Abstract
Background:
Rheumatoid arthritis (RA) is complicated with interstitial lung disease (ILD). Gastroesophageal reflux disease is prevented by Helicobacter pylori infection and is a predisposing factor for idiopathic pulmonary fibrosis. However, the prevalence of H. pylori infection in RA patients with ILD has not been sufficiently investigated.
Objective:
In this study, we analyzed anti-H. pylori antibodies in RA patients with ILD.
Design:
Case-control observational study
Methods:
Anti-H. pylori antibodies were analyzed in the sera of RA patients using a commercially available enzyme-linked immunosorbent assay kit.
Results:
The positivity of anti-H. pylori antibodies in RA with ILD (n = 30 [18.0%], P = .0227), usual interstitial pneumonia (n = 10 [14.3%], P = .0212), and airway disease (n = 30 [18.0%], P = .0227) was significantly lower than that of RA without chronic lung disease (n = 78 [27.5%]). The positivity of anti-H. pylori antibodies was also lower in RA with chronic lung disease (n = 68 [18.2%], P = .0059). Multiple logistic regression analyses showed that the presence of anti-H. pylori antibodies was independently and protectively associated with chronic lung disease in RA.
Conclusion:
The seroprevalence of H. pylori was lower in RA with ILD. H. pylori infection prevented ILD in patients with RA by protecting them from gastroesophageal reflux disease.
Keywords: Rheumatoid arthritis, anti-Helicobacter pylori antibodies, interstitial lung disease, seroprevalence, gastroesophageal reflux disease
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by the destruction of synovial joints; 1 although the pathogenesis of RA is unknown, it is believed to involve genetic and environmental factors. RA is complicated with extra-articular manifestations including chronic lung diseases (CLD), pleuritis, pericarditis, or vasculitis. CLD in RA mainly includes airway disease (AD), interstitial lung disease (ILD), and emphysema. 2 The complication of ILD or AD in RA confers a poor prognosis on the patient.3 -7 Thus, it is necessary to elucidate risk factors for ILD or AD in RA.
Helicobacter pylori is a gram-negative bacterium and childhood infections persist unless eradication is conducted. Chronic gastric infection by H. pylori causes peptic ulcers, 8 gastric adenocarcinoma, 9 and lymphoma. 10 However, H. pylori infection reduced the risk of asthma, 11 gastroesophageal reflux disease,12,13 and cardiovascular disease.14,15 H. pylori infection might also influence the risk of autoimmune diseases including RA, although this is still controversial.16 -23 It was suggested that the eradication of H. pylori might reduce the severity of RA.24,25 Although gastroesophageal reflux disease is a risk factor for idiopathic pulmonary fibrosis,26 -29 the prevalence of H. pylori in idiopathic pulmonary fibrosis was comparable.30,31 Furthermore, the prevalence of H. pylori in patients with AD or chronic obstructive pulmonary disease was increased.32 -34 This study aims to investigate the seroprevalence of H. pylori in RA with ILD.
Of note, the prevalence of H. pylori infection in the young is decreasing in industrialized countries, resulting in a reduced incidence of gastric cancer.35 -37 Higher anti-H. pylori antibody levels have been detected in sera from H. pylori-infected individuals, and anti-H. pylori antibody levels were decreased several months after eradication by antimicrobial therapy. 38 Thus, the measurement of serum anti-H. pylori antibodies can be used to reflect H. pylori infection. However, there are few reports on the seroprevalence of H. pylori in RA with ILD. In the present study, we investigated the seroprevalence of H. pylori in RA with ILD.
