Abstract
Mosquito-borne diseases pose a significant public health threat, prompting the need to pinpoint high-risk areas for targeted interventions and environmental control measures. Culex quinquefasciatus is the primary vector for several mosquito-borne pathogens, including West Nile virus. Using spatial analysis and modeling techniques, we investigated the geospatial distribution of Culex quinquefasciatus abundance in the large metropolis of Harris County, Texas, from 2020 to 2022. Our geospatial analysis revealed clusters of high mosquito abundance, predominantly located in central Houston and the north-northwestern regions of Harris County, with lower mosquito abundance observed in the western and southeastern areas. We identified persistent high mosquito abundance in some of Houston’s oldest neighborhoods, highlighting the importance of considering socioeconomic factors, the built environment, and historical urban development patterns in understanding vector ecology. Additionally, we observed a positive correlation between mosquito abundance and neighborhood-level socioeconomic status with the area deprivation index explaining between 22 and 38% of the variation in mosquito abundance (p-value < 0.001). This further underscores the influence of the built environment on vector populations. Our study emphasizes the utility of spatial analysis, including hotspot analysis and geostatistical interpolation, for understanding mosquito abundance patterns to guide resource allocation and surveillance efforts. Using geostatistical analysis, we discerned fine-scale geospatial patterns of Culex quinquefasciatus abundance in Harris County, Texas, to inform targeted interventions in vulnerable communities, ultimately reducing the risk of mosquito exposure and mosquito-borne disease transmission. By integrating spatial analysis with epidemiologic risk assessment, we can enhance public health preparedness and response efforts to prevent and control mosquito-borne disease.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12942-024-00385-4.
Keywords: Mosquito abundance, Spatial analysis, Built environment, Environmental control, Public health, Houston, Texas
Introduction
Culex quinquefasciatus (Say), commonly known as the southern house mosquito, is the primary vector of several mosquito-borne diseases, including West Nile virus (WNV) [1–4]. While hypothesized to originate from tropical regions of Southeast Asia, Cx. quinquefasciatus is now established throughout the subtropical and tropical areas of the world [5, 6]. This mosquito species has adapted to thrive in warm urban environments with an optimal temperature range of 75–82 °F (23.9–27.8 °C) [7, 8]. It lays its eggs in “dirty,” stagnant, water sources rich in organic material, with breeding sites encompassing wastewater, ditches, ponds, discarded tires, and abandoned swimming pools. While primarily ornithophilic, Cx. quinquefasciatus can feed on various mammalian, amphibian, and reptilian hosts, emphasizing its importance in zoonotic pathogen transmission [3, 9]. Due to ongoing urbanization, rising temperatures, and other environmental factors, the habitat range of Cx. quinquefasciatus has grown significantly and is projected to continue spreading throughout North America [10, 11].
WNV is the primary cause of autochthonous mosquito-borne illness in the United States (USA) and poses a significant public health threat [12]. While the majority of WNV infections are asymptomatic, around 20% of infected individuals will develop mild febrile disease, and < 1% will progress to neuroinvasive disease. Neuroinvasive disease can result in significant morbidity and mortality, including the development of encephalitis, meningitis, and acute flaccid paralysis [13, 14]. Those who recover from neuroinvasive disease are often left with significant disability [15]. Since its emergence in North America in 1999, there have been over 56,500 reported cases of WNV, resulting in more than 25,700 hospitalizations and 2,776 deaths (as of 2022). Texas accounted for 10% (5,901) of these cases [12]. Beyond its impact on health, WNV can inflict a substantial economic burden on affected individuals and communities [16, 17]. For instance, the 2014 outbreak in Harris County, Texas, resulted in 139 cases and two deaths, incurring acute medical care and lost productivity costs estimated at USD 6 million [16]. Given the absence of a vaccine or definitive treatment for WNV, effective prevention and control strategies targeting both the virus and its mosquito vectors are critical public health measures [18].
Mosquito abundance and distribution are influenced by each species’ specific biological and ecological requirements, which are affected by a variety of factors, including climate, land use, land cover, and the built environment [19]. Other factors, such as housing density, housing age, foreclosures, and income levels, have also been associated with transmission dynamics, reflecting socioeconomic and environmental conditions conducive to mosquito proliferation [20–23]. These variables have served as proxies for characteristics that are challenging to measure, such as inadequate storm drainage systems, aging sewer infrastructure, abandoned swimming pools, lack of landscaping maintenance, and other environmental conditions contributing to the growth of Culex spp. mosquitoes [14, 19, 24–32]. Because these associations can vary geographically, understanding region-specific geospatial patterns of adult mosquito vectors is imperative for developing targeted surveillance and control strategies. This information may facilitate informed decision-making, contribute to localized control measures and educational initiatives, and allow for targeted abatement efforts, potentially reducing the risk of insecticide resistance [33–35].
Harris County, Texas, is a focal point for understanding the dynamics of mosquitoes and mosquito-borne diseases. This county is home to a diverse range of over 50 mosquito species, including vectors responsible for transmitting chikungunya, dengue, Saint Louis encephalitis, West Nile, yellow fever, and Zika viruses [36]. Since its introduction in 2002, Harris County has reported at least one WNV human case and has detected WNV in mosquitoes every year [16]. Harris County’s geographic and demographic conditions create suitable conditions for the proliferation of mosquitoes and mosquito-borne pathogens. Located along the Gulf of Mexico with major seaports and international airports, the county is a hub for constantly moving goods and people. However, this role also exposes the county to the introduction of new pathogens and infected individuals. Furthermore, Harris County has a population of about 4.7 million people, and an additional one million individuals are projected to migrate into the county over the next three decades [37]. This influx further increases the population density, accelerates urbanization, and leads to increased garbage production, including artificial containers and food waste [11, 19, 20, 38]. The region’s long summer season, characterized by high temperatures and humidity, is a fertile ground for mosquito breeding. Predictions of increased annual precipitation in Harris County and shifts in daily rainfall patterns could further influence the abundance and distribution of mosquitoes [39]. Here, we present spatial and statistical analyses to examine the patterns of adult female Cx. quinquefasciatus abundance in Harris County, Texas, from 2020 to 2022 to identify areas with statistically significant clustering of mosquito abundance. Our research aims to provide critical insights into mosquito population dynamics for public health officials and local vector control agencies. These insights will support targeted mosquito control and education efforts in Harris County and offer a valuable framework for similar cities along the southern coast of the United States.
