Abstract
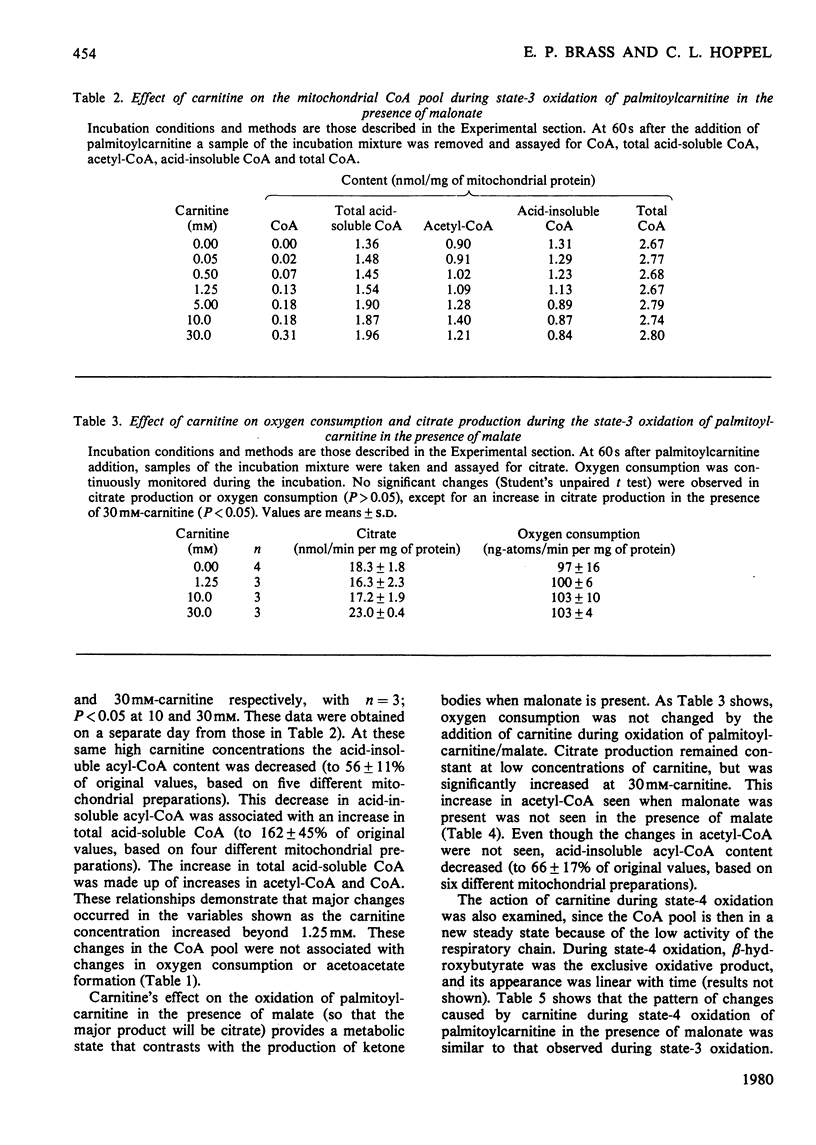
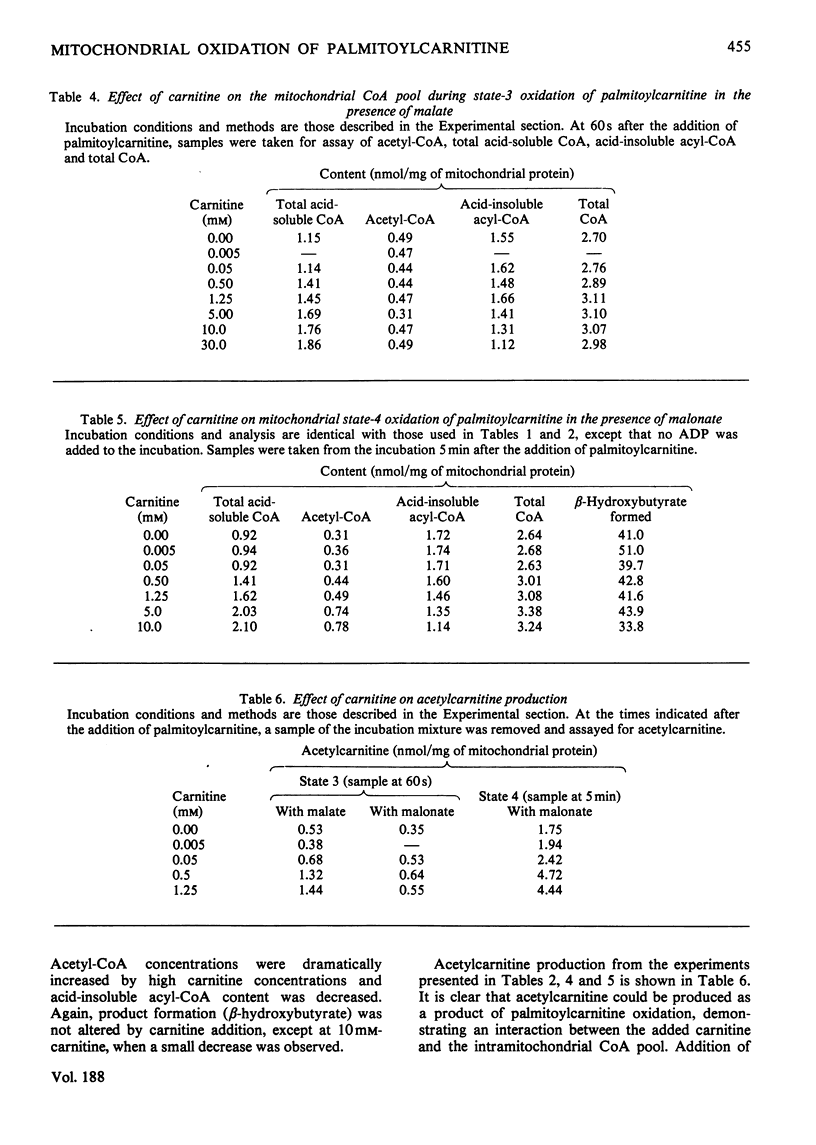
The effects of carnitine on the metabolism of palmitoylcarnitine were studied by using isolated rat liver mitochondria. Particular attention was given to carnitine acyltransferase-mediated interactions between carnitine and the mitochondrial CoA pool. Carnitine concentrations less than 1.25mm resulted in an increased production of acetylcarnitine during palmitoylcarnitine oxidation. Despite this shunting of C2 units to acetylcarnitine formation, no change was observed in the rate of oxygen consumption or major product formation (citrate or acetoacetate). Further, no changes were observed in the mitochondrial content of acetyl-CoA, total acid-soluble CoA or acid-insoluble acyl-CoA. These observations support the concept, based on studies in vivo, that the carnitine/acylcarnitine pool is metabolically sluggish and the acyl-group flux low as compared with the CoA/acyl-CoA pool. Acid-insoluble acyl-CoA content was decreased and CoA content increased at carnitine concentrations greater than 1.25mm. When [14C]carnitine was used in the incubations, it was demonstrated that this resulted from acid-insoluble acylcarnitine formation from intramitochondrial acid-insoluble acyl-CoA mediated by carnitine palmitoyltransferase B. Again, the higher carnitine concentrations resulted in no changes in the rates of oxygen consumption or major product formation. The above effects of carnitine were observed whether citrate or acetoacetate was the major product of oxidation. In contrast, an increase in acetyl-CoA concentration was observed at high carnitine concentrations only when acetoacetate was the product. Since the rate of acetoacetate production was not changed, these higher acetyl-CoA concentrations suggest that a new steady state had been established to maintain acetoacetate-production rates. Since there was no change in acetyl-CoA concentration when citrate was the major product, a change in the activity of the pathway utilizing acetyl-CoA for ketone-body synthesis and the potential regulation of this pathway must be considered.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred J. B., Guy D. G. Determination of coenzyme A and acetyl CoA in tissue extracts. Anal Biochem. 1969 May;29(2):293–299. doi: 10.1016/0003-2697(69)90312-1. [DOI] [PubMed] [Google Scholar]