Material and Methods
Patients
A total of 657 patients with RA and 52 healthy controls (healthy individuals, mean age ± standard deviation: 35.4 ± 11.1, male number: 2 [3.8%], no matching or selection was performed.) were recruited at NHO Sagamihara National Hospital, NHO Tokyo National Hospital, NHO Miyakonojo Medical Center, NHO Himeji Medical Center, NHO Nagoya Medical Center, and NHO Nagasaki Medical Center. All the patients with RA fulfilled the Rheumatoid Arthritis Classification Criteria 39 or American College of Rheumatology criteria for RA. 40 RA patients with computed tomography images were recruited. A diagnosis of usual interstitial pneumonia (UIP, irregular linear opacities and honeycombing), nonspecific interstitial pneumonia (NSIP, bilateral ground-glass attenuation patterns predominantly in subpleural and basal regions), AD (centrilobular or peribronchial nodules, branching linear structures, bronchial dilatation, bronchial wall thickening, or atelectasis), emphysema (low attenuation area or bullae), or no CLD [CLD(−), no abnormalities in computed tomography images] was determined based on the predominant findings of chest computed tomography images of RA patients.41 -43 The CLD(+) group included patients with UIP, NSIP, AD, and emphysema. The ILD group included patients with UIP and NSIP. Steinbrocker stages of the RA patients were evaluated as previously described. 44 Because the collection of the sera was not conducted in the same year and the seroprevalence of H. pylori in the young generations is decreasing, birth year was used in the analyses.
This study was conducted in accordance with the principles expressed in the Declaration of Helsinki and was approved by the Research Ethics Committees of NHO Tokyo National Hospital (190010, 29 May 2019), NHO Sagamihara National Hospital, and the Central Institutional Review Board of the National Hospital Organization. Written informed consent was obtained from each participant.
Measurement of serum anti-H. pylori antibodies
Serum anti-H. pylori antibodies were detected by a commercially available enzyme-linked immunosorbent assay (ELISA) kit (E plate ‘Eiken’ H. pylori antibody II, Eiken Chemical Co., Ltd., Tokyo, Japan), according to the manufacturer’s instructions. The recommended cut-off level of the kit is 10 U/mL. The standard anti-H. pylori antibodies (3, 10, 30, 100 U/mL) and the horseradish peroxidase-labeled mouse anti-human IgG monoclonal antibody were used in the ELISA kit (https://www.info.pmda.go.jp/downfiles/ivd/PDF/170005_22200AMX00935000_A_01_03.pdf).
Statistical analysis
The presence of anti-H. pylori antibodies was compared with CLD(−) RA patients by Fisher’s exact test using 2 × 2 contingency tables. The clinical characteristics of RA patients with or without anti-H. pylori antibodies were compared by Student’s t-test or Fisher’s exact test using 2 × 2 contingency tables. Multiple logistic regression analysis was conducted to evaluate whether the presence of anti-H. pylori antibodies was independently associated with CLD or RA from other clinical information, including birth year, sex, Steinbrocker stage, and smoking status. Statistical significance was considered to be two-sided P-values less than .05.
Results
Detection of anti-H. pylori antibodies in RA patients and controls
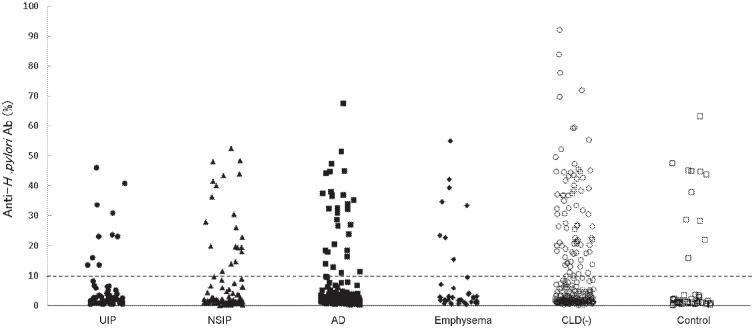
Titers of anti-H. pylori antibodies were measured in the sera of RA patients (Figure 1). The presence of anti-H. pylori antibodies in each group was compared with those in CLD(−) RA patients (Table 1). The positivity of anti-H. pylori antibodies in RA with ILD (n = 30 [18.0%], P = .0227), UIP (n = 10 [14.3%], P = .0212), and AD (n = 30 [18.0%], P = .0227) was significantly lower than that in CLD(−) (n = 78 [27.5%]). The positivity of anti-H. pylori antibodies was also lower in RA with CLD (n = 68 [18.2%], P = .0059). Anti-H. pylori antibody levels in RA with CLD were also analyzed by Student’s t-test and were lower when compared with CLD(−) RA (mean ± SD, 7.3 ± 12.2 vs 10.6 ± 16.4 U/mL, P = .0048). Thus, the positivity of anti-H. pylori antibody was lower in RA with ILD, UIP, AD, or CLD.