Methods
Study region
Harris County is located in southeast Texas, adjacent to the Gulf of Mexico at sea-level elevation (29° 44’ N, 95° 27’ W). With Houston serving as the county seat, Harris County ranks as the nation’s third-largest county, with a population of over 4.7 million individuals as of the 2020 census [40]. The county spans 1,707 square miles (~ 4421 sq. km) and contains 34 cities and towns (Fig. 1) [41]. The county’s humid, subtropical climate features hot summers, mild winters, and highly variable rainfall. Climate norms for the region from 2006 to 2020 include a mean minimum temperature of 43.1 °F (6.2 °C) during the coldest month (January) and a mean maximum temperature of 95.4 °F (35.2 °C) during the hottest month (August). The mean annual rainfall is about 52 inches (132 cm), with 62% of the precipitation accumulating from May through October [42]. For comparison, the average precipitation for the contiguous U.S. from 2018 to 2023 was 30.71 inches (78 cm) [43].
Fig. 1.
Map of Texas highlighting Harris County. The inset shows a zoomed-in version of Harris County with all city limits distinguished in gray
Data source
Mosquito surveillance data collected by Harris County Mosquito and Vector Control Division (HCMVCD) from 2020 to 2022 were used. Routine surveillance involves sampling from systematically placed traps within 268 stable polygons known as Mosquito Control Operational Areas (MCOAs). Trapping efforts were relatively consistent during the three years of this study. However, some sites were surveyed more frequently within and between years due to the availability of resources. For this study, we utilized data collected using a modified CDC Reiter gravid trap (J.W. Hock and Co., Gainesville, FL) baited with a modified Reiter medium (fermented hay infusion) [44]. The gravid trap is helpful in sampling ovipositing Culex spp. mosquitoes and was designed to maximize the collection of Cx. quinquefasciatus for WNV surveillance [45, 46]. Gravid traps are placed in the afternoon and collected the following morning, allowing a collection time of approximately 18 h. The mosquitoes are then sorted, sexed, identified morphologically to genus and species level, and placed in cold holding at -80 °C. The HCMVCD Virology Laboratory then tests mosquito pools for arboviruses that are significant to human health [44]. For this analysis, we only used Cx. quinquefasciatus abundance and WNV testing data.
Statistical analyses
We focused analyses on female Cx. quinquefasciatus due to their role in the transmission of mosquito-borne pathogens. To account for variations in sampling effort, we computed the average (mean) number of adult female Cx. quinquefasciatus mosquitoes per number of nights that traps were employed (“trap-nights”) for each gravid trap site. Average abundance was compared between years using a Kruskal-Wallis test with a post hoc Dunn’s test using a False Discovery Rate (FDR) correction for multiple comparisons. Statistical analyses were conducted using R version 4.3.1 (R Core Team 2023).
Geostatistical analysis
Spatial analyses were conducted using ArcGIS Pro (version 3.2.0, ESRI, Redlands, CA). For spatial analyses, we subset the data to include mosquito season from May to October, incorporating all regular trapping routes. First, trap locations were geocoded using coordinates. While trapping efforts were relatively consistent during the three years of this study, some sites were surveyed more frequently within and between years due to the availability of resources. On average, a trap site was sampled 12 ± 9 times per year, and 215 (80.2%) trap sites were sampled every year. To adjust for trapping effort and to obtain one representative abundance for each trap site, data for each year (May to October) were aggregated based on the female Cx. quinquefasciatus abundance per trap-night for spatial analysis. We assessed the spatial autocorrelation of mosquito abundance for each year using Global Moran’s I. This statistic evaluates whether trap sites exhibit clustered, dispersed, or random distribution based on location and Cx. quinquefasciatus abundance (standardized by trap-night) [47]. Significant spatial autocorrelation prompted further hotspot analysis for each year, with significance evaluated at α = 0.05 [48].
The Getis-Ord Gi* statistic was used to identify significant clusters of high or low Cx. quinquefasciatus abundance per trap-night with trap sites aggregated into Mosquito Control Operational Areas (MCOA). Using Getis-Ord Gi*, a statistically significant hot spot is defined as an MCOA with a high mosquito abundance that is surrounded by MCOAs with high abundance as well. MCOAs without a trap site were not included in the hotspot analysis. If an MCOA contained more than one trap site during a year, then traps were aggregated to mean abundance per trap-night for all traps in the MCOA. Utilizing the Spatial Autocorrelation (Global Moran’s I) tool in ArcGIS Pro, the Gi* statistic was computed for each MCOA. A sensitivity analysis using inverse distance, inverse distance squared, and K nearest neighbors was conducted to ensure that the conceptualization of spatial relationships was robust. For the current study, we proceeded with K nearest neighbors. Hotspot maps were generated using standard Z-score cut-offs to pinpoint areas of unexpectedly high or low mosquito abundance, given an equal sampling effort. MCOAs with high positive Z-scores were identified as hotspots, indicating a clustering of high abundance, and areas with negative Z-scores were identified as cold spots, indicating a clustering of low abundance. MCOAs with a p-value > 0.05 were considered to be not significant. We compared the results of the hotspot analyses among study years to identify changes in statistically significant hotspots and cold spots.
Kriging and variography
After confirming spatial autocorrelation using Global Moran’s I, we conducted a spatial interpolation of abundance per trap-night to generate predictive surfaces for Harris County. Spatial interpolation is useful to depict a variable of interest (e.g., annual mosquito abundance per trap-night) when there are a finite number of sampling locations (e.g., trap sites). Kriging is a geostatistical interpolation method that estimates values at unsampled locations based on the observed spatial autocorrelation among sampled data points [48]. Empirical Bayesian kriging is more robust than other kriging methods due to its ability to estimate errors associated with the semivariogram model, its ability to handle moderate non-stationarity, and the fact that there are fewer semivariogram model assumptions [49]. Empirical Bayesian kriging was chosen based on cross-validation (leave-one-out resampling method) to select an appropriate semivariogram model [49]. Predictive surfaces were generated using this method in ArcGIS Pro (Version 3.2.0., ESRI, Redlands, CA) to estimate the average abundance per trap-night at unsampled locations throughout Harris County for each year. The resulting predictive surfaces were visualized for interpretation. Community Tabulation Areas for 2020, akin to Super Neighborhoods, were included to visualize social community boundaries for easier interpretation [50].
Correlation with area deprivation index
The area deprivation index (ADI) was used as an indicator of neighborhood-level socioeconomic status. ADI is a composite score that measures neighborhood disadvantage in the context of income, education, employment, and housing quality [22, 51]. We utilized the 2021 ADI census block group rankings at the state level within Texas. In addition, we obtained shapefiles for 2021 census block groups from the United States Census Bureau [52]. Mosquito traps were joined with the census block group shapefile for correlation with ADI. Census block groups without a trap site were not included in this analysis. Using Spearman’s correlation coefficient, we investigated the association between the ADI and Cx. quinquefasciatus abundance at trap sites by census block group. The analysis was conducted separately for each year of the study period to examine temporal variations in the relationship between ADI and mosquito abundance.
