Dear Editor,
BK Virus-associated nephropathy (BKVAN) is an early posttransplant complicationassociated with increased risk of all-cause and death-censored graft loss.1 Treatment options are limited, and optimizing immunosuppressive therapy is the cornerstone of treatment. The goal of screening is to identify a point of potential intervention much early in the natural history of BKV, when timely intervention can be done to prevent the development of overt nephropathy.
KDIGO 20092 and American Society of Transplantation guidelines 20193 recommend plasma for BKV screening. The literature for recommending BKV viruria screening is limited to few observational studies.4 Because of the low quality of evidence available, there still exists controversy between plasma versus urine for BKV screening. The positive predictive value for urine and plasma BKV PCR are 40% and 50–60%, respectively, but the negative predictive value of urine BKV is 100%.5 Y. Funahashi et al. demonstrated that BK virus was detected more frequently in serum (74% versus 9%) when present in urine at ≥10^7 copies/mL.6 Viremia and BKV nephropathy do not develop without higher levels of viruria. Based on these findings, our institutional protocol for BKV screening was developed [Figure 1].
Figure 1:
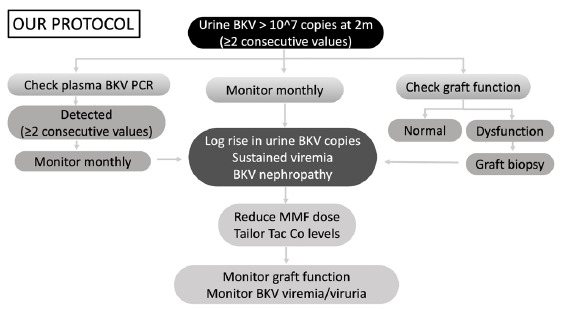
Protocol for BKV screening. BKV PCR: BK Polyoma virus polymerase chain reaction; Tac Co levels: Tacrolimus trough levels; MMF: Mycophenolate mofetil.
Previous studies showed that polyomavirus reactivation in the blood was detected earlier by urine screening protocol.S1,S2 In a retrospective analysis of BKV screening with urine BKV viral load, urine cytology, and serum viral load in 192 kidney transplant recipients (KTR), it was found that a two-stage screening with urine BKV at viral load threshold ≥10^4 copies/mL has superior PPV and cost-effective compared to either urine cytology or plasma BKV PCR alone.S3 Periodic screening for BKV viruria is an efficient method for earlier detection of BKV-infected patients. To our understanding, ours is the first Indian study comparing urine versus plasma for BKV screening.
Our study elaborates on an efficient screening protocol aimed at early detection and timely intervention for preventing BKVAN. Our protocol involves universal screening with urine BKV PCR at 2 months posttransplant. Those testing positive for viruria are tested for viremia with a PCR-based assay. Patients testing positive for viruria alone and viruria with viremia are followed up monthly with urine BKV PCR and urine with plasma BKV PCR, respectively. Intervention by immunosuppression optimization was done if any of these criteria were fulfilled: significant persistent (≥2 values) viruria (≥10^7 copies/mL), persistent viremia (≥2 values), or biopsy-proven BKVAN. Our goal was clearance of viremia/significant viruria by optimizing immunosuppression while balancing the risk of the development of rejection. The protocol followed for managing patients with BKV viremia and viruria is summarized in Figure 1.
Contrary to KDIGO and AST guidelines, we are proposing screening for BKV in urine. The protocol is unique as it is aimed at identifying patients at the viruric stage, which is prior to the viremic stage in the natural history of BKV infection. The lead time obtained from earlier detection enables the clinician to identify at-risk patients and institute therapeutic modifications.
It is a retrospective single-center cohort study of 529 kidney transplants performed at the IQRAA International Hospital and Research Centre, Kerala, India from October 2016 till July 2022. The medical records were reviewed for demographics such as age, gender, diabetes, induction therapy, ABO incompatible transplant, desensitization, rejection, infection episodes, tacrolimus trough levels, graft function, BK viruria and viremia status, treatment modification, and outcomes. Quantitative variables were expressed as mean and standard variation, and qualitative variables were expressed in percentages. Two qualitative variables were tested using chi-square test, and other quantitative variables were tested using the student t test.
