Abstract
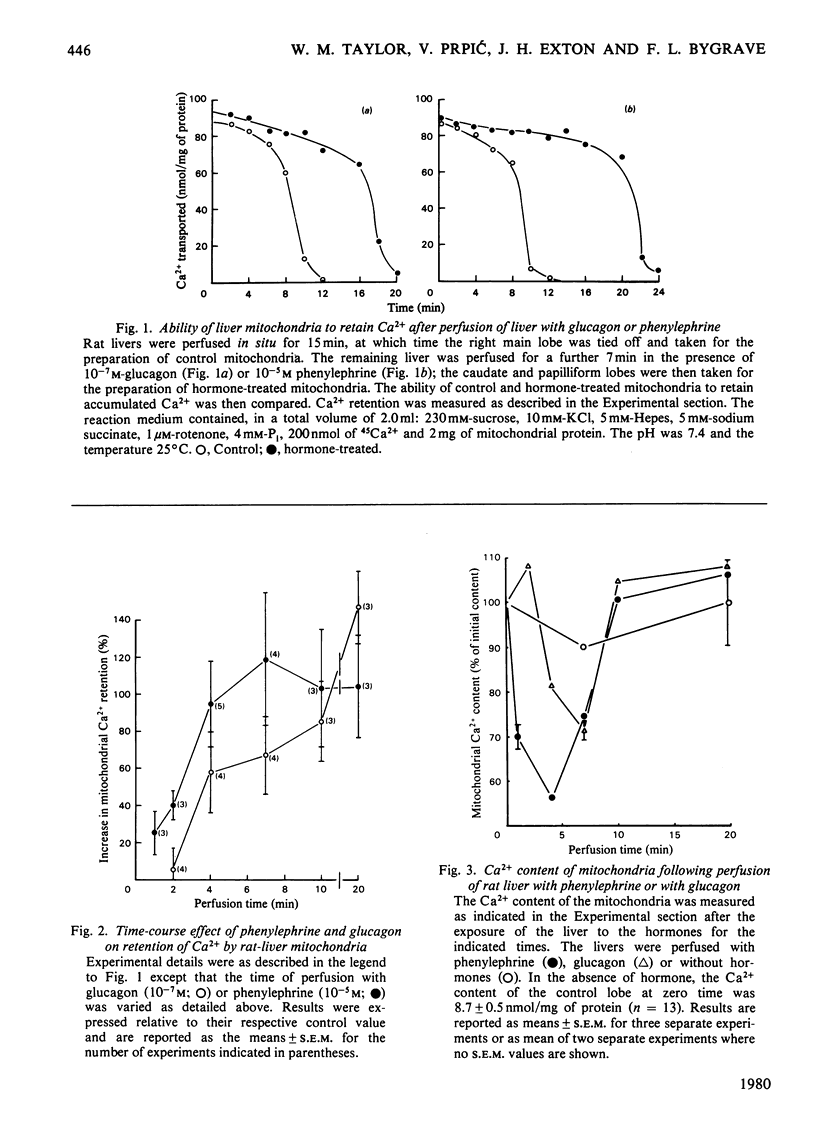
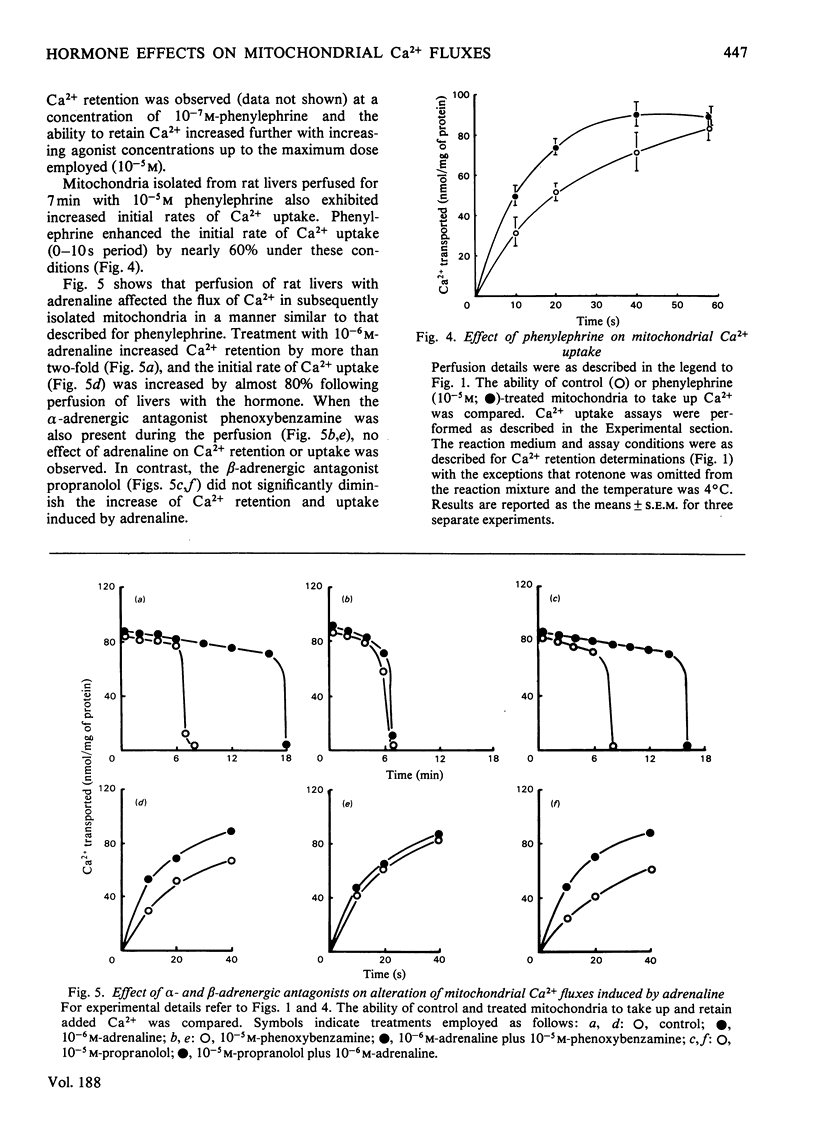
Mitochondria isolated from rat liver after a short-term perfusion with the α-adrenergic agonist phenylephrine or with glucagon exhibited enhanced rates of uptake of Ca2+ and prolonged retention of Ca2+ in the presence of 4mm-Pi. The effect of Ca2+ retention was apparent after perfusion with phenylephrine for only 1min and was maximal after 7min of treatment. The changes induced by glucagon, although similar, were less rapid. Adrenaline caused similar changes to phenylephrine and its effects were blocked by the α-adrenergic antagonist phenoxybenzamine, but not by the β-antagonist propranolol. The Ca2+ content of the isolated mitochondria decreased by 30% 1min after the onset of perfusion with phenylephrine; by 6min it had begun to return to the original value which was reached at 10min. A similar loss in calcium content was induced by glucagon but the changes were not as great and occurred more slowly. Mitochondria from phenylephrine-treated livers exhibited decreased rates of Ca2+ efflux induced by addition of 2mm-EGTA, a 50% increase in the contents of ADP and total adenine nucleotides, a small increase in the transmembrane pH gradient, and a reduced rate of oxaloacetate-induced NADPH oxidation. This study thus shows that stimulation of liver by α-adrenergic agonists, like that by glucagon, induces within minutes a stable modification of mitochondria leading to alterations in the Ca2+-translocation cycle (increased Ca2+ uptake and retention) and alterations in mitochondrial energy-linked reactions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- BRADLEY L. B., JACOB M., JACOBS E. E., SANADI D. R. Uncoupling of oxidative phosphorylation by cadmium ion. J Biol Chem. 1956 Nov;223(1):147–156. [PubMed] [Google Scholar]
- Blackmore P. F., Brumley F. T., Marks J. L., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Relationship between alpha-adrenergic stimulation of calcium efflux and activation of phosphorylase in isolated rat liver parenchymal cells. J Biol Chem. 1978 Jul 25;253(14):4851–4858. [PubMed] [Google Scholar]