Results
Mosquito collection data
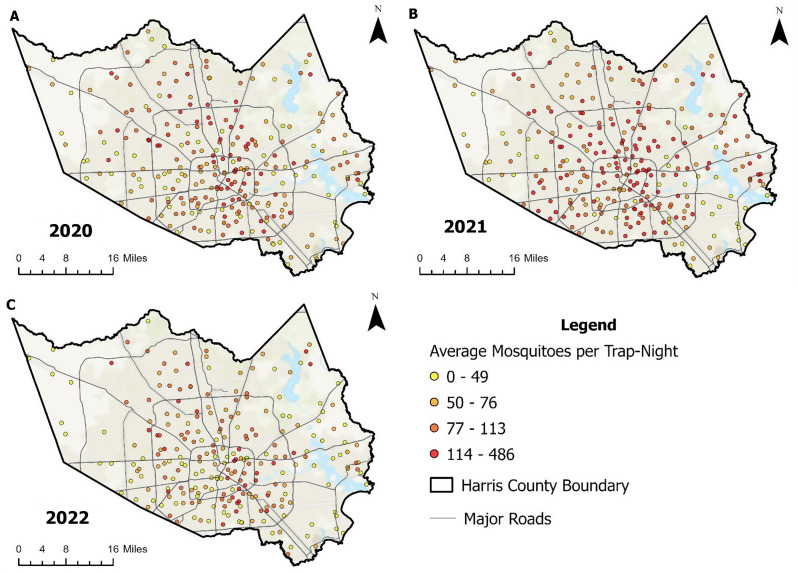
From 2020 to 2022, more than 800,000 adult female Cx. quinquefasciatus mosquitoes were collected from 8,830 trap-nights included in the analysis (Table 1). Each month, there was an average of 491 ± 141 trap-nights at 174 ± 48 locations (Fig. 2).
Table 1.
Number of female Culex quinquefasciatus mosquitoes collected by month in Harris County, Texas, 2020–2022
| Year | Month | Trap locations (N) | Mosquitoes collected (N) | Trap-nights (N) |
|---|---|---|---|---|
| 2020 | May | 134 | 31,691 | 224 |
| June | 187 | 56,141 | 374 | |
| July | 171 | 47,474 | 548 | |
| August | 135 | 43,539 | 459 | |
| September | 225 | 40,013 | 667 | |
| October | 137 | 40,872 | 597 | |
| 2021 | May | 230 | 40,153 | 500 |
| June | 205 | 121,604 | 642 | |
| July | 259 | 96,347 | 678 | |
| August | 151 | 53,747 | 589 | |
| September | 258 | 60,806 | 649 | |
| October | 135 | 64,652 | 547 | |
| 2022 | May | 122 | 22,966 | 289 |
| June | 135 | 27,328 | 348 | |
| July | 138 | 18,790 | 368 | |
| August | 232 | 32,773 | 588 | |
| September | 139 | 32,252 | 408 | |
| October | 135 | 24,224 | 355 |
Fig. 2.
Average abundance of female Culex quinquefasciatus at gravid trap locations in Harris County, Texas, from 2020 to 2022. Panels A (2020), B (2021), and C (2022) show the average female Culex quinquefasciatus abundance per trap-night collected at gravid traps included in the analysis for each year
Mosquito abundance over study period
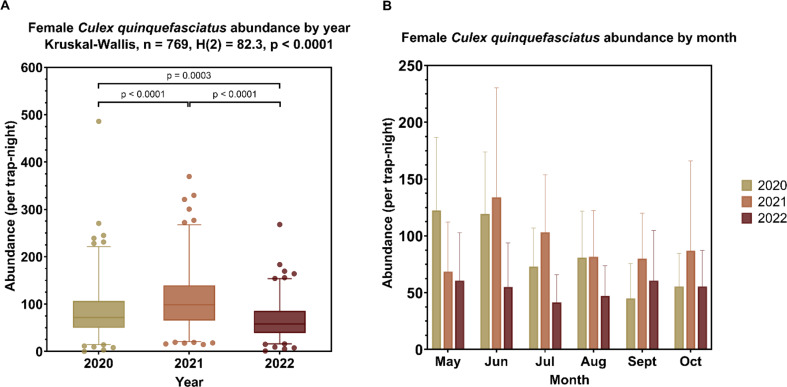
Temporal analysis revealed significant variation in the abundance of Cx. quinquefasciatus over the three-year period (Kruskal Wallis: X2(2) = 82.08, p < 0.0001) (Fig. 3A). A post hoc Dunn’s test using an FDR correction demonstrated that mosquito abundance per trap-night was significantly higher in 2021 (median: 98.5; IQR: 65.6–139) compared to 2020 [(median: 71.5; IQR: 50–106), adjusted p < 0.0001]. However, both 2020 and 2021 had a significantly higher abundance than 2022 [(median: 57.9; IQR: 39–85.5), adjusted p = 0.0003 and adjusted p < 0.0001, respectively]. The seasonal distribution generally indicated peak abundance early in the summer season (May), a decrease in mid-summer (June through August), and a subsequent increase in late summer (September and October) (Fig. 3B).
Fig. 3.
Abundance of female Culex quinquefasciatus mosquitoes by (A) year and (B) month. (A) Boxplots and Kruskal-Wallis test results to test for significant differences in median mosquito abundance per trap-night between the years. For each box plot, the central line is the median; the box encompasses the upper and lower quartiles; the lines extend to the 2.5 and 97.5 percentile, with the points representing values outside this range (outliers). (B) The bar graph depicts the median female Cx. quinquefasciatus abundance per trap-night by month and year. The error bar represents the upper quartile
Spatial autocorrelation (Global Moran’s I)
Spatial autocorrelation analysis using Global Moran’s I identified statistically significant clustering of Cx. quinquefasciatus abundance per trap-night during each study year from May 2020 to October 2022. Positive Global Moran’s I statistics were observed for all study years, indicating significant clustering and non-random distribution of mosquito abundance in Harris County [2020 (Moran’s Index = 0.38, z-score = 5.38, p < 0.0001), 2021 (Moran’s Index = 0.25, z-score = 8.82, p < 0.0001), and 2022 (Moran’s Index = 0.11, z-score = 3.84, p = 0.0001)].
