Abstract
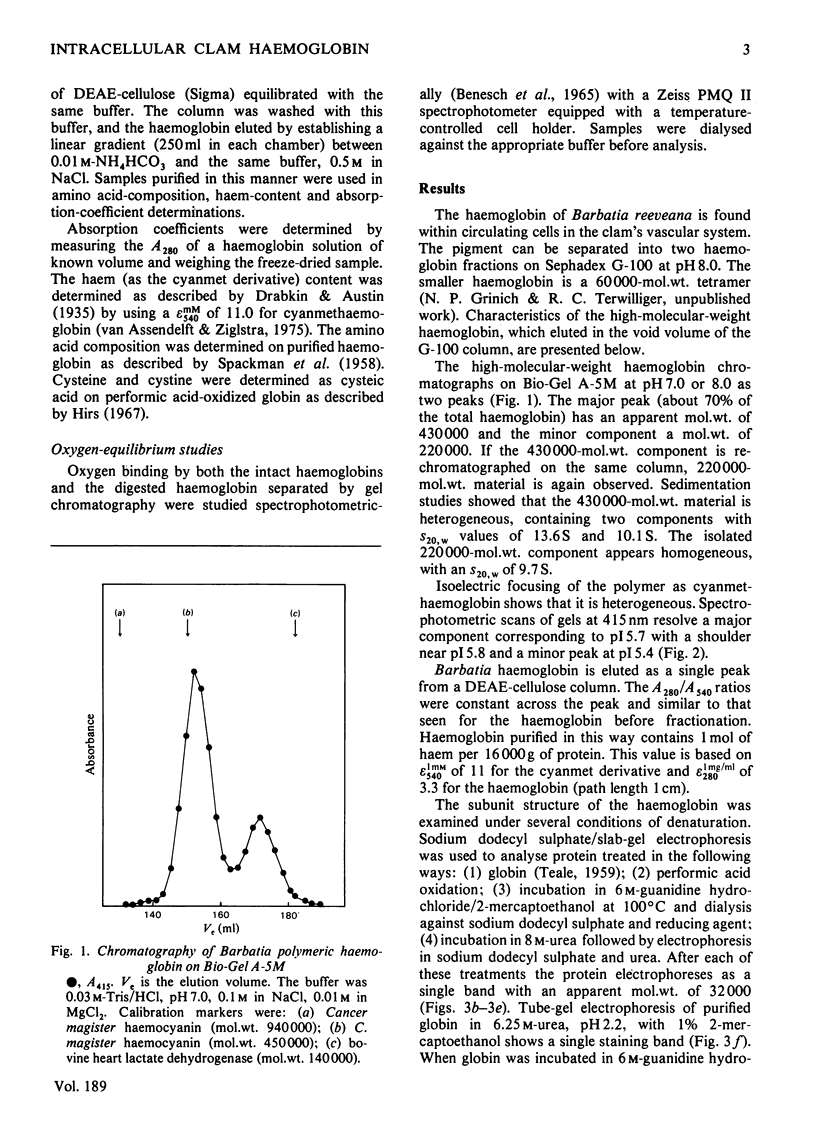
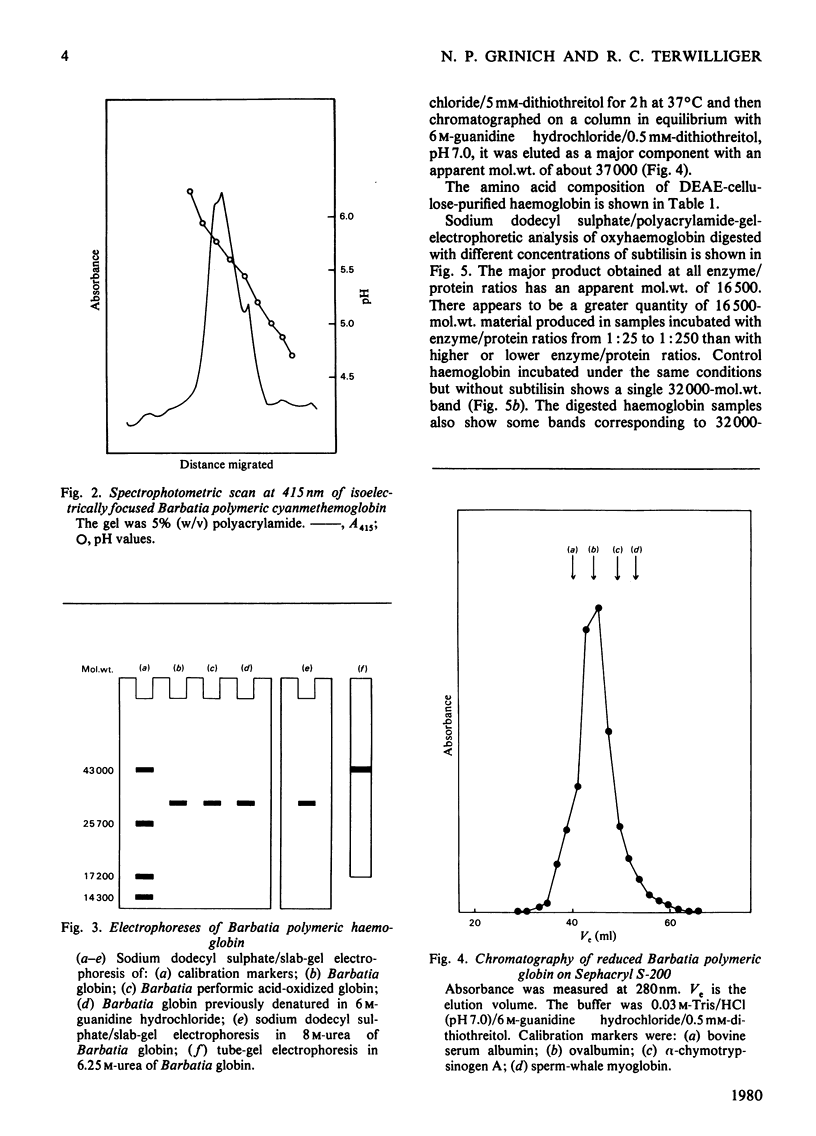
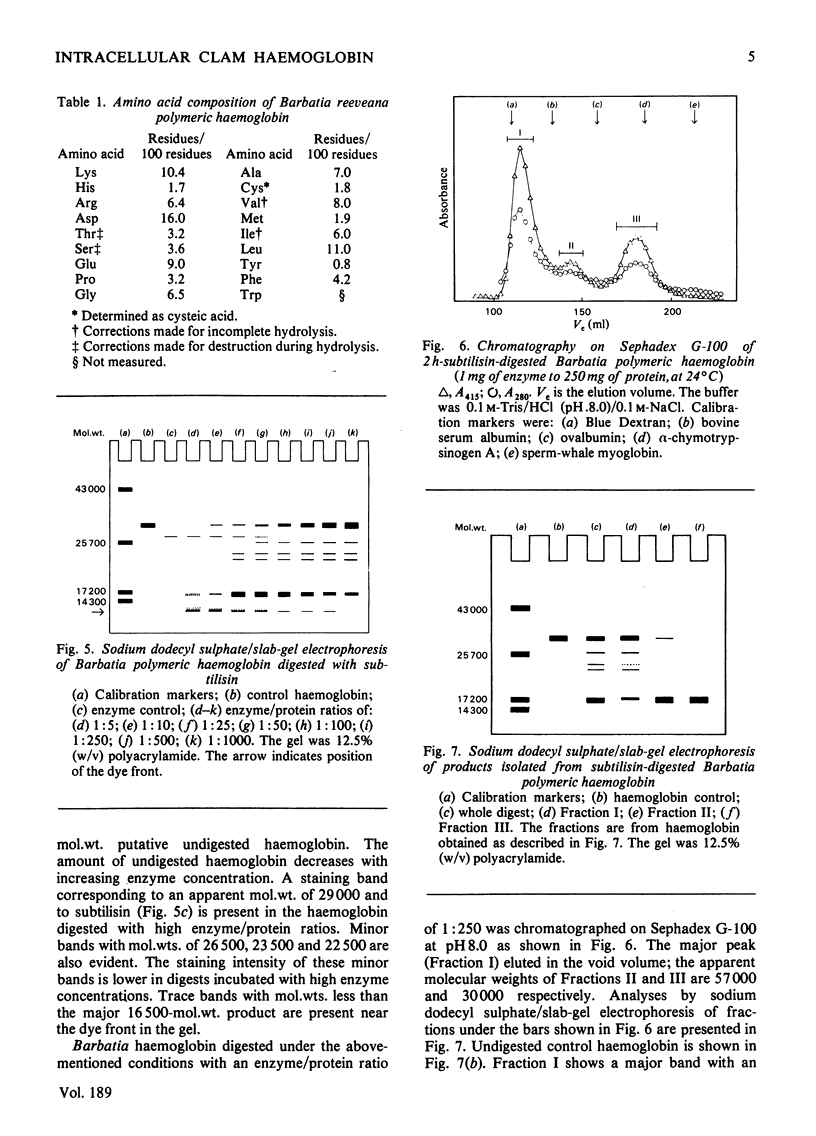
The arcid clam Barbatia reeveana contains an intracellular haemoglobin with an unusual structure. First, compared with other intracellular haemoglobins, it is extremely large, with a mol.wt. of 430000 and an s20,w of 13.6S. A minor component (mol.wt.=220000; s20,w=9.7S) is also present as a probable dissociation product of the major component. Secondly, this haemoglobin has an unusual subunit structure. It contains 1mol of haem per 16000g of protein, in common with most other haemoglobins. However, the smallest polypeptide that could be obtained after treatment with sodium dodecyl sulphate or 6m-guanidine with reducing agent has a mol.wt. of 32000–37000. Digestion of the haemoglobin with the proteinase subtilisin produces both 57000- and 30000-mol.wt. aggregates that contain 1mol of haem per 16000g of protein and that can be dissociated into 16500-mol.wt. polypeptides by treatment with sodium dodecyl sulphate. The intact polymer shows slight co-operativity (h=1.7), lacks a Bohr effect between pH7 and 8, and has a low oxygen affinity [P50=4.8kPa (36mmHg) at 20°C] relative to other haemoglobins. The 30000-mol.wt. aggregate obtained by digestion of the polymer binds oxygen reversibly with an affinity greater than that of the polymer, but with some co-operativity (h=1.7). These results are consistent with the hypothesis that the subunits of this unusually large intracellular haemoglobin are 32000-mol.wt. polypeptides that in turn are composed of two covalently linked haem-containing oxygen-binding domains. This is the first report of an intracellular haemoglobin with such a