- Blackmore P. F., Dehaye J. P., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. The role of mitochondrial calcium release in alpha-adrenergic activation of phosphorylase in perfused rat liver. J Biol Chem. 1979 Aug 10;254(15):6945–6950. [PubMed] [Google Scholar]
- Blackmore P. F., Dehaye J. P., Strickland W. G., Exton J. H. alpha-Adrenergic mobilization of hepatic mitochondrial calcium. FEBS Lett. 1979 Apr 1;100(1):117–120. doi: 10.1016/0014-5793(79)81144-8. [DOI] [PubMed] [Google Scholar]
- Bryla J., Harris E. J., Plumb J. A. The stimulatory effect of glucagon and dibutyryl cyclic AMP on ureogenesis and gluconeogenesis in relation to the mitochondrial ATP content. FEBS Lett. 1977 Aug 15;80(2):443–448. doi: 10.1016/0014-5793(77)80494-8. [DOI] [PubMed] [Google Scholar]
- Bygrave F. L., Heaney T. P., Ramachandran C. Submitochondrial location of ruthenium red-sensitive calcium-ion transport and evidence for its enrichment in a specific population of rat liver mitochondria. Biochem J. 1978 Sep 15;174(3):1011–1019. doi: 10.1042/bj1741011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L. Mitochondria and the control of intracellular calcium. Biol Rev Camb Philos Soc. 1978 Feb;53(1):43–79. doi: 10.1111/j.1469-185x.1978.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Assimacopoulos F. D., Harper S. C., Corbin J. D., Park C. R., Exton J. H. Studies on the alpha-andrenergic activation of hepatic glucose output. II. Investigation of the roles of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in the actions of phenylephrine in isolated hepatocytes. J Biol Chem. 1976 Sep 10;251(17):5209–5218. [PubMed] [Google Scholar]
- Dorman D. M., Barritt G. J., Bygrave F. L. Stimulation of hepatic mitochondrial calcium transport by elevated plasma insulin concentrations. Biochem J. 1975 Sep;150(3):389–395. doi: 10.1042/bj1500389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J. C. The effects of glucagon, catecholamines, and the calcium ionophore A23187 on the phosphorylation of rat hepatocyte cytosolic proteins. J Biol Chem. 1978 Oct 10;253(19):7091–7100. [PubMed] [Google Scholar]