Hotspot analysis
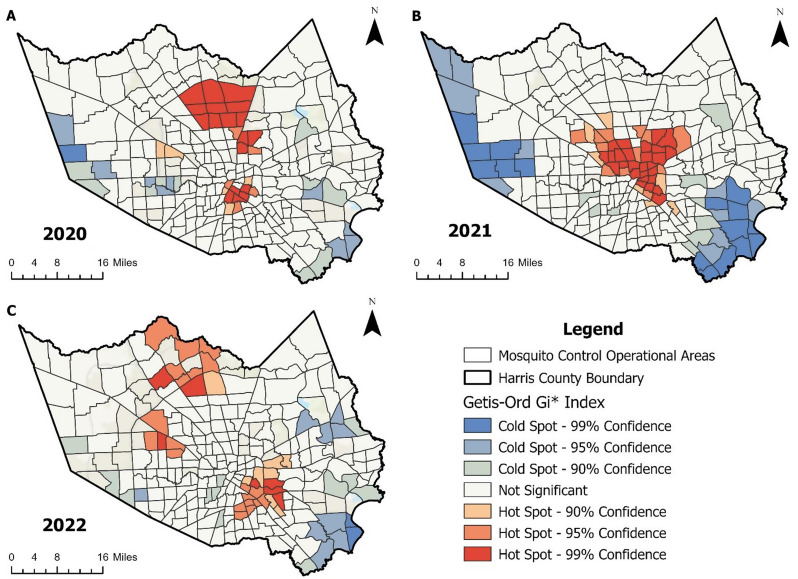
The Getis-Ord Gi* hotspot analysis identified 28 hotspots and 20 cold spots in 2020 (n = 243; Fig. 4A), 46 hotspots and 35 cold spots in 2021 (n = 266; Fig. 4B), and 35 hotspots and 23 cold spots in 2022 (n = 236; Fig. 4C). Hotspots were predominantly observed near central Houston and expanded towards the north-northwest region of the county. In contrast, cold spots were primarily observed in the western and southeastern areas of Harris County. Changes in significant hotspots across the study period were noted, with transitions from not significant to hot in far north and southeast Houston. Three Mosquito Control Operational Areas (MCOA) were identified as hotspots during all three years. These hotspots were located in the Magnolia Park, Second Ward, and Lawndale/Wayside community tabulation areas (Supplementary Figure S1). Hotspots for at least two years during the study period were located in East Little York/Homestead, East Houston, Pecan Park, Eastwood, Aldine Northwest, Aldine Southeast, Denver Harbor/Port Houston, and Carverdale/Westbranch. Seven MCOAs were identified as cold spots during all three years. These cold spots were located in the community tabulation areas of Friendswood, La Porte/Shoreacres, Nassau Bay, El Lago, Katy North, and Seabrook.
Fig. 4.
Changes in Hotspots by year for (A) 2020, (B) 2021, and (C) 2022. The Getis-Ord Gi* statistic identified significant clusters of high or low female Cx. quinquefasciatus normalized by trap-night within Mosquito Control Operational Areas. Hotspots and cold spots were determined using standard Z-score cut-offs. The number of hotspots and cold spots varied across years: 28 hotspots and 20 cold spots in 2020, 46 hotspots and 35 cold spots in 2021, and 35 hotspots and 23 cold spots in 2022. Analysis and mapping were conducted using ArcGIS Pro 3.2.0 (Esri, Redlands, CA)
Kriging and variography
Empirical Bayesian kriging (EBK) was performed to estimate the female Cx. quinquefasciatus abundance at unsampled locations and produce a map of estimated abundances in Harris County, Texas. The best-fit semivariogram model for the mosquito abundance data was K-Bessel with a standard circular neighborhood using K nearest neighbors. The cross-validation results and error analysis of the interpolation results are shown in Supplementary Table S1. The mean prediction error and mean standardized error for 2020, 2021, and 2022 indicate that the models are unbiased. The root mean square standardized error was close to one for all models, demonstrating that the standard errors are generally accurate. The root mean square error, a measurement of prediction accuracy, is 52.24 for 2020, 54.54 for 2021, and 35.68 for 2022. The average standard error was relatively close to the root mean square error for all models, with some indication of slight overestimation and underestimation of the variability in prediction. While the kriging models generally exhibit unbiased estimations for the three years, the cross-validation results suggest that integrating additional factors into future models may refine abundance estimates.
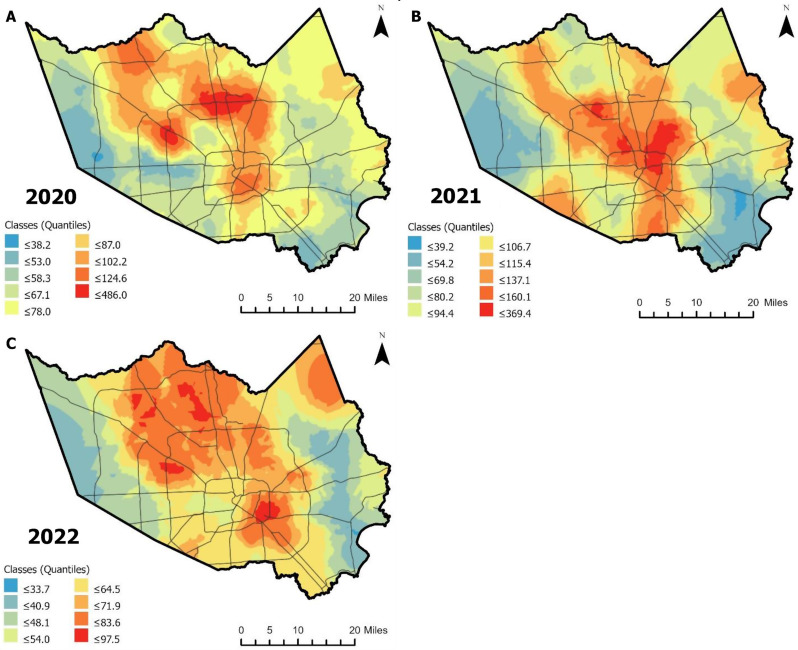
Spatial distribution of female Cx. quinquefasciatus abundance
Predictive maps of female Cx. quinquefasciatus abundance demonstrated that the distribution varied spatially, even after accounting for sampling variation (Fig. 5A-C). Specifically, the predictive surfaces illustrated that female Cx. quinquefasciatus abundance was generally higher near central Houston, expanding into north and northwest Harris County. Areas with lower abundance were typically observed on the western and southeastern borders of Harris County. The overall predictive surfaces reflected Cx. quinquefasciatus abundance consistent with our hotspot analysis. In 2020, Cx. quinquefasciatus showed especially strong spatial clustering near Greenspoint and Carverdale/Westbranch neighborhoods. In 2021, higher abundances were observed near Aldine West, Kashmere Gardens, and Independence Heights neighborhoods. In 2022, higher abundance was observed near Carverdale/Westbranch, Magnolia Park, and Spring Southwest. Notably, these regions tend to coincide with areas characterized by older infrastructure, which may hint at underlying spatial processes influencing patterns in mosquito abundance.