Figure 1.
Anti-H. pylori antibody levels in RA patients and controls.
Abbreviations: AD, airway disease; CLD, chronic lung disease; NSIP, nonspecific interstitial pneumonia; RA, rheumatoid arthritis; UIP, usual interstitial pneumonia.
The distribution of anti-H. pylori antibody levels is shown. Filled circles, filled triangles, filled squares, filled diamonds, empty circles, and empty squares represent RA with UIP, RA with NSIP, RA with AD, RA with emphysema, RA without CLD, and controls, respectively. A dotted line indicates the recommended cut-off level of the kit.
Table 1.
The positivity of anti-H. pylori antibody in RA patients.
| n | Anti-H. pylori antibody positive, n (%) | P-value | |
|---|---|---|---|
| ILD | 167 | 30 (18.0) | .0227 |
| UIP | 70 | 10 (14.3) | .0212 |
| NSIP | 97 | 20 (20.6) | .2259 |
| AD | 167 | 30 (18.0) | .0227 |
| Emphysema | 39 | 8 (20.5) | .4416 |
| CLD(+) | 373 | 68 (18.2) | .0059 |
| CLD(−) | 284 | 78 (27.5) | |
| Total RA | 657 | 146 (22.2) |
Abbreviations: AD, airway disease; CLD, chronic lung disease; ILD, interstitial lung disease; NSIP, nonspecific interstitial pneumonia; RA, rheumatoid arthritis; UIP, usual interstitial pneumonia.
ILD group includes UIP and NSIP groups. The CLD(+) group includes UIP, NSIP, AD, and emphysema patients. The positive number in each group is shown. Percentages are shown in parentheses. Differences compared with the CLD(−) population were tested by Fisher’s exact test using 2 × 2 contingency tables.
Clinical characteristics of RA patients with or without anti-H. pylori antibodies
A potential association of the clinical characteristics of RA patients with the presence of anti-H. pylori antibodies was analyzed (Table 2). CLD was associated with the absence of anti-H. pylori antibodies (P = .0059) whereas other clinical manifestations were not associated with the presence or absence of anti-H. pylori antibodies.
Table 2.
Association of anti-H. pylori antibody positivity with the clinical characteristics of RA.
| Clinical manifestations | Total RA (n = 657) | Anti-H. pylori antibody (+) RA (n = 146) | Anti-H. pylori antibody (−) RA (n = 511) | P-value |
|---|---|---|---|---|
| Age, years (SD) | 65.4 (11.1) | 65.0 (9.8) | 65.5 (11.5) | *.5483 |
| Birth year, years (SD) | 1947 (10.6) | 1946 (9.7) | 1947 (10.9) | *.3937 |
| Male n (%) | 155 (23.6) | 29 (19.9) | 126 (24.7) | .2691 |
| Age at onset, years (SD) | 52.3 (15.0) | 53.2 (14.2) | 52.1 (15.2) | *.4234 |
| Steinbrocker stage 3, 4, n (%) | 318 (49.5) | 61 (42.4) | 257 (51.5) | .0586 |
| Underweight (body mass index <18.5 kg/m2), n (%) | 494 (82.1) | 121 (87.7) | 373 (80.4) | .0576 |
| Smoker or past smoker, n (%) | 239 (39.6) | 59 (42.8) | 180 (38.7) | .5448 |
| CLD (+), n (%) | 373 (56.8) | 68 (46.6) | 305 (59.7) | .0059 |
| Rheumatoid factor positive, n (%) | 567 (86.3) | 123 (84.2) | 444 (86.9) | .4146 |
| Anti-citrullinated peptide antibody positive, n (%) | 592 (90.1) | 134 (91.8) | 458 (89.6) | .5305 |
| Krebs von den lungen-6 positive, n (%) | 115 (25.4) | 21 (24.1) | 94 (25.8) | .8911 |
| Surfactant protein-D positive, n (%) | 98 (25.3) | 17 (22.7) | 81 (26.0) | .6577 |
Abbreviations: CLD, chronic lung disease; RA, rheumatoid arthritis; SD, standard deviation.
