Abstract
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder occurring in women of reproductive age. The disease is caused by a complex interplay of genetic and environmental factors including genes encoding components of the hypothalamic-pituitary-adrenal (HPA) axis. We have recently reported the association of melanocortin receptor genes (MC1R, MC2R, MC3R, MC4R, and MC5R) with the risk of type 2 diabetes (T2D) and/or major depressive disorder (MDD). The latter 2 disorders are comorbid with PCOS. In this study, we used microarray to test 12 single nucleotide polymorphisms (SNPs) in the MC1R gene, 10 SNPs in the MC2R gene, 5 SNPs in the MC3R gene, 6 SNPs in the MC4R gene, and 4 SNPs in the MC5R gene in 212 original Italian families with PCOS. We identified 1 SNP in MC1R, 1 SNP in MC2R, 2 SNPs in MC3R, and 2 SNPs in MC5R significantly linked and/or associated to/with the risk of PCOS in Italian families. This is the first study to report the novel implication of melanocortin receptor genes (MC1R, MC2R, and MC5R) in PCOS. MC3R and MC4R were previously reported in PCOS. However, functional studies are needed to validate these results.
Keywords: Polycystic ovary syndrome, PCOS, Melanocortin receptor gene, Melanocortin receptor 1 gene, MC1R, Melanocortin receptor 2 gene, MC2R, Melanocortin receptor 3 gene, MC3R, Melanocortin receptor 4 gene, MC4R, Melanocortin receptor 5 gene, MC5R, Hypothalamic-pituitary-adrenal axis, HPA axis, Linkage disequilibrium, LD, Association, Novel, Variant, Single nucleotide polymorphism, SNP, Microarray, Type 2 diabetes, Depression, Anxiety, Insomnia
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age, characterized by hyperandrogenism, irregular menses, anovulation, and polycystic ovaries. It is associated with an increased risk of infertility [1], type 2 diabetes (T2D) [2], hypertension [3], depression, anxiety [4, 5], and insomnia [6], and insulin resistance (IR) [7], which may be linked to impaired stress response [8]. In addition to metabolic and hormonal factors, the neuroendocrine system plays an essential and central role in the pathophysiology of PCOS and remains up-to-date in recent research. An imbalance in the pattern of gonadotropin-releasing hormone production can lead to a disruption in the hypothalamic-pituitary-ovarian or adrenal axis, which in turn has been associated with the development of PCOS [9].
The hypothalamic-pituitary-adrenal (HPA) axis, which regulates stress response [10], shows increased activation, pro-inflammatory mediators, and psychological distress and maladaptive stress-driven HPA axis activation in women with PCOS [11]. HPA dysfunction is implicated in the metabolic and inflammatory aspects of PCOS, including IR [12, 13].
In some publications on PCOS, genetic mechanisms are particularly emphasized, and it is stated that these genetic changes may be related to some metabolic consequences of PCOS [13, 14]. Genes potentially contributing to HPA axis-related predisposition to T2D, major depressive disorder (MDD), and possibly PCOS include the corticotropin-releasing hormone receptors (CRHR1 and CRHR2) [15, 16], melanocortin receptors (MC1R–MC5R) [17], glucocorticoid receptor (NR3C1) [18], and mineralocorticoid receptor (NR3C2) [19]. Melanocortin receptor genes encode key feeding and metabolic regulators (MC3R, MC4R, and MC5R) [20–23] and components of the HPA axis (MC2R) [24]. While MC1R is primarily known for its role in skin pigmentation [25], it also contributes to the inflammatory response [26] and obesity [27]. The MC4R gene is overexpressed in the hypothalamus of PCOS rat models [28], and variants in MC3R and MC4R have been identified in Turkish and Chinese individuals with PCOS [29, 30]. Mutations or variations in the MC4R gene have also been associated with obesity [31], a common comorbidity in PCOS [32]. The MC4R gene may influence the development of insulin resistance [33] and hyperandrogenism [34], key features of PCOS [35], by affecting metabolic pathways that regulate glucose and lipid metabolism [36, 37]. Dysfunction in the melanocortin signaling pathway can, therefore, exacerbate the metabolic and reproductive abnormalities seen in women with PCOS. This study aims to investigate the association of melanocortin receptor genes (MC1R–MC5R) with PCOS in Italian families.