Fig. 5.
Geospatial distribution of female Culex quinquefasciatus abundance standardized by trap-nights in Harris County, Texas, for (A) 2020, (B) 2021, and (C) 2022. Predictive surfaces were generated using Empirical Bayesian kriging, with the resulting classes (split by quantiles) shown in each legend. The Harris County boundary and major roads are overlayed. Analysis and mapping were conducted using ArcGIS Pro 3.2.0 (Esri, Redlands, CA)
West Nile virus positivity
14,101 mosquito pools were tested for WNV during the study period. In 2020, 10 out of 4,520 pools (0.2%) were WNV-positive. In 2021, 230 out of 5,973 pools (3.9%) were WNV-positive. In 2022, 25 out of 3,608 pools (0.7%) were WNV-positive. These findings correlate with human case data for Harris County, Texas, with one case reported in 2020, 23 cases reported in 2021 (10 symptomatic and 13 viremic blood donors), and six cases reported in 2022 (5 symptomatic and one viremic blood donor) [53]. Of the positive mosquito pools, 15 were collected in June, 115 in July, 96 in August, 36 in September, and three in October. The highest positivity rate was observed in August 2021 (8.6%), July 2021 (7.9%), September 2021 (3.2%), and July 2022 (2.8%). In all other months, less than 1.3% of mosquito pools tested positive for WNV. No WNV-positive mosquito pools were collected in May during the study period (Table 2).
Table 2.
Number of female Culex quinquefasciatus mosquito pools collected and tested for West Nile virus by month in Harris County, Texas, 2020–2022
| Year | Month | WNV-Positive mosquito pools (N) | Mosquito pools tested (N) | WNV-Positive mosquito pools (%) |
|---|---|---|---|---|
| 2020 | May | 0 | 354 | 0% |
| June | 0 | 692 | 0% | |
| July | 5 | 945 | 0.5% | |
| August | 3 | 779 | 0.4% | |
| September | 2 | 993 | 0.2% | |
| October | 0 | 757 | 0% | |
| 2021 | May | 0 | 722 | 0% |
| June | 14 | 1,116 | 1.3% | |
| July | 95 | 1,210 | 7.9% | |
| August | 86 | 1,002 | 8.6% | |
| September | 33 | 1,045 | 3.2% | |
| October | 2 | 878 | 0.2% | |
| 2022 | May | 0 | 360 | 0% |
| June | 1 | 621 | 0.2% | |
| July | 15 | 543 | 2.8% | |
| August | 7 | 884 | 0.8% | |
| September | 1 | 650 | 0.2% | |
| October | 1 | 550 | 0.2% |
Correlation with area deprivation index
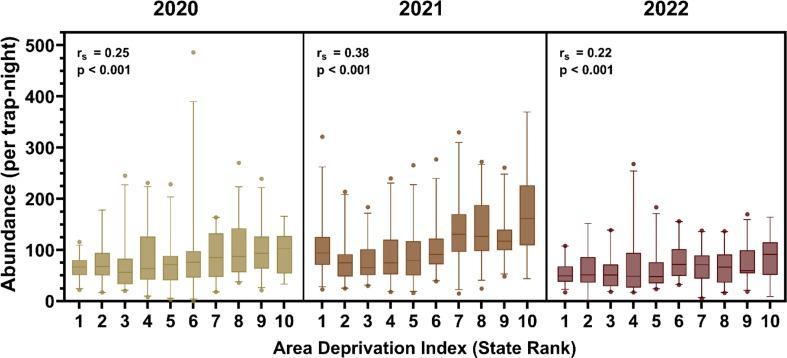
We explored the relationship between mosquito abundance and ADI, revealing consistent positive correlations across the years 2020, 2021, and 2022. Overall, we observed a positive correlation between Cx. quinquefasciatus abundance and census block group ADI. Spearman’s correlation coefficients indicated significant associations between mosquito abundance and state ranking of census block group ADI, underscoring the influence of socioeconomic factors on vector populations. In 2020, the correlation coefficient was ρ = 0.25 (p < 0.001); in 2021, ρ = 0.38 (p < 0.001); and in 2022, ρ = 0.22 (p < 0.001) (Fig. 6). These findings highlight the importance of considering socioeconomic disparities and culturally relevant educational initiatives when developing mosquito surveillance and control efforts.
Fig. 6.
Correlation between Area Deprivation Index (ADI) and average Culex quinquefasciatus abundance for each year. Census block group rankings for 2021 ADI were utilized, and Spearman’s correlation coefficient was employed for analysis. Consistent positive correlations between mosquito abundance and ADI were observed across the study years. For each box plot, the central line is the median; the box encompasses the upper and lower quartiles; the lines extend to the 5th and 95th percentile, with the points representing values outside this range
Discussion
Having foundational knowledge about the contemporary abundance and distribution of Cx. quinquefasciatus in a major metropolitan area like Harris County, Texas, is crucial for developing effective mitigation strategies and evaluating changes in mosquito ecology that could result from climate change. By utilizing extensive mosquito surveillance data and robust geostatistical analysis, our study yielded significant findings related to the spatial and temporal distribution of Cx. quinquefasciatus in Harris County, Texas, from 2020 to 2022.
Our findings revealed a heterogeneous distribution of Cx. quinquefasciatus throughout Harris County, highlighting the importance of understanding the underlying drivers of these geospatial patterns for effective vector control [35]. The non-random distribution of mosquito abundance indicates that environmental factors, such as the built environment, land use, and land cover, play an essential role in the risk of WNV transmission [1, 4, 54–56]. Leveraging the inherent spatial autocorrelation in mosquito abundance data, we used empirical Bayesian kriging to estimate mosquito abundance at locations beyond trapping sites. Kriging may be particularly useful in areas with limited surveillance resources and could empower vector control programs to identify high-risk areas for targeted resource allocation and mosquito control efforts.
Our hotspot analysis identified areas within Houston that may have a higher abundance of Cx. quinquefasciatus, potentially warranting educational initiatives and targeted surveillance and control efforts. In our study, hotspots were generally observed in the population-dense area of central Houston and the north and northwest regions of Harris County. Northwest Harris County was previously identified as having a statistically significant cluster of WNV cases in 2012–2014, with over 68% of cases clustered in west and northwest Harris County [57]. Conversely, areas with lower mosquito abundance (cold spots), such as the western and southeastern borders of Harris County, may require different intervention strategies tailored to their specific ecological and environmental context.