The CLD(+) group includes usual interstitial pneumonia, nonspecific interstitial pneumonia, airway disease, and emphysema patients. The number or mean of each group is shown. Percentages or SDs are shown in parentheses. Differences between anti-H. pylori antibody(+) and (−) RA populations were tested with Student’s t-test or Fisher’s exact test using 2 × 2 contingency tables.
Student’s t-test was used.
Multiple logistic regression analysis of anti-H. pylori antibodies
Multiple logistic regression analysis was conducted to exclude the influence of clinical characteristics on the protective association of the presence of anti-H. pylori antibodies with CLD (Table 3). In univariate analysis, the presence of anti-H. pylori antibodies was significantly associated with CLD (P = .0050, odds ratio [OR] 0.59, 95% confidence interval [CI] 0.41, 0.85). The association of the presence of anti-H. pylori antibodies remained significant (Padjusted = .0022, ORadjusted 0.53, 95% CI 0.35, 0.79), when conditioned on the other clinical characteristics. Thus, this suggested that the presence of anti-H. pylori antibodies was independently and protectively associated with CLD in RA.
Table 3.
Multiple logistic regression analysis of anti-H. pylori antibody positivity and clinical manifestations of CLD in RA.
| Clinical manifestations | Unconditioned | Conditioned on other factors | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P-value | ORadjusted | 95%CI | P adjusted | |
| Anti-H. pylori antibody, positive | 0.59 | (0.41, 0.85) | .0050 | 0.50 | (0.33, 0.76) | .0012 |
| Birth year, years | 0.96 | (0.95, 0.98) | 1.03 × 10−6 | 0.95 | (0.94, 0.97) | 1.28 × 10−7 |
| Male | 1.83 | (1.25, 2.67) | .0019 | 1.32 | (0.84, 2.08) | .2239 |
| Steinbrocker stage | 0.84 | (0.74, 0.95) | .0073 | 0.83 | (0.72, 0.96) | .0141 |
| Smoking status | 1.41 | (1.13, 1.76) | .0025 | 1.53 | (1.20, 1.96) | .0006 |
Abbreviations: CI, confidence interval; CLD, chronic lung diseases; OR, odds ratio; RA, rheumatoid arthritis.
P, OR, 95%CI, Padjusted, and ORadjusted were calculated by logistic regression analysis on RA patients.
Comparison of the presence of anti-H. pylori antibodies between RA patients and controls
The presence of anti-H. pylori antibodies was compared between RA patients and healthy controls (Supplementary Table S1), and no difference was revealed. In order to exclude the effects of other clinical features including birth year and sex on the association between the presence of anti-H. pylori antibodies and RA, multiple logistic regression analysis was conducted, and no association was detected. Thus, no association was observed between the presence of anti-H. pylori antibodies and RA.
Discussion
In this study, the positivity of anti-H. pylori antibodies in RA with ILD, UIP, and AD was lower compared with RA without CLD. We also revealed that the presence of anti-H. pylori antibodies was independently and protectively associated with CLD in RA. However, the presence of anti-H. pylori antibodies was not associated with RA, per se.