- Halestrap A. P. Stimulation of the respiratory chain of rat liver mitochondria between cytochrome c1 and cytochrome c by glucagon treatment of rats. Biochem J. 1978 Jun 15;172(3):399–405. doi: 10.1042/bj1720399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson N. J., Brumley F. T., Assimacopoulos F. D., Harper S. C., Exton J. H. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem. 1976 Sep 10;251(17):5200–5208. [PubMed] [Google Scholar]
- Lehninger A. L., Vercesi A., Bababunmi E. A. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Prpić V., Spencer T. L., Bygrave F. L. Stable enhancement of calcium retention in mitochondria isolated from rat liver after the administration of glucagon to the intact animal. Biochem J. 1978 Dec 15;176(3):705–714. doi: 10.1042/bj1760705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. Methodology for in vitro studies of Ca-2+ transport. Anal Biochem. 1975 Jul;67(1):44–54. doi: 10.1016/0003-2697(75)90270-5. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974 May;140(2):143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZARKOWSKA L., KLINGENBERG M. ON THE ROLE OF UBIQUINONE IN MITOCHONDRIA. SPECTROPHOTOMETRIC AND CHEMICAL MEASUREMENTS OF ITS REDOX REACTIONS. Biochem Z. 1963;338:674–697. [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Isolated hepatocytes as a model for the study of stable glucagon effects on mitochondrial respiratory functions. FEBS Lett. 1979 May 15;101(2):277–281. doi: 10.1016/0014-5793(79)81025-x. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K. Glucagon stimulation of mitochondrial respiration. J Biol Chem. 1975 Oct 10;250(19):7924–7930. [PubMed] [Google Scholar]
- Yamazaki R. K., Sax R. D., Hauser M. A. Glucagon stimulation of mitochondrial ATPase and potassium ion transport. FEBS Lett. 1977 Mar 15;75(1):295–299. doi: 10.1016/0014-5793(77)80106-3. [DOI] [PubMed] [Google Scholar]