A few communities consistently emerged as hotspots throughout the study period. Magnolia Park, Lawndale/Wayside, and Second Ward contained MCOAs identified as hotspots during all three years, which was particularly noteworthy due to their higher ADI ranks and older homes. Each community has unique characteristics that may place its residents at greater risk of mosquito exposure.
Magnolia Park is one of Houston’s oldest communities. It is located in eastern Harris County, just south of some initial wharves constructed as the Houston Ship Channel transitioned into a major deep-water port in 1913. The community has multiple land use types, including single-family and multi-family residences, commercial, industrial, undeveloped land, and open water [58]. Magnolia Park is home to the earliest constructed homes in Houston, with a median home construction year of 1936 (median in Harris County = 1979). Lawndale/Wayside is a community within Houston’s inner loop, containing heavily wooded preserves and Houston’s first country club. Land use in this community is a mixture of single-family and multi-family residences, commercial, parks, open space, undeveloped land, and a small proportion of open water (a bayou) [59]. The median home construction year in Lawndale/Wayside is 1946. Second Ward, one of Houston’s original neighborhoods, is bounded by rail lines and serves as a commercial district for industrial services. Second Ward’s land use includes single-family and multi-family residences, commercial, a larger industrial area (numerous vacant), and a small proportion of open water (a bayou) [60]. The median home construction year in Second Ward is 1940. These findings demonstrate that neighborhoods with older homes may be at heightened risk of mosquito exposure due to aging infrastructure.
Furthermore, Spearman’s correlation coefficients indicated significant associations between mosquito abundance and state ranking of census block group ADI, underscoring the influence of socioeconomic and built environment factors on vector populations. These findings corroborate previous research demonstrating that areas with increased urbanization and lower socioeconomic status tend to have a higher burden of Culex spp. mosquitoes [4, 21]. For example, an Atlanta-based study found that pre-1960 homes, the proportion of low-income households, and housing density significantly correlated with an increased risk of WNV infection [14]. Similarly, a multi-year Chicago-based study revealed that areas with pre-1990 homes and greater population density were associated with increased human WNV cases [61]. Our study findings underscore the importance of targeted interventions and public health initiatives, especially within vulnerable communities. The identification of hotspots can assist in allocating limited resources and developing culturally informed interventions for the communities where they are needed most.
The proportion of WNV-positive mosquito pools was substantially higher in 2021 (3.9%) compared to 2020 (0.2%) and 2022 (0.7%). Within 2021, the highest positivity was observed in July (7.9%) and August (8.6%), corresponding with the highest average monthly temperatures across the study period (July average temperature: 84.9 °F/29.4 °C; August average temperature: 84.9 °F/29.4 °C). Previous studies on temperature and WNV transmission have demonstrated that increased temperatures can result in a shortened incubation period, a decreased period between mosquito bloodmeals, and a higher rate of WNV infection and dissemination [62–65].
We observed that WNV-positive mosquito pools generally occurred in regions estimated to have a moderate mosquito abundance based on the kriged surfaces. While the estimated abundance of Cx. quinquefasciatus is a critical indicator in calculating the transmission risk of mosquito-borne diseases; other factors should also be considered when assessing transmission risk. Other determinants of WNV transmission risk, in addition to mosquito abundance, include human density, human-vector contact, and the presence of competent bird species [66–68].
Climate variation is also a critical factor in the abundance of Cx. quinquefasciatus and WNV transmission. Previous studies have identified the optimal temperature range for Cx. quinquefasciatus development to be between 75.2 °F (24 °C) to 82.4 °F (28 °C) [1]. As Harris County regularly exceeds this temperature range during May to October, the negative relationship between peak temperature and peak abundance in our study reflects the critical temperature limits of the species [1]. The average maximum temperature from May to October was slightly lower in 2021 (average maximum temperature for May – Oct 2021: 89.3 °F/27.2 °C) compared to 2020 (average temperature for May – Oct 2020: 90.0 °F/32.2 °C) and 2022 (average temperature for May – Oct 2022: 92.2 °F/33.4 °C) in Harris County. This cool temperature anomaly during the summer of 2021 could have provided a slight reprieve from the typical hot summer temperatures in Houston, allowing a proliferation in the abundance of Cx. quinquefasciatus mosquitoes. Precipitation can have varying effects on mosquito abundance depending on the amount of rainfall, percentage of impervious surfaces, and quality of drainage systems in an environment [65]. In addition, aging infrastructure and vacant properties moderate the impact of normal to above-normal precipitation events [69]. As a result, further research is needed to elucidate the association between temperature, rainfall, and mosquito abundance across various environments in Harris County.
Our results demonstrate how geostatistical analysis using mosquito surveillance datasets can provide valuable information about local vector ecology and support for developing targeted abatement strategies. In addition, our study confirmed the presence of significant spatial autocorrelation within the data, underscoring the importance of accounting for spatial dependencies in future modeling approaches [47, 70, 71]. The study’s strengths include the systematic placement of traps among MCOAs and robust spatial analysis techniques. One limitation is that an unequal sampling of trap sites across the county within the study period may have introduced bias. Moreover, empirical Bayesian kriging assumes that the underlying spatial process is constant across the study area [49]. However, spatial processes are often non-stationary due to various factors, including land use, land cover, and the built environment, including urban development patterns. The cross-validation results indicate that the kriging models are generally unbiased in their predictions. However, future models may benefit from incorporating environmental factors into abundance estimates. Model prediction errors should also be considered when the results are utilized in decision-making. Finally, while our study provides valuable insights into the geospatial distribution of Cx. quinquefasciatus, further research is needed to understand the complex interactions between environmental factors, vector ecology, the built environment, and WNV transmission dynamics in large urban centers.
Conclusion
Our study contributes to the growing literature on the geospatial epidemiology of mosquito-borne diseases and their vectors. This information offers valuable insights into areas that may require additional surveillance or outreach and education initiatives [25]. By elucidating the geospatial and temporal distribution of Cx. quinquefasciatus in Harris County, Texas, our findings support evidence-based decision-making and targeted interventions in high-risk areas. These findings also highlight the importance of considering socioeconomic disparities and culturally relevant educational initiatives when developing mosquito surveillance and control efforts. The resulting interventions should be designed using an integrated approach, fostering collaboration across multiple county sectors, including public health, housing, planning and development, and public works, to effectively manage WNV and other mosquito-borne diseases in our most vulnerable communities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization: M.J. and S.M.G.; Data curation: M.V.; Formal analysis: M.J.; Funding acquisition: M.S.N. and S.M.G.; Investigation: M.J.; Methodology: M.J., R.R., A.O. and S.M.G.; Project administration: S.M.G.; Resources: M.S.N. and S.M.G.; Software: M.J.; Supervision: E.L.B. and S.M.G.; Validation: M.J.; Visualization: M.J.; Writing – original draft: M.J.; Writing - review & editing: M.J., M.S.N., R.R., H.T.E., A.O., E.L.B., M.V. and S.M.G.