In some previous studies, the prevalence of H. pylori infection was comparable between RA patients and healthy individuals.16 -18,45 However, it was decreased in another study. 22 Furthermore, H. pylori infection increased the risk of RA in women less than 30 years old. 23 In the present study, the seroprevalence of H. pylori in RA was similar to that in healthy individuals, suggesting no association between H. pylori infection and RA, per se.
It is known that H. pylori infection prevents gastroesophageal reflux disease by decreasing gastric acid secretion.12,13 Gastroesophageal reflux disease with microaspiration was revealed to be a predisposing factor for idiopathic pulmonary fibrosis.26,27 Thus, the prevalence of H. pylori in idiopathic pulmonary fibrosis was estimated to be lower; however, it was not significantly lower than that in healthy controls, though there was a lower trend.30,31 In our study, the seroprevalence of H. pylori in RA patients with ILD was significantly lower compared with RA without CLD. Thus, H. pylori infection might prevent the development of ILD in RA probably by protecting patients from gastroesophageal reflux disease.
The prevalence of H. pylori in patients with AD was increased in previous studies.32,33 However, in this study, the seroprevalence of H. pylori in RA patients with AD was significantly lower compared with RA without CLD. These data suggested that the pathogenesis of AD in RA is different from that in AD without RA.
It was reported that the prevalence of H. pylori infection was influenced by birth year36,37 suggesting the associations between the presence of anti-H. pylori antibodies and CLD in our study could be influenced by birth year. After the effects of birth year were adjusted for in multiple logistic regression analyses, the associations between the presence of anti-H. pylori antibodies and CLD remained significant. Thus, the presence of anti-H. pylori antibody was independently and protectively associated with CLD in RA.
To the best of our knowledge, this is the first study to report the seroprevalence of H. pylori in RA patients with ILD. The results of the study indicated the lower seroprevalence of H. pylori in RA patients with ILD, UIP, AD, or CLD. This study includes some limitations. The sample size of the study was modest and this study was performed in Japanese populations. Larger-scale studies must be performed in different populations to replicate the results. Because information on gastroesophageal reflux disease in RA patients was not obtained in this study, the potential confounding effects of gastroesophageal reflux disease could not be excluded. The potential correlations between H. pylori infection, gastroesophageal reflux disease, and ILD in RA patients should be investigated in future studies with sufficient clinical information.
Conclusion
The seroprevalence of H. pylori was lower in RA with ILD. H. pylori infection prevented ILD in patients with RA by protecting them from gastroesophageal reflux disease.
Abbreviations
AD: airway disease
CLD: chronic lung diseases
ELISA : enzyme-linked immunosorbent assay
ILD: interstitial lung disease
NSIP: nonspecific interstitial pneumonia
RA: rheumatoid arthritis
UIP: usual interstitial pneumonia
Supplemental Material
Supplemental material, sj-docx-1-bmi-10.1177_11772719241297171 for Helicobacter pylori Seroprevalence in Rheumatoid Arthritis Patients with Interstitial Lung Disease by Shomi Oka, Takashi Higuchi, Hiroshi Furukawa, Kota Shimada, Akira Okamoto, Misuzu Fujimori, Atsushi Hashimoto, Akiko Komiya, Koichiro Saisho, Norie Yoshikawa, Masao Katayama, Toshihiro Matsui, Naoshi Fukui, Kiyoshi Migita and Shigeto Tohma in Biomarker Insights
Acknowledgments
None.
Declarations
Ethics approval and consent to participate: This study was conducted in accordance with the principles expressed in the Declaration of Helsinki and was approved by the Research Ethics Committees of NHO Tokyo National Hospital (190010, 29 May 2019), NHO Sagamihara National Hospital, and the Central Institutional Review Board of the National Hospital Organization. Written informed consent was obtained from each participant.
Consent for publication: Not applicable.