Materials and methods
We used microarray to test 12 single nucleotide polymorphisms (SNPs) in the MC1R gene, 10 SNPs in the MC2R gene, 5 SNPs in the MC3R gene, 6 SNPs in the MC4R gene, and 4 SNPs in the MC5R gene in 212 original Italian families with familial history of T2D. The average age at T2D diagnosis was 47.85 years, ranging from 7 to 81, with a median age of 41. The male-to-female ratio was 1.04:1, and the average family size was 5.45. The families were additionally diagnosed with PCOS according to the Rotterdam diagnostic criteria (presence of at least two of the following three characteristics: chronic anovulation or oligomenorrhea, clinical or biological hyperandrogenism, and/or polycystic ovaries) [38]. To diagnose a subject with PCOS, conditions such as thyroid hormonal disorders, hyperprolactinemia, hypothalamic amenorrhea, and congenital adrenal hyperplasia were ruled out. Additionally, two or more of the following criteria had to be met: chronic anovulation or oligomenorrhea, clinical or biochemical hyperandrogenism, and/or the presence of polycystic ovaries. In the familial dataset with T2D, 11% of families had at least one member diagnosed with PCOS. The average BMI of PCOS patients at age 20 was 24.73 (range 19.53–34.08), with 30% classified as overweight (BMI ≥ 25) and 13% as obese (BMI ≥ 30). The average maximum lifetime BMI for these patients was 32.51 (range 20.57–69.85), with 74% being overweight (BMI ≥ 25) and 39% obese (BMI ≥ 30). The average increase in BMI from age 20 to maximum lifetime BMI was 8.91, with a mean BMI increment of 1.36. The SNPs were selected according to their genomic positions as well as minimum allele frequency (MAF) to ensure adequate representation of the gene. The average MAF was 0.06. Families were recruited based on Italian-only ancestry for at least 3 generations, inherited familial T2D, and characterized for additional phenotypes (e.g., PCOS) and traits (e.g., blood pressure). After the exclusion of Mendelian inheritance errors and/or uncertain paternity via the PLINK software [39], we tested the SNPs via the Pseudomarker software [40] for parametric linkage to and/or linkage disequilibrium (LD) – the latter testing linkage and association – with PCOS via the recessive model with complete penetrance (R1) and incomplete penetrance (R2). We then ran a secondary analysis under the dominant models with complete (D1) and incomplete penetrance (D2). The recessive and dominant models were selected based on the genetic architecture of the SNPs studied, as these models can help capture potential associations with PCOS by accounting for different modes of inheritance. We used these models to explore the effects of SNPs under varying genetic assumptions, enhancing the robustness of our findings. PLINK was used for its efficiency and widespread application in genetic studies’ quality control and data management, making it particularly suitable to run quality checks and exclude bad inheritance, uncertain paternity, and errors in genotypes by also analyzing SNP data within a familial dataset [39]. Pseudomarker was chosen for its ability to perform combined linkage and association analysis, which is valuable in family-based studies, as it allows to leverage both linkage and association information to identify genetic variants potentially contributing to PCOS [40]. Tested variants were computed for the presence of LD blocks using correlations from SNPs available in the Toscani Italian population from the 1000 Genomes Project (https://www.internationalgenome.org/data-portal/population/TSI).
We conducted a bioinformatic analysis to predict the significant variant’s role in the expression or function of the corresponding proteins (transcription-factor binding (SNP2TFBS [41]), splicing (SNP-function prediction [42]), miRNA binding (mirSNP [43]), and regulatory potential (RegulomeDB [44]). We selected these tools because they are specifically designed to predict the functional impact of SNPs on transcription-factor binding sites (SNP2TFBS [41] and RegulomeDB [44]), and miRNA target sites (mirSNP [43]), and splicing (SNP-function prediction [42]) These analyses are particularly relevant for our study, given the focus on understanding how genetic variants in melanocortin receptor genes might affect gene regulation and contribute to PCOS pathogenesis.