structure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki T., Okazaki T., Kajita A., Shukuya R. Polymerization of oxygenated and deoxygenated bullfrog hemoglobins. Biochim Biophys Acta. 1974 Jun 7;351(2):427–436. doi: 10.1016/0005-2795(74)90207-4. [DOI] [PubMed] [Google Scholar]
- BENESCH R., MACDUFF G., BENESCH R. E. DETERMINATION OF OXYGEN EQUILIBRIA WITH A VERSATILE NEW TONOMETER. Anal Biochem. 1965 Apr;11:81–87. doi: 10.1016/0003-2697(65)90045-x. [DOI] [PubMed] [Google Scholar]
- Dangott L. J., Terwilliger R. C. Structural studies of a branchiopod crustacean (Lepidurus bilobatus) extracellular hemoglobin. Evidence for oxygen-binding domains. Biochim Biophys Acta. 1979 Aug 28;579(2):452–461. doi: 10.1016/0005-2795(79)90072-2. [DOI] [PubMed] [Google Scholar]
- Ellerton H. D., Carpenter D. E., Van Holde K. E. Physical studies of hemocyanins. V. Characterization and subunit structure of the hemocyanin of Cancer magister. Biochemistry. 1970 May 26;9(11):2225–2232. doi: 10.1021/bi00813a002. [DOI] [PubMed] [Google Scholar]
- Elli R., Giuliani A., Tentori L., Chiancone E., Antonini E. The hemoglobin of amphibia. X. Sedimentation behaviour of frog, triton and axolotl hemoglobins. Comp Biochem Physiol. 1970 Sep 1;36(1):163–171. doi: 10.1016/0010-406x(70)90662-6. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Furuta H., Ohe M., Kajita A. Subunit structure of hemoglobins from erythrocytes of the blood clam, Anadara broughtonii. J Biochem. 1977 Dec;82(6):1723–1730. doi: 10.1093/oxfordjournals.jbchem.a131870. [DOI] [PubMed] [Google Scholar]