Author contributions: HF and ST conceived and designed the experiments. SO, TH, and HF performed the experiments. SO and HF analyzed the data. HF, KS, AO, MF, AH, AK, KS, NY, MK, TM, NF, KM, and ST contributed reagents/materials/analysis tools. SO, HF, and ST contributed to the writing of the manuscript. All authors read and approved the final version of the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by Health and Labour Science Research Grants from the Ministry of Health, Labour, and Welfare of Japan, Grants-in-Aid for Scientific Research (B, C) (22591090, 26293123, 15K09543, 18K08402) and for Young Scientists (B) (24791018) from the Japan Society for the Promotion of Science, Grants-in-Aid of the Practical Research Project for Allergic Diseases and Immunology (Research on Allergic Diseases and Immunology) from the Japan Agency for Medical Research and Development, Grants-in-Aid for Clinical Research from the National Hospital Organization, Research Grants from the Daiwa Securities Health Foundation, Research Grants from the Japan Research Foundation for Clinical Pharmacology, Research Grants from The Nakatomi Foundation, Research Grants from the Takeda Science Foundation, Research Grants from the Mitsui Sumitomo Insurance Welfare Foundation, and Bristol-Myers K.K. RA Clinical Investigation Grant from Bristol-Myers Squibb Co. Research grants were also received from the following pharmaceutical companies: Abbott Japan Co., Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Merck Sharp and Dohme Inc., Pfizer Japan Inc., Takeda Pharmaceutical Company Limited, and Teijin Pharma Limited.
Competing interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HF has the following conflicts of interest and the following funders are supported wholly or in part by the indicated pharmaceutical companies. The Japan Research Foundation for Clinical Pharmacology is run by Daiichi Sankyo, the Takeda Science Foundation is supported by an endowment from Takeda Pharmaceutical Company and the Nakatomi Foundation was established by Hisamitsu Pharmaceutical Co. The Daiwa Securities Health Foundation was established by Daiwa Securities Group Inc. and Mitsui Sumitomo Insurance Welfare Foundation was established by Mitsui Sumitomo Insurance Co., Ltd. HF was supported by research grants from Bristol-Myers Squibb Co. HF received honoraria from Ajinomoto Co., Inc., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Company, Luminex Japan Corporation Ltd, and Ayumi Pharmaceutical Corporation. ST was supported by research grants from the following pharmaceutical companies: Abbott Japan Co., Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Merck Sharp and Dohme Inc., Pfizer Japan Inc., Takeda Pharmaceutical Company Limited, and Teijin Pharma Limited. ST received honoraria from Asahi Kasei Pharma Corporation, Astellas Pharma Inc., AbbVie GK., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Pfizer Japan Inc. The other authors have no financial or commercial conflict of interest to declare.
Availability of data and materials: Data supporting the findings of the present study are presented in the paper and the supplementary file. Other data are available from the corresponding author upon reasonable request. However, the clinical information and antibody data of each participant are not available under the condition of informed consent acquisition, which was mandated by the Act on the Protection of Personal Information.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023-2038. [DOI] [PubMed] [Google Scholar]
- 2. Stainer A, Tonutti A, De Santis M, et al. Unmet needs and perspectives in rheumatoid arthritis-associated interstitial lung disease: a critical review. Front Med (Lausanne). 2023;10(1129939):1129939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hakala M. Poor prognosis in patients with rheumatoid arthritis hospitalized for interstitial lung fibrosis. Chest. 1988;93(1):114-118. [DOI] [PubMed] [Google Scholar]