Results
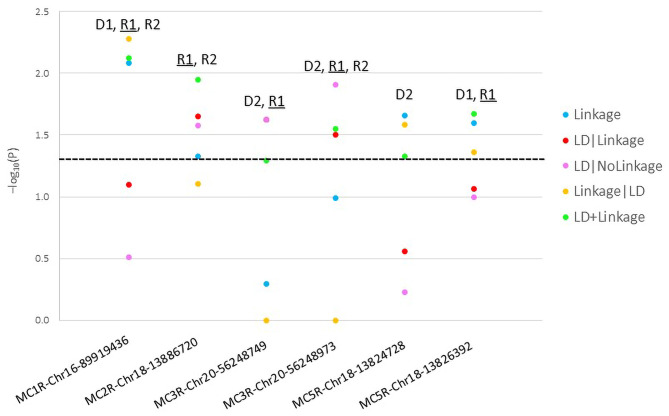
We identified 1 SNP in MC1R, 1 SNP in MC2R, 2 SNPs in MC3R, and 2 SNPs in MC5R significantly linked and/or associated to/with the risk of PCOS in Italian families (Table 1; Fig. 1). Table 1 summarizes the genetic variants studied, their positions, alleles, significant models, potential effects, presence or absence of LD block, and whether they have been previously documented, providing a detailed snapshot of the genetic factors considered in this research. Figure 1 presents the results of the parametric analysis of the risk-associated SNPs in melanocortin receptor genes related to PCOS. None of the variants in MC4R gene was significant. The two SNPs in the MC3R gene were in an LD block (Set01). None of the detected risk variants was previously reported with the risk of PCOS. However, the same risk allele of the variants MC1R-rs1805005, MC3R-rs3746619, and MC3R-rs3827103 were previously associated with the risk of T2D, and the same risk allele of the variant MC5R-rs2236700 was previously associated with the risk of MDD in the same Italian families in this study [17].
Table 1.
Polycystic ovary syndrome (PCOS) melanocortin receptor genes risk single nucleotide polymorphisms (SNPs)
| Gene | Model1 | SNP | Position | REF | ALT | Risk allele | Consequence | LD block | Reported? |
|---|---|---|---|---|---|---|---|---|---|
| MC1R | D1,R1,R2 | rs1805005 | 16:89919436 | G | T | G | Missense (p.R151G) | Independent | T2D [14] |
| MC2R | R1,R2 | rs28926173 | 18:13886720 | G | A | A | Intronic | Independent | Novel |
| MC3R | D2,R1 | rs3746619 | 20:56248749 | C | A | C | 5’-UTR | Set01 | T2D [14] |
| D2,R1,R2 | rs3827103 | 20:56248973 | G | A | G | Missense (p.V44I) | Set01 | T2D [14] | |
| MC5R | D2 | rs59999658 | 18:13824728 | T | G | T | Intronic | NA | Novel |
| D1,R1 | rs2236700 | 18:13826392 | C | G | C | Missense (p.F209L) | Independent | MDD [14] |
Legend: 1Models: D1: dominant, complete penetrance, D2, dominant, incomplete penetrance: R1: recessive, complete penetrance, R2: recessive, incomplete penetrance. LD: linkage-disequilibrium block
Fig. 1.
Parametric analysis results of melanocortin receptor genes risk single nucleotide polymorphisms (SNPs) in polycystic ovary syndrome (PCOS)
Discussion
Our findings highlight the potential role of melanocortin receptor genes in PCOS, building on existing evidence of their involvement in other metabolic and psychological disorders due to their wide tissue distribution and pleiotropic roles [45] The melanocortin receptor genes (MC1R, MC2R, MC3R, MC4R, and MC5R) have been studied in various mental, metabolic, and endocrine disorders [46–48]. We have recently reported the implication of melanocortin receptor genes in the risk of T2D and MDD [17]. In this study, we report the additional implication of melaocortin receptor genes in the susceptibility to PCOS. We reported single variants in each of MC1R and MC2R genes, and two variants in each of MC3R and MC5R genes significantly linked to and associated with PCOS in Italian families. Only the MC3R and MC4R genes were previously reported in PCOS [29, 30]. Therefore, the association that we report in this study of MC1R, MC2R, and MC5R genes with PCOS is novel. Variants in these genes, however, were previously reported in patients with obesity [27, 49] and T2D [17]. Interestingly, no MC4R variant was associated with PCOS in our study despite its well-known involvement in several metabolic derangements such as obesity [49], T2D [50], BMI in PCOS patients [51]. The statistically non-significant association between MC4R and PCOS in our study does not constitute a contradiction but reflects a lack of replication within our dataset.