Funding
This work was partially supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI165560. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moser SK, et al. Scoping review of Culex mosquito life history trait heterogeneity in response to temperature. Parasit Vectors. 2023;16:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lillibridge KM, et al. The 2002 introduction of West Nile virus into Harris County, Texas, an area historically endemic for St. Louis encephalitis. Am J Trop Med Hyg. 2004;70:676–81. [PubMed] [Google Scholar]
- 3.Molaei G, et al. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County. Tex Am J Trop Med Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- 4.Poh KC, Medeiros MCI, Hamer GL. Landscape and demographic determinants of Culex infection with West Nile virus during the 2012 epidemic in Dallas County, TX. Spat. Spatio-Temporal Epidemiol. 2020;33:100336. [DOI] [PubMed] [Google Scholar]
- 5.Burke R, Barrera R, Lewis M, Kluchinsky T, Claborn D. Septic tanks as larval habitats for the mosquitoes aedes aegypti and Culex quinquefasciatus in Playa-Playita, Puerto Rico. Med Vet Entomol. 2010;24:117–23. [DOI] [PubMed] [Google Scholar]
- 6.Reisen WK. The contrasting Bionomics of Culex mosquitoes in Western North America. J Am Mosq Control Assoc. 2012;28:82–91. [DOI] [PubMed] [Google Scholar]
- 7.Ciota AT, Matacchiero AC, Kilpatrick AM, Kramer LD. The Effect of temperature on Life History traits of Culex mosquitoes. J Med Entomol. 2014;51:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerberg EJ, Barnard DR, Ward RA. Manual for Mosquito Rearing and experimental techniques. Lake Charles, La: American Mosquito Control Association; 1994. [Google Scholar]
- 9.Mann JG, et al. Feeding habits of Vector mosquitoes in Harris County, TX, 2018. J Med Entomol. 2020;57:1920–9. [DOI] [PubMed] [Google Scholar]
- 10.Gorris ME, et al. Updated distribution maps of predominant Culex mosquitoes across the Americas. Parasit Vectors. 2021;14:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilke ABB, et al. Urbanization favors the proliferation of Aedes aegypti and Culex quinquefasciatus in urban areas of Miami-Dade County, Florida. Sci Rep. 2021;11:22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Historic Data. (1999–2022) | West Nile Virus | CDC. https://www.cdc.gov/westnile/statsmaps/historic-data.html (2023).
- 13.Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases. West Nile Virus. West Nile Virus https://www.cdc.gov/westnile/statsmaps/data-and-maps.html (2023).
- 14.Soto RA, Hughes ML, Staples JE, Lindsey NP. West Nile Virus and Other Domestic Nationally Notifiable Arboviral Diseases — United States, 2020. 71, (2022). [DOI] [PMC free article] [PubMed]
- 15.Garcia MN, Hasbun R, Murray KO. Persistence of West Nile virus. Microbes Infect. 2015;17:163–8. [DOI] [PubMed] [Google Scholar]
- 16.Martinez D, et al. West Nile Virus Outbreak in Houston and Harris County, Texas, USA, 2014. Emerg Infect Dis. 2017;23:1372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Economic Cost Analysis of West Nile Virus Outbreak. Sacramento County, California, USA, 2005 - PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3322011/ [DOI] [PMC free article] [PubMed]
- 18.Sejvar JJ. West Nile Virus: an historical overview. Ochsner J. 2003;5:6–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Moise IK, Riegel C, Muturi EJ. Environmental and social-demographic predictors of the southern house mosquito Culex quinquefasciatus in New Orleans, Louisiana. Parasit Vectors. 2018;11:249–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockaby G, et al. Climatic, ecological, and Socioeconomic Factors Associated with West Nile Virus incidence in Atlanta, Georgia, U.S.A. J Vector Ecol. 2016;41:232–43. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz MO, Walker ED, Foster ES, Haramis LD, Kitron UD. Association of West Nile virus illness and urban landscapes in Chicago and Detroit. Int J Health Geogr. 2007;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible — the Neighborhood Atlas. N Engl J Med. 2018;378:2456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. West Nile virus. https://www.who.int/news-room/fact-sheets/detail/west-nile-virus (2017).
- 24.Flanagan BE, Gregory EW, Hallisey EJ, Heitgerd JL, Lewis B. A Social Vulnerability Index for Disaster Management. J Homel Secur Emerg Manag 8, (2011).
- 25.Bondo KJ, et al. Spatial modeling of two mosquito vectors of West Nile virus using integrated nested Laplace approximations. Ecosphere. 2023;14:e4346. [Google Scholar]
- 26.Caillouët KA, Carlson JC, Wesson D, Jordan F. Colonization of abandoned swimming pools by larval mosquitoes and their predators following Hurricane Katrina. J Vector Ecol J Soc Vector Ecol. 2008;33:166–72. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz MO, Tedesco C, McTighe TJ, Austin C, Kitron U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int J Health Geogr. 2004;3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troyo A, Fuller DO, Calderón-Arguedas O, Beier JC. A geographical sampling method for surveys of mosquito larvae in an urban area using high-resolution satellite imagery. J Vector Ecol J Soc Vector Ecol. 2008;33:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezenwa VO, et al. Land cover variation and West Nile virus prevalence: patterns, processes, and implications for disease control. Vector-Borne Zoonotic Dis. 2007;7:173–80. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz MO, et al. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit Vectors. 2010;3:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moise IK, Brown KS, Riegel C, Kalipeni E, Ruiz M. O. Geographic assessment of unattended swimming pools in post-katrina New Orleans, 2006–2008. Ann Assoc Am Geogr. 2013;103:1160–75. [Google Scholar]
- 32.Reisen WK, Takahashi RM, Carroll BD, Quiring R. Delinquent mortgages, neglected swimming pools, and West Nile virus, California. Emerg Infect Dis. 2008;14:1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batistella Pasini MP, Dal’Col Lúcio A, Cargnelutti AF. Semivariogram models for estimating fig fly population density throughout the year. Pesqui Agropecuária Bras. 2014;49:493–505. [Google Scholar]
- 34.Ryan PA, Lyons SA, Alsemgeest D, Thomas P, Kay BH. Spatial statistical analysis of adult mosquito (Diptera: Culicidae) counts: an example using light trap data, in Redland Shire, southeastern Queensland, Australia. J Med Entomol. 2004;41:1143–56. [DOI] [PubMed] [Google Scholar]
- 35.Wilson AL, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14:e0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah UA, Daguma D, Fredregill C. Harris County Public Health Uses Global Health Innovations to Prevent Infectious Mosquito-Borne Diseases in Harris County. (2019).