- 4. Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology. 2010;49(8):1483-1489. [DOI] [PubMed] [Google Scholar]
- 5. Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010;35(6):1322-1328. [DOI] [PubMed] [Google Scholar]
- 6. Vergnenegre A, Pugnere N, Antonini MT, et al. Airway obstruction and rheumatoid arthritis. Eur Respir J. 1997;10(5):1072-1078. [DOI] [PubMed] [Google Scholar]
- 7. Swinson DR, Symmons D, Suresh U, Jones M, Booth J. Decreased survival in patients with co-existent rheumatoid arthritis and bronchiectasis. Br J Rheumatol. 1997;36(6):689-691. [DOI] [PubMed] [Google Scholar]
- 8. Nomura A, Stemmermann GN, Chyou PH, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120(12):977-981. [DOI] [PubMed] [Google Scholar]
- 9. Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127-1131. [DOI] [PubMed] [Google Scholar]
- 10. Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330(18):1267-1271. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198(4):553-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koike T, Ohara S, Sekine H, et al. Helicobacter pylori infection prevents erosive reflux oesophagitis by decreasing gastric acid secretion. Gut. 2001;49(3):330-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326(7392):737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mayr M, Kiechl S, Mendall MA, Willeit J, Wick G, Xu Q. Increased risk of atherosclerosis is confined to CagA-positive Helicobacter pylori strains: prospective results from the Bruneck study. Stroke. 2003;34(3):610-615. [DOI] [PubMed] [Google Scholar]
- 15. Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut. 2013;62(9):1262-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartels LE, Pedersen AB, Kristensen NR, et al. Helicobacter pylori infection is not associated with rheumatoid arthritis. Scand J Rheumatol. 2019;48(1):24-31. [DOI] [PubMed] [Google Scholar]
- 17. Showji Y, Nozawa R, Sato K, Suzuki H. Seroprevalence of Helicobacter pylori infection in patients with connective tissue diseases. Microbiol Immunol. 1996;40(7):499-503. [DOI] [PubMed] [Google Scholar]
- 18. Meron MK, Amital H, Shepshelovich D, et al. Infectious aspects and the etiopathogenesis of rheumatoid arthritis. Clin Rev Allergy Immunol. 2010;38(2-3):287-291. [DOI] [PubMed] [Google Scholar]
- 19. Smyk DS, Koutsoumpas AL, Mytilinaiou MG, Rigopoulou EI, Sakkas LI, Bogdanos DP. Helicobacter pylori and autoimmune disease: cause or bystander. World J Gastroenterol. 2014;20(3):613-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang L, Cao ZM, Zhang LL, et al. Helicobacter Pylori and autoimmune diseases: involving multiple systems. Front Immunol. 2022;13(833424):833424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Etchegaray-Morales I, Jiménez-Herrera EA, Mendoza-Pinto C, et al. Helicobacter pylori and its association with autoimmune diseases: systemic lupus erythematosus, rheumatoid arthritis and Sjögren syndrome. J Transl Autoimmun. 2021;4(100135):100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanaka E, Singh G, Saito A, et al. Prevalence of Helicobacter pylori infection and risk of upper gastrointestinal ulcer in patients with rheumatoid arthritis in Japan. Mod Rheumatol. 2005;15(5):340-345. [DOI] [PubMed] [Google Scholar]
- 23. Lee TH, Wu MC, Lee MH, Liao PL, Lin CC, Wei JC. Influence of Helicobacter pylori infection on risk of rheumatoid arthritis: a nationwide population-based study. Sci Rep. 2023;13(1):15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seriolo B, Cutolo M, Zentilin P, Savarino V. Helicobacter pylori infection in rheumatoid arthritis. J Rheumatol. 2001;28(5):1195-1196. [PubMed] [Google Scholar]
- 25. Zentilin P, Seriolo B, Dulbecco P, et al. Eradication of Helicobacter pylori may reduce disease severity in rheumatoid arthritis. Aliment Pharmacol Ther. 2002;16(7):1291-1299. [DOI] [PubMed] [Google Scholar]
