Abstract
Background:
The prevalence of central nervous system tuberculosis (TB) is about 1–2% of all TB cases. Atypical cases like the present case, being interpreted as leptomeningeal metastasis in magnetic resonance imaging (MRI), can pose a dilemma, delaying or even leading to mistreatment.
Case Description:
A 19-year-old male presented with acute onset paraparesis and bowel bladder involvement presented with an MRI lumbar spine suggesting leptomeningeal metastasis from D11–L5 levels who underwent decompression biopsy which on histopathological examination revealed to be tubercular granulomatous infection. Anti-tubercular drug (ATD) started, and significant improvement was seen in the lower limb power and tone. The outcome of treatment has been unpredictable. Previous case studies having neurological deficits due to severe compression, including ours, show good recovery after surgical decompression and ATD regime.
Conclusion:
Such cases should be managed with high suspicion as they can be easily misdiagnosed to be tumors, leading to mistreatment or delayed treatment.
Keywords: Arachnoiditis, Paraparesis, Radiculomyelopathy, Tuberculosis

INTRODUCTION
The prevalence of central nervous system tuberculosis (TB) is about 1–2% of all TB cases.[3] Considering ours is an endemic zone, it is not uncommon to see a variety of cases of extrapulmonary TB in daily practice. The musculoskeletal system is the most commonly involved extrapulmonary site, and among this, thoracic, and lumbar sacral are involved in decreasing frequency. Few of these are so atypical as to be confused with some malignancy and unnecessarily operated on, adding to the morbidity of the patient and further to the misery is the delay in starting of proper anti-tubercular drug (ATD) regime. Here, we illustrate an atypical presentation which was referred to us as a case of multiple leptomeningeal metastases and later revealed itself to be TB arachnoiditis.
CASE REPORT
A 19-year-old young male student by occupation presented with dull, aching, progressively increasing back pain, radiating to both the lower limbs up to the foot for 4 months. This was accompanied by tingling and paresthesia of both the lower limbs. Three months later, he noticed the asymmetric onset of weakness in both the lower limbs involving proximal and distal muscles. He had developed numbness in the lower half of the body, including the perianal areas, only to progress to bladder disturbances, for which he used to give pressure in the suprapubic region to pass urine with constipation and erectile dysfunction for 2 weeks before admission. On clinical examination, he had asymmetric lower motor neuron type paraparesis with bladder and bowel involvement. Sensory loss (up to 50%) was present below the D12 dermatome, including the perianal region. Spinal tenderness and deformity were absent. He had a normal hemogram but an increased erythrocyte sedimentation rate (98 mm/1st h), being the only suspicious finding. X-rays of the lumbosacral spine and chest were normal. Contrast MRI (lumbosacral) revealed an ill-defined lesion in the lumbar region involving conus, with multiple leptomeningeal enhancements, in the intradural space at D12–S1, which was heterointence both in T1W and T2W [Figure 1]. The lesion took up contrast, and areas of cerebrospinal fluid (CSF) entrapment were seen inside the lesion [Figures 2 and 3]. The conus was bulky with abnormal signals. Intervertebral discs show mild disc desiccation, no pre-paravertebral collection/inflammation seen. The features were suggestive of neoplastic/metastatic etiology. The features suggested neoplastic lesion/metastasis. Positron emission tomography-computed tomography was done to rule out any other lesions. It revealed multiple metabolically active ill-defined dural based and intramedullary soft-tissue lesions and thickenings involving the spinal cord from D11–L5 vertebra, including cauda equine nerve roots-could be granulomatous/metastatic with no other metabolically active disease elsewhere. As a result, it was planned to tackle the lumbar pathology first. He underwent lower half L2–L5 laminectomy. On durotomy, the mass was found to be densely adhered with arachnoid filling up the entire diameter of the spinal canal and with densely adhered nerve roots. The lesion was pale pink with yellowish areas, mildly vascular, and partly suckable. At L2–L3 levels, on further dissection, a granuloma like lesion with pus-pockets was seen. Pus was aspirated and sent for acid-fast bacilli staining, and culture–sensitivity and decompression biopsy was attempted to prevent inadvertent damage to the entangled nerve roots (as visible in [Figure 3a and b]). Histopathological examination (HPE) of the resected mass revealed caseating granuloma with Langerhans giant cells suggestive of tubercular etiology. The pus reports suggested no growth. The patient was started on antitubercular medication. One month has passed, and the patient is showing significant improvement in his limb powers and bladder control. A postoperative MRI lumbar spine was done, showing [Figure 4] lesser contrast uptake than previously. Furthermore, no gross collection/CSF leak was there. The patient had an uneventful recovery from surgery.
Figure 1:
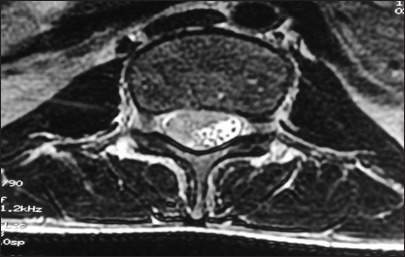
Axial sections showing diffuse intradural mass entrapping the nerve roots and features of compression with no pre-paravertebral collection/inflammation.