Comparing our results with studies from other populations reveals both overlaps and unique findings, suggesting potential genetic and environmental interactions influencing PCOS phenotypes [52]. For instance, studies in Asian and Middle Eastern populations have identified other gene variants associated with PCOS, such as FTO and CAPN10, reflecting distinct genetic risk profiles [53, 54]. Further research in diverse cohorts will be critical to validate our findings and understand the broader applicability of melanocortin receptor gene involvement in PCOS.
The functional role of the detected risk variants reported in our study is yet to be determined. No results were predicted frr in-silico functional analysis. Interestingly all risk variants were mostly significant across the R1 model (except for MC5R-Chr18-13824728 [rs59999658]) (Fig. 1). This suggests that the disease mechanism is probably related to abnormal receptor density mediated by the recessive genotypes. Dysfunctional adrenal melanocortin receptor 2 could also possibly divert cellular steroids towards excess androgen production [55]. However, functional studies are needed to confirm these results.
Our study has potential therapeutic implications. A recent study has shown that metformin, which is effective in PCOS by improving metabolic control and ovulatory cycles [56], acts specifically through MC2R and MC3R, potentially mediating an anti-androgenic and weight control effect [57]. However, for clarity, the effect mediated by metformin has not been investigated in the MC2R and MC3R variants identified in our study. Understanding the genetic predisposition involving melanocortin receptors could guide personalized treatment strategies, especially targeting specific pathways affected by these genes. For example, therapies enhancing MC2R function could potentially modulate androgen excess, a core feature of PCOS.
However, our study has limitations. The sample size and familial recruitment approach may limit the robustness of associations observed to this Italian peninsular familial dataset, and replication in larger and ethnically diverse populations is warranted. Additionally, the lack of functional validation of the implicated variants restricts our understanding of their functional role in PCOS pathophysiology. Future studies should aim to explore these genetic associations in functional models and across varied populations to establish a more comprehensive picture of the role of melanocortin receptor genes in PCOS.
Acknowledgements
We thank the families who participated in the study, and we thank Bios Biotech Multi-Diagnostic Health Center, Rome, Italy, for data access and for financial, medical, and laboratory staff support.
Author contributions
C.G. conceived and supervised the project, including statistical analysis and manuscript drafting. R.W. critically helped in data interpretation and critical revision of the manuscript. All authors have approved the final manuscript.
Data availability
The study data are available on reasonable request, and due to lacking specific patients’ consent and privacy restrictions, they are not publicly available.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hart R. PCOS and infertility. Panminerva Med. 2008;50(4):305–14. [PubMed] [Google Scholar]
- 2.Ehrmann DA, et al. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22(1):141–6. [DOI] [PubMed] [Google Scholar]
- 3.Marchesan LB, Spritzer PM. ACC/AHA 2017 definition of high blood pressure: implications for women with polycystic ovary syndrome. Fertil Steril. 2019;111(3):579–e5871. [DOI] [PubMed] [Google Scholar]
- 4.Kolhe JV et al. PCOS and Depression: Common Links and Potential Targets. Reprod Sci, 2021. [DOI] [PubMed]
- 5.Cooney LG, Dokras A. Depression and anxiety in polycystic ovary syndrome: etiology and treatment. Curr Psychiatry Rep. 2017;19(11):83. [DOI] [PubMed] [Google Scholar]
- 6.Franik G, et al. Sleep disturbances in women with polycystic ovary syndrome. Gynecol Endocrinol. 2016;32(12):1014–7. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Qiao J. Association of Insulin Resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): a review of literature. J Healthc Eng. 2022;2022:p9240569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30(1):1–10. [DOI] [PubMed] [Google Scholar]
- 9.Camili FE et al. Oncostatin M is related to polycystic ovary syndrome-case control study. Biomedicines, 2024. 12(2). [DOI] [PMC free article] [PubMed]
- 10.Herman JP, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamanti-Kandarakis E, Economou F. Stress in women: metabolic syndrome and polycystic ovary syndrome. Ann N Y Acad Sci. 2006;1083:54–62. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77(4):300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokmak A, et al. Effect of obesity on clinical parameters and pregnancy rates in women with polycystic ovary syndrome undergoing ovulation induction cycles. J Reprod Med. 2017;62(5–6):300–4. [PubMed] [Google Scholar]
- 14.Guney G, et al. The role of ERK-1 and ERK-2 gene polymorphisms in PCOS pathogenesis. Reprod Biol Endocrinol. 2022;20(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin M, et al. Comorbidity of Novel CRHR2 gene variants in type 2 diabetes and depression. Int J Mol Sci. 2022;23(17):9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gragnoli C. Hypothesis of the neuroendocrine cortisol pathway gene role in the comorbidity of depression, type 2 diabetes, and metabolic syndrome. Appl Clin Genet. 2014;7:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin M et al. Implication of Melanocortin Receptor Genes in the familial comorbidity of type 2 diabetes and depression. Int J Mol Sci, 2022;23(15). [DOI] [PMC free article] [PubMed]
- 18.Amin M et al. Familial linkage and association of the NR3C1 gene with type 2 diabetes and Depression Comorbidity. Int J Mol Sci, 2022;23(19). [DOI] [PMC free article] [PubMed]
- 19.Amin M et al. Novel linkage and association of the mineralocorticoid receptor gene (NR3C2) with familial type 2 diabetes and depression and their comorbidity. Aspects Mol Med, 2023;1.
