Butler W. H. Some effects of acute and chronic dosing with aflatoxin B1 on rat liver nuclei. Cancer Res. 1976 May;36(5):1771–1778. [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Glick M. R., Reddy W. J. Separation of beating cardiac myocytes from suspensions of heart cells. Am J Pathol. 1972 May;67(2):215–226. [PMC free article] [PubMed] [Google Scholar]
- Sasaki R., Morishita T., Yamagata S. Determination of deoxyribonucleic acid content of heart muscle in experimental cardiac hypertrophy. Tohoku J Exp Med. 1970 Jun;101(2):153–159. doi: 10.1620/tjem.101.153. [DOI] [PubMed] [Google Scholar]
- Sasaki R., Morishita T., Yamagata S. Estimation of the cell number of heart muscle in experimental cardiac hypertrophy. Tohoku J Exp Med. 1970 Jun;101(2):147–152. doi: 10.1620/tjem.101.147. [DOI] [PubMed] [Google Scholar]
- Shibko S., Koivistoinen P., Tratnyek C. A., Newhall A. R., Friedman L. A method for sequential quantitative separation and determination of protein, RNA, DNA, lipid, and glycogen from a single rat liver homogenate or from a subcellular fraction. Anal Biochem. 1967 Jun;19(3):514–528. doi: 10.1016/0003-2697(67)90242-4. [DOI] [PubMed] [Google Scholar]
- Stambolova M. A., Cox D., Mathias A. P. The activity of deoxyribonucleic acid polymerase and deoxyribonucleic acid synthesis in nuclei from brain fractionated by zonal centrifugation. Biochem J. 1973 Nov;136(3):685–695. doi: 10.1042/bj1360685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahouny G. V., Wei R., Starkweather R., Davis C. Preparation of beating heart cells from adult rats. Science. 1970 Mar 20;167(3925):1616–1618. doi: 10.1126/science.167.3925.1616. [DOI] [PubMed] [Google Scholar]
- Zak R. Cell proliferation during cardiac growth. Am J Cardiol. 1973 Feb;31(2):211–219. doi: 10.1016/0002-9149(73)91034-5. [DOI] [PubMed] [Google Scholar]