Figure 2:
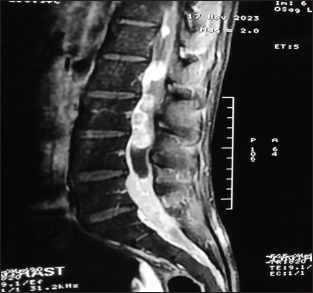
Sagittal sections contrast study suggesting long-segment intradural lesion involving nerve roots, cerebrospinal fluid like entrapments in between, and leptomeningeal enhancements at multiple sites with apparently normal intervertebral discs and paravertebral regions intraoperative image.
Figure 3:
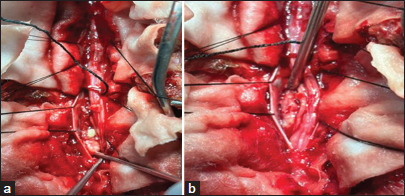
(a and b) Densely adhered nerve roots with granulation tissue and pus pocket visible inside on deeper dissection.
Figure 4:
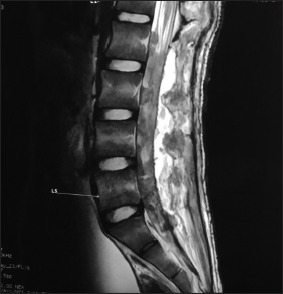
Postoperative contrast image with visible nerve roots at L1–L2 levels with comparatively lesser enhancement as compared to the preoperative state, and laminectomy defects visible. Arrow: L5 Vertebral level.
He is able to stand and walk with support. Furthermore, he is able to pass urine voluntarily better than previously (he still has to give mild suprapubic pressure for complete evacuation of the bladder).
Unusual characteristics outlined in prior reports regarding spinal TB include (1) spinal posterior element involvement while sparing the anterior column, (2) occurrence of skip lesions, and (3) compression of neural components due to TB granuloma. The pedicle is the most frequently affected site within the posterior element of the spine. Intradural TB has been referred to by various terms, including intradural intramedullary/extramedullary TB, spinal arachnoiditis, and chronic adhesive arachnoiditis. It has been proposed to classify all these atypical TB forms under the term “tuberculous radiculomyelopathy” (TBRM).[4,11]
TBRM may develop from three different sources.
Primary TB lesion arising in the spinal meninges
A downward extension from the intracranial TB meningitis
A secondary spread from adjacent vertebrae disease.
Among these, downward spread from TBM is the most common. The thoracic cord is more frequently involved, followed by lumbar and cervical cords.
TBRM passes through three stages.[1]
Radiculitis–inflammation of pia arachnoid with associated hyperemia and swelling of roots.
Arachnoiditis–progressive fibroblast proliferation and collagen deposition leading to nerve root adhesions to each other and pia arachnoid.
Adhesive arachnoiditis–dense collagen deposition with encapsulation of atrophied nerve roots. Ours was in the second stage as per the features stated, extending from D11–L5, with densely adhered but not atrophied nerve roots. Furthermore, no other focus of TB, either in the brain or lungs could be ascertained.
The clinical manifestations are mostly either monoradicular or polyradicular pain syndromes. Rapidly evolving cases have been documented rarely as it usually takes several years.[5] This rapid onset of symptoms, as happened in our case (<3 months) along with the brain lesions, led us to the misdiagnosis of intradural tumor/metastasis rather than arachnoiditis.
Classically, three MR patterns of arachnoiditis have been described involving cauda equina.[7]
Type 1: Central type–roots are clumped to the center of thecal sac
Type 2: Peripheral type–roots are adherent to the margins of the dural sac
Type 3: Adherent roots to one side of thecal sac resembling a soft-tissue mass.
MRI of our case is of type 3, showing adherent roots filling the entire subarachnoid space resembling the conus cauda tumor. Differential diagnoses of contrast enhancing tumors in the conus cauda lesions in this age group include myxopapillary ependymoma, schwannoma, and paraganglioma. Also, to add to the dilemma were apparently normal intervertebral discs, and no pre/paravertebral collection/or any other inflammatory changes. Hence, all the findings never pointed to tubercular etiology. Before surgery, the dura was thick, roots of the cauda equina were adherent to each other and to the overlying dura. The roots of cauda equina were entangled in the granulation tissue. Similar intraoperative findings have been reported by Takahashi et al.,[9] and Sree Harsha et al.,[8] for cases of spinal arachnoiditis.