- 37.Texas Water Development Board. 2022 Texas State Water Plan. https://texasstatewaterplan.org/county/Harris
- 38.Morandeira NS, et al. An interdisciplinary approach to assess human health risk in an urban environment: a case study in temperate Argentina. Heliyon. 2019;5:e02555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen-Gammon J et al. Assessment of Historic and Future Trends of Extreme Weather in Texas, 1900-2036-2024 Update. (2024).
- 40.U.S. Census Bureau QuickFacts. Harris County, Texas. https://www.census.gov/quickfacts/fact/table/harriscountytexas/PST045222
- 41.Census Bureau US, Population Division. Incorporated Places and Minor Civil Divisions Datasets: Subcounty Resident Population Estimates: April 1, 2020 to July 1, 2022 (SUB-EST2022). (2023).
- 42.National Oceanic and Atmospheric Administration. National Weather Service Forecast Office Houston/Galveston, TX.
- 43.NOAA National Centers for Environmental Information. Climate at a Glance: National Mapping. (2024).
- 44.Vigilant M, Battle-Freeman C, Braumuller KC, Riley R, Fredregill CL. Harris County Public Health Mosquito and Vector Control Division Emergency Response to Hurricane Harvey: Vector-Borne Disease Surveillance and Control. J Am Mosq Control Assoc. 2020;36:15–27. [DOI] [PubMed] [Google Scholar]
- 45.Environmental Surveillance | Mosquitoes | CDC. https://www.cdc.gov/mosquitoes/guidelines/west-nile/surveillance/environmental-surveillance.html (2022).
- 46.Karki S, et al. Effect of trapping methods, Weather, and Landscape on estimates of the Culex Vector Mosquito abundance. Environ Health Insights. 2016;10:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao J. Fundamentals of spatial analysis and modelling. Taylor and Francis; 2021.
- 48.Chang K-T. Introduction to Geographic Information Systems. New York: McGraw-Hill Education; 2019. [Google Scholar]
- 49.Krivoruchko K. Empirical bayesian kriging. ArcUser Fall. 2012;6:1145. [Google Scholar]
- 50.Kinder Institute For Urban Research - Urban Data Platform Team. Community Tabulation Areas 2020 (Version 1). 10.25612/837.6EP1WPN43RED (2022).
- 51.University of Wisconsin School of Medicine and Public Health. 2021 Area Deprivation Index v4. (2021).
- 52.US Census Bureau. TIGER/Line Shapefiles and TIGER/Line Files. Census.gov https://www.census.gov/programs-surveys/geography/technical-documentation/complete-technical-documentation/tiger-geo-line.html
- 53.Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases. Historic Data (1999–2023). West Nile Virus Historical Data. https://www.cdc.gov/west-nile-virus/data-maps/historic-data.html (2024).
- 54.Nielsen CF, et al. Risk factors associated with human infection during the 2006 West Nile virus outbreak in Davis, a residential community in Northern California. Am J Trop Med Hyg. 2008;78:53–62. [PMC free article] [PubMed] [Google Scholar]
- 55.Soh S, Aik J. The abundance of Culex mosquito vectors for West Nile Virus and other flaviviruses: a time-series analysis of rainfall and temperature dependence in Singapore. Sci Total Environ. 2021;754:142420. [DOI] [PubMed] [Google Scholar]
- 56.Gardner AM, Lampman RL, Muturi EJ. Land use patterns and the risk of West Nile virus transmission in central Illinois. Vector Borne Zoonotic Dis Larchmt N. 2014;14:338–45. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, et al. Integrated West Nile Virus Surveillance in Harris County, Texas, 2003 to 2018. Online J Public Health Inf. 2019;11:e392. [Google Scholar]
- 58.City of Houston Planning and Development Department. Super Neighborhood 82 - Magnolia Park. https://www.houstontx.gov/superneighborhoods/82.html (2021).
- 59.City of Houston Planning and Development Department. Super Neighborhood 88 - Lawndale/Wayside. https://www.houstontx.gov/superneighborhoods/88.html (2021).
- 60.City of Houston Planning and Development Department. Super Neighborhood 63 - Second Ward. https://www.houstontx.gov/superneighborhoods/63.html (2021).
- 61.Karki S, Brown WM, Uelmen J, Ruiz MO, Smith RL. The drivers of West Nile virus human illness in the Chicago, Illinois, USA area: fine scale dynamic effects of weather, mosquito infection, social, and biological conditions. PLoS ONE. 2020;15:e0227160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skaff NK et al. Thermal thresholds heighten sensitivity of West Nile virus transmission to changing temperatures in coastal California. Proc. Biol. Sci. 287, 20201065 (2020). [DOI] [PMC free article] [PubMed]
- 63.Paz S. Climate change impacts on West Nile virus transmission in a global context. Philos Trans R Soc B Biol Sci. 2015;370:20130561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens Quinquefasciatus say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis Larchmt N. 2007;7:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reisen WK. Ecology of West Nile Virus in North America. Viruses. 2013;5:2079–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang X, Athrey GN, Kaufman PE, Fredregill C, Slotman MA. Effective population size of Culex quinquefasciatus under insecticide-based vector management and following Hurricane Harvey in Harris County, Texas. Front Genet. 2023;14:1297271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thongsripong P, Hyman JM, Kapan DD, Bennett SN. Human–mosquito contact: a missing link in our understanding of Mosquito-Borne Disease Transmission dynamics. Ann Entomol Soc Am. 2021;114:397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poh KC, et al. The influence of weather and weather variability on mosquito abundance and infection with West Nile virus in Harris County, Texas, USA. Sci Total Environ. 2019;675:260–72. [DOI] [PubMed] [Google Scholar]
- 69.Bisanzio D, et al. Arboviral diseases and poverty in Alabama, 2007–2017. PLoS Negl Trop Dis. 2021;15:e0009535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chun Y, Griffith DA. Spatial statistics and geostatistics: theory and applications for Geographic Information Science and Technology. Sage; 2013.
- 71.Bataineh AL, et al. Spatial autocorrelation and pseudoreplication in fire ecology. Fire Ecol. 2006;2:107–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.