Out of 529 KTR, 424 (66.8%) were males. The mean recipient and donor age were 38.7 and 48.2 years, respectively. One hundred and ninety-one (36.1%) patients were diabetic. Eighty-one (15.3%) patients received antithymocyte globulin induction, and 108 (20.4%) patients were desensitized with Rituximab and/or PLEX for HLA/ABO incompatibility. Eighty-nine (16.8%) patients had viruria ≥10^7 copies/mL, and 56 (10.5%) had viremia. Graft biopsy was performed as per standard indications, and 14 (2.6%) were found to have BKV polyomavirus nephropathy. As per the protocol, immunosuppression reduction was done in 58 (10.9%) patients, of which all had significant viruria, 56 had sustained viremia, and 14 had BKV nephropathy.
Viremic patients were found to have a higher rate of hospitalization for other infections compared to nonviremic patients (12.2% versus 6.7%, p = 0.016). However, no such correlation was found among viruric and nonviruric patients. Graft function at 6 months and 1 year was comparable between those with and without significant BKV viruria or BKV viremia. Estimated GFR was comparable between those with and without viremia; 63 and 68.6 mL/min at 6 months, and 61.6 and 67.6% at 12 months posttransplant. Till date, there has not been any graft loss due to BKVAN. With the above protocol for immunosuppression reduction, more than two-thirds of viremic patients and three-fourths of viruric patients cleared the virus by 6 months [Figure 2]. No correlation was found between average tacrolimus trough levels and viremia status, neither was there any correlation of viremic status with tacrolimus metabolizer status. The results have been summarized in Table 1.
Figure 2:
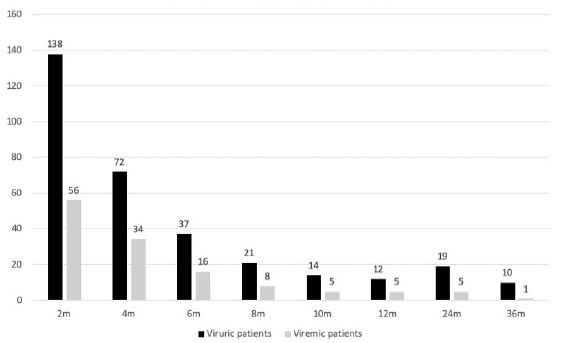
Number of viruric and viremic patients at various timelines.
Table 1:
Distribution of variables in patients with and without BKV viremia and viruria
| Total (N = 529) | BKV viruria ≥ 10^7 (n = 89) | No viruria (n = 440) | Viremia (n = 56) | No viremia (n = 473) | |
|---|---|---|---|---|---|
| Recipient age | 38.7±10.5 | 39.4±10.9 | 38.6±10.5 | 39.7±11.8 | 38.6±10.4 |
| Males | 424 (80.1%) | 71 (79.8%) | 353 (80.2%) | 41 (73.2%) | 378 (79.9%) |
| Donor age | 48.2±9.6 | 49.9 ± 10.1 | 47.9±9.5 | 51.8 ± 9.2 | 47.8 ± 9.6 |
| Diabetes | 191 (36.1%) | 30 (33.7%) | 161 (36.6%) | 6 (10.7%) | 65 (13.7%) |
| ABOi Tx/Desensitization | 108 (20.4%) | 19 (21.3%) | 89 (20.2%) | 15 (26.8%) | 93 (19.7%) |
| BPAR | 93 (17.6%) | 20 (22.5%) | 73 (16.6%) | 16 (28.6%) | 77 (16.2%) |
| Induction | 81 (15.3%) | 14 (15.7%) | 67 (15.2%) | 11 (19.6%) | 70 (14.8%) |
| CMV | 31 (4.9%) | 4 (4.5%) | 27 (6.1%) | 4 (7.1%) | 52 (10.9%) |
| Tacrolimus metabolizer | |||||
| Extensive | 39 (7.4%) | 5 (5.6%) | 34 (7.7%) | 4 (7.1%) | 35 (7.3%) |
| Intermediate | 147 (27.7%) | 23 (25.8%) | 124 (28.2%) | 14 (25%) | 133 (28.1%) |
| Poor | 181 (34.2%) | 33 (37.1%) | 148 (33.6%) | 23 (41.1%) | 158 (33.4%) |
| Age Tac Co levels over first 2 months | 7.9 | 8 | 7.9 | 8.05 | 7.88 |
| GFR 6 m | 68±19.4 | 64.7±19 | 68.7±19.5 | 63±18.2 | 68.6±19.5 |
| GFR 12 m | 66.9±21.2 | 63.8±21 | 67.6±21.2 | 61.6±21.3 | 67.6±21 |
| Hospitalization or infection | 236 (44.6%) | 47 (52.8%) | 189 (42.9%) | 32 (57.1%) | 230 (48.6%) |
BPAR: Biopsy-proven acute rejection, ABOi Tx: ABO incompatible transplant, CMV: Cytomegalovirus, GFR: Glomerular filtration rate.