- Harrington J. P., Suarez G., Borgese T. A., Nagel R. L. Subunit interactions of Glycera dibranchiata hemoglobin. J Biol Chem. 1978 Oct 10;253(19):6820–6825. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Onoki S., Mitomi Y., Hata R., Satake K. Heterogeneity of hemoglobin from Arca (Anadara satowi). Molecular weights and oxygen equilibria of Arca Hb I and II. J Biochem. 1973 Apr;73(4):717–725. doi: 10.1093/oxfordjournals.jbchem.a130134. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Poole T., Leach B. S., Fish W. W. Analysis of polypeptide molecular weights by electrophoresis in urea. Anal Biochem. 1974 Aug;60(2):596–607. doi: 10.1016/0003-2697(74)90272-3. [DOI] [PubMed] [Google Scholar]
- Sasakawa S., Satake K. On the molecular weight of hemoglobin from Anadara inflata at various pH. J Biochem. 1967 Jul;62(1):139–140. doi: 10.1093/oxfordjournals.jbchem.a128629. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Terwilliger N. B., Terwilliger R. C. Oxygen binding domains of a clam (Cardita borealis) extracellular hemoglobin. Biochim Biophys Acta. 1978 Nov 20;537(1):77–85. doi: 10.1016/0005-2795(78)90604-9. [DOI] [PubMed] [Google Scholar]
- Terwilliger N. B., Terwilliger R. C., Schabtach E. The quaternary structure of a molluscan (Helisoma trivolvis) extracellular hemoglobin. Biochim Biophys Acta. 1976 Nov 26;453(1):101–110. doi: 10.1016/0005-2795(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Wood E. J., Gullick W. J. Planorbis corneus haemoglobin. Circular dichroism and susceptibility to proteases. Biochim Biophys Acta. 1979 Feb 26;576(2):456–465. doi: 10.1016/0005-2795(79)90420-3. [DOI] [PubMed] [Google Scholar]
- van Assendelft O. W., Zijlstra W. G. Extinction coefficients for use in equations for the spectrophotometric analysis of haemoglobin mixtures. Anal Biochem. 1975 Nov;69(1):43–48. doi: 10.1016/0003-2697(75)90563-1. [DOI] [PubMed] [Google Scholar]