- 26. Lee JS, Ryu JH, Elicker BM, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(12):1390-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bédard Méthot D, Leblanc É, Lacasse Y. Meta-analysis of gastroesophageal reflux disease and idiopathic pulmonary fibrosis. Chest. 2019;155(1):33-43. [DOI] [PubMed] [Google Scholar]
- 28. Fidler L, Sitzer N, Shapera S, Shah PS. Treatment of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest. 2018;153(6):1405-1415. [DOI] [PubMed] [Google Scholar]
- 29. Chen B, You WJ, Liu XQ, Xue S, Qin H, Jiang HD. Chronic microaspiration of bile acids induces lung fibrosis through multiple mechanisms in rats. Clin Sci (Lond). 2017;131(10):951-963. [DOI] [PubMed] [Google Scholar]
- 30. Bennett D, Bargagli E, Refini RM, et al. Helicobacter pylori seroprevalence in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2014;43(2):635-638. [DOI] [PubMed] [Google Scholar]
- 31. Fahim A, Dettmar PW, Morice AH, Hart SP. Gastroesophageal reflux and idiopathic pulmonary fibrosis: a prospective study. Medicina (Kaunas). 2011;47(4):200-205. [PubMed] [Google Scholar]
- 32. Kanbay M, Gur G, Akcay S, Yilmaz U. Helicobacter pylori seroprevalence in patients with chronic bronchitis. Respir Med. 2005;99(10):1213-1216. [DOI] [PubMed] [Google Scholar]
- 33. Tsang KW, Lam SK, Lam WK, et al. High seroprevalence of Helicobacter pylori in active bronchiectasis. Am J Respir Crit Care Med. 1998;158(4):1047-1051. [DOI] [PubMed] [Google Scholar]
- 34. Gencer M, Ceylan E, Yildiz Zeyrek F, Aksoy N. Helicobacter pylori seroprevalence in patients with chronic obstructive pulmonary disease and its relation to pulmonary function tests. Respiration. 2007;74(2):170-175. [DOI] [PubMed] [Google Scholar]
- 35. Kikuchi S, Nakajima T, Kobayashi O, et al. Effect of age on the relationship between gastric cancer and Helicobacter pylori. Tokyo Research Group of Prevention for Gastric Cancer. Jpn J Cancer Res. 2000;91(8):774-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ueda J, Gosho M, Inui Y, et al. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter. 2014;19(2):105-110. [DOI] [PubMed] [Google Scholar]
- 37. Abiko S, Hirayama Y, Otaki J, et al. Changes in prevalence of Helicobacter pylori in Japan from 2008 to 2018: a repeated cross-sectional study. BMJ Open. 2022;12(9):e058774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanaka S, Goto A, Yamagishi K, et al. Long-term response of Helicobacter pylori antibody titer after eradication treatment in middle-aged Japanese: JPHC-NEXT study. J Epidemiol. 2023;33(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581. [DOI] [PubMed] [Google Scholar]
- 40. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315-324. [DOI] [PubMed] [Google Scholar]
- 41. Tanaka N, Kim JS, Newell JD, et al. Rheumatoid arthritis-related lung diseases: CT findings. Radiology. 2004;232(1):81-91. [DOI] [PubMed] [Google Scholar]
- 42. Mori S, Cho I, Koga Y, Sugimoto M. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol. 2008;35(8):1513-1521. [PubMed] [Google Scholar]
- 43. Oka S, Furukawa H, Shimada K, et al. Association of human leukocyte antigen alleles with chronic lung diseases in rheumatoid arthritis. Rheumatology (Oxford). 2016;55(7):1301-1307. [DOI] [PubMed] [Google Scholar]
- 44. Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc. 1949;140(8):659-662. [DOI] [PubMed] [Google Scholar]
- 45. Youssefi M, Tafaghodi M, Farsiani H, Ghazvini K, Keikha M. Helicobacter pylori infection and autoimmune diseases; is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. J Microbiol Immunol Infect. 2021;54(3):359-369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-bmi-10.1177_11772719241297171 for Helicobacter pylori Seroprevalence in Rheumatoid Arthritis Patients with Interstitial Lung Disease by Shomi Oka, Takashi Higuchi, Hiroshi Furukawa, Kota Shimada, Akira Okamoto, Misuzu Fujimori, Atsushi Hashimoto, Akiko Komiya, Koichiro Saisho, Norie Yoshikawa, Masao Katayama, Toshihiro Matsui, Naoshi Fukui, Kiyoshi Migita and Shigeto Tohma in Biomarker Insights