This study demonstrates the efficiency of urine BKV screening strategy for early identification of at-risk patients, thereby facilitating timely therapeutic modifications. More than half of the patients who had persistent significant viruria also have concurrent viremia; therefore, persistent significant viruria alerts the clinician of possible viremia. It is not the mere presence but the trend of magnitude of viruria that should decide the treatment. There is established evidence that patients with persistent viremia are at high risk of developing BKVAN. The decline in viruria and viremia are parallel [Figure 2]; therefore, by optimizing immunosuppression and decreasing viruria, viremia is also controlled.
Viremic patients have a higher rate of hospitalization for other infections vis-à-vis nonviremic patients. However, no such correlation was found among viruric and nonviruric patients. From this, it may be extrapolated that by the time the patient reaches the viremic stage, they’re already in a state of over-immunosuppression. Identifying them at the viruric stage enables timely intervention so that viremia may be prevented. This strategy is significant in that it identifies patients who are at risk for other infections as well. Our approach is more efficient than the current recommended approach in that timely detection at viruric stage presents a wider lead time, which enables earlier identification and treatment modifications so that they do not develop viremia, at which stage when the patient is already over-immunosuppressed. This way, we can prevent not only BKV nephropathy but also KTR from being hospitalized due to other infections.
There are a few limitations to viruria screening. It requires multiple and serial screening, adding to the cost and resulting in more clinic visits. However, it may be argued that it may be compensated by the prevention of over-immunosuppression and avoidance of hospitalizations for other infections.
In conclusion, monitoring of the trend of urine BKV levels, starting at 8 weeks posttransplant, alerts nephrologists of possible viremia and BKV nephropathy. It is complementary rather than a substitute, which helps us fine-tune the doses of immunosuppressive medications of KTR. With periodic close monitoring of urinary BKV levels and timely intervention, the graft function between those with and without BKV viremia and viruria are comparable.
Acknowledgements
We thank the study participants, their families, and caregivers.
Footnotes
How to cite this article: Sulaiman S, Aziz F, Hafeeq B, Anoop KP M, Uvais NA, Narayanan R, et al. Early Detection Strategy of BK Polyoma Virus Infection in Kidney Transplant Recipients. 2024;34:648-51. Indian J Nephrol. doi: 10.25259/ijn_481_23
Conflicts of interest
There are no conflicts of interest.
Supplementary Files
References
- 1.Gately R, Milanzi E, Lim W, Teixeira-Pinto A, Clayton P, Isbel N, et al. Incidence, risk factors, and outcomes of kidney transplant recipients with BK polyomavirus-associated nephropathy. Kidney Int Rep. 2023;8:531–43. doi: 10.1016/j.ekir.2022.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckardt KU, Kasiske BL, Zeier MG. Special issue: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HH, Randhawa PS. AST infectious diseases community of practice BK polyomavirus in solid organ transplantation—Guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33:e13528. doi: 10.1111/ctr.13528. [DOI] [PubMed] [Google Scholar]
- 4.Elfadawy N, Flechner SM, Liu X, Schold J, Tian D, Srinivas TR, et al. The impact of surveillance and rapid reduction in immunosuppression to control BK virus-related graft injury in kidney transplantation. Transpl Int. 2013;26:822–32. doi: 10.1111/tri.12134. [DOI] [PubMed] [Google Scholar]
- 5.Sawinski D, Goral S. BK virus infection: An update on diagnosis and treatment. Nephrol Dial Transplant. 2015;30:209–17. doi: 10.1093/ndt/gfu023. [DOI] [PubMed] [Google Scholar]
- 6.Funahashi Y, Kato M, Fujita T, Tsuruta K, Inoue S, Gotoh M. Correlation between urine and serum BK virus levels after renal transplantation. Transplant Proc. 2014;46:567–9. doi: 10.1016/j.transproceed.2013.11.154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Files