- BREMER J. Carnitine in intermediary metabolism. The metabolism of fatty acid esters of carnitine by mitochondria. J Biol Chem. 1962 Dec;237:3628–3632. [PubMed] [Google Scholar]
- Brass E. P., Hoppel C. L. Carnitine metabolism in the fasting rat. J Biol Chem. 1978 Apr 25;253(8):2688–2693. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Choi Y. R., Fogle P. J., Clarke P. R., Bieber L. L. Quantitation of water-soluble acylcarnitines and carnitine acyltransferases in rat tissues. J Biol Chem. 1977 Nov 25;252(22):7930–7931. [PubMed] [Google Scholar]
- DiMarco J. P., Hoppel C. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. J Clin Invest. 1975 Jun;55(6):1237–1244. doi: 10.1172/JCI108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITZ I. B., YUE K. T. LONG-CHAIN CARNITINE ACYLTRANSFERASE AND THE ROLE OF ACYLCARNITINE DERIVATIVES IN THE CATALYTIC INCREASE OF FATTY ACID OXIDATION INDUCED BY CARNITINE. J Lipid Res. 1963 Jul;4:279–288. [PubMed] [Google Scholar]
- Lee L. P., Fritz I. B. Factors controlling ketogenesis by rat liver mitochondria. Can J Biochem. 1972 Feb;50(2):120–127. doi: 10.1139/o72-015. [DOI] [PubMed] [Google Scholar]
- Lopes-Cardozo M., Klazinga W., van den Bergh S. G. Accumulation of carnitine esters of beta-oxidation intermediates during palmitate oxidation by rat-liver mitochondria. Eur J Biochem. 1978 Feb;83(2):629–634. doi: 10.1111/j.1432-1033.1978.tb12132.x. [DOI] [PubMed] [Google Scholar]
- Olsen C. An enzymatic fluorimetric micromethod for the determination of acetoacetate, -hydroxybutyrate, pyruvate and lactate. Clin Chim Acta. 1971 Jul;33(2):293–300. doi: 10.1016/0009-8981(71)90486-4. [DOI] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Effect of carnitine on branched-chain amino acid oxidation by liver and skeletal muscle. Am J Physiol. 1978 May;234(5):E494–E499. doi: 10.1152/ajpendo.1978.234.5.E494. [DOI] [PubMed] [Google Scholar]
- Pearson D. J., Tubbs P. K. Carnitine and derivatives in rat tissues. Biochem J. 1967 Dec;105(3):953–963. doi: 10.1042/bj1050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrede S., Bremer J. The compartmentation of CoA and fatty acid activating enzymes in rat liver mitochondria. Eur J Biochem. 1970 Jul;14(3):465–472. doi: 10.1111/j.1432-1033.1970.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Stokke O., Bremer J. A simple method for preparation of methyl-labelled (-) carnitine. Biochim Biophys Acta. 1970 Dec 15;218(3):552–554. doi: 10.1016/0005-2760(70)90021-4. [DOI] [PubMed] [Google Scholar]