The management of TBRM primarily involves medical treatment, typically lasting 9–12 months. Surgical intervention should be considered only when HPE confirmation is necessary or in cases of spinal cord compression with neurological deficits or spinal instability. Treatment for spinal tuberculous arachnoiditis may involve either medical or surgical approaches, though medical treatment remains the cornerstone. Anti-TB therapy, using a combination of drugs, should be initiated once the diagnosis is confirmed.[10] Previous studies have indicated promising outcomes with intrathecal hyaluronidase, an enzyme that breaks down hyaluronic acid and other mucopolysaccharides of the ground substance. High-dose corticosteroids serve as another effective adjunctive medical treatment, administered orally or, in rare cases, through the intrathecal route. The entire treatment regimen should span at least 9–12 months, aligning with recent TB updates, as longer courses have not shown significantly improved results in many trials and may increase the risk of drug-induced toxicities.[2,12]
The outcome of treatment has been unpredictable, depending on the response of ATT on the individual patients; however, mostly favorable results are seen.[9] Previous case studies, including ours, show good recovery after surgical decompression. A similar case of tubercular arachnoiditis was reported with good outcomes following subtotal decompression and ATD use; however, there were no suspicious brain findings like this.[6] Hence, surgery-decompressive laminectomy-should be considered if the histologic diagnosis is necessary or there is evidence of spinal cord compression with neurologic deficit or spinal instability.
CONCLUSION
Cases of tubercular arachnoiditis pose a great challenge to be diagnosed in the first look if there are no other foci of TB. Hence, such cases should be managed with high suspicion as they can be easily misdiagnosed to be tumors that may lead to mistreatment. MRI interpretation should be better, especially in endemic zones of TB, so that such cases are not missed, and collectively, we can contribute to achieving the TB eradication goal of the World Health Organization.
Footnotes
How to cite this article: Kumar J, Roy K, Chakraborty A, Patra R. Tubercular arachnoiditis: A rare culprit of paraparesis in a young adult – A case report and review of literature. Surg Neurol Int. 2024;15:432. doi: 10.25259/SNI_598_2024
Contributor Information
Jiwesh Kumar, Email: jjiwesh@gmail.com.
Kaushik Roy, Email: dr.kaushik@rediffmail.com.
Abhirup Chakraborty, Email: draabhirup@gmail.com.
Ritankar Patra, Email: rpatra760@gmail.com.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Burton CV. Lumbosacral arachnoiditis. Spine (Phila Pa 1976) 1978;3:24–30. doi: 10.1097/00007632-197803000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Gourie-Devi M, Satishchandra P. Hyaluronidase as an adjuvant in the management of tuberculous spinal arachnoiditis. J Neurol Sci. 1991;102:105–11. doi: 10.1016/0022-510x(91)90100-l. [DOI] [PubMed] [Google Scholar]
- 3.Guleria R, Kavitha Central nervous system tuberculosis. Indian J Tuberc. 2014;61:195–9. [PubMed] [Google Scholar]
- 4.Hasegawa K, Murata H, Naitoh K, Nagano A. Spinal tuberculosis: Report of an atypical presentation. Clin Orthop Relat Res. 2002;403:100–103. [PubMed] [Google Scholar]
- 5.Hoffman GS. Spinal arachnoiditis: What is the clinical spectrum? Spine (Phila Pa 1976) 1983;8:538–40. [PubMed] [Google Scholar]
- 6.Konar SK, Rao KN, Mahadevan A, Devi BI. Tuberculous lumbar arachnoiditis mimicking conus cauda tumor: A case report and review of literature. J Neurosci Rural Pract. 2011;2:93–6. doi: 10.4103/0976-3147.80098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross J, Masaryk T, Modic M, Delamater R, Bohlman H, Wilbur G, et al. MR imaging of lumbar arachnoiditis. AJR Am J Roentgenol. 1987;149:1025–32. doi: 10.2214/ajr.149.5.1025. [DOI] [PubMed] [Google Scholar]
- 8.Sree Harsha CK, Shetty AP, Rajasekaran S. Intradural spinal tuberculosis in the absence of vertebral or meningeal tuberculosis: A case report. J Orthop Surg (Hong Kong) 2006;14:71–5. doi: 10.1177/230949900601400116. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi H, Ito S, Kojima S, Tanno T, Hattori T. Intradural extramedullary tuberculoma of the thoracic spine: Paradoxical response to antituberculous therapy. Intern Med. 2008;47:797–8. doi: 10.2169/internalmedicine.47.0839. [DOI] [PubMed] [Google Scholar]
- 10.Vidyasagar C, Murthy HK. Spinal tuberculosis with neurological deficits. Natl Med J India. 1996;9:25–7. [PubMed] [Google Scholar]
- 11.Wadia NH, Dastur DK. Spinal meningitides with radiculomyelopathy: Clinical and radiological features. J Neurol Sci. 1969;8:239–60. doi: 10.1016/0022-510x(69)90112-9. [DOI] [PubMed] [Google Scholar]
- 12.WHO consolidated guidelines on tuberculosis . Geneva: World Health Organization; 2021. Module 2: Screening-systematic screening for tuberculosis disease. Available from: https://www.who.int/publications/i/item/9789240022676 [Last accessed on 2024 Jul 20] [PubMed] [Google Scholar]