- 20.Girardet C, Butler AA. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim Biophys Acta. 2014;1842(3):482–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayers KL, et al. Melanocortin 4 receptor pathway dysfunction in obesity: patient stratification aimed at MC4R agonist treatment. J Clin Endocrinol Metab. 2018;103(7):2601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srisai D, et al. Characterization of the Hyperphagic response to Dietary Fat in the MC4R knockout mouse. Endocrinology. 2011;152(3):890–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji LQ, Hong Y, Tao YX. Melanocortin-5 receptor: Pharmacology and its regulation of Energy Metabolism. Int J Mol Sci, 2022;23(15). [DOI] [PMC free article] [PubMed]
- 24.Novoselova TV, Chan LF, Clark AJL. Pathophysiology of melanocortin receptors and their accessory proteins. Best practice & research. Clinical endocrinology & metabolism; 2018;32(2)93–106. [DOI] [PubMed]
- 25.Healy E. Melanocortin 1 receptor variants, pigmentation, and skin cancer susceptibility. Photodermatol Photoimmunol Photomed. 2004;20(6):283–8. [DOI] [PubMed] [Google Scholar]
- 26.Mun Y, Kim W, Shin D. Melanocortin 1 receptor (MC1R): pharmacological and therapeutic aspects. Int J Mol Sci, 2023;24(15). [DOI] [PMC free article] [PubMed]
- 27.Gerhard GS, et al. Next-generation sequence analysis of genes associated with obesity and nonalcoholic fatty liver disease-related cirrhosis in extreme obesity. Human Hered. 2013;75(2–4):144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nooranizadeh MH, et al. Enhancement of Melanocortin-4 receptor (MC4R) and constancy of Kiss1 mRNAs expression in the Hypothalamic Arcuate Nucleus in a model of polycystic ovary syndrome rat. Galen Med J. 2018;7:e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hepsen S, et al. Melanocortin 3 receptor gene polymorphism is associated with polycystic ovary syndrome in Turkish population. Gynecol Endocrinol. 2019;35(8):685–90. [DOI] [PubMed] [Google Scholar]
- 30.Yuan H, et al. Interaction between common variants of FTO and MC4R is associated with risk of PCOS. Reprod Biol Endocrinol. 2015;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu K, et al. Association between MC4R rs17782313 genotype and obesity: a meta-analysis. Gene. 2020;733:144372. [DOI] [PubMed] [Google Scholar]
- 32.Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med. 2012;30(6):496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sull JW, Kim G, Jee SH. Association of MC4R (rs17782313) with diabetes and cardiovascular disease in Korean men and women. BMC Med Genet. 2020;21(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roldan Martin MB, et al. Mutational analysis of the melanocortin-4 receptor (MC4R) gene in children with premature pubarche and adolescent girls with hyperandrogenism. Fertil Steril. 2004;82(5):1460–2. [DOI] [PubMed] [Google Scholar]
- 35.Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. [DOI] [PubMed] [Google Scholar]
- 36.Morgan DA, et al. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes. 2015;64(6):1976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzger PJ et al. A human obesity-associated MC4R mutation with defective Gq/11α signaling leads to hyperphagia in mice. J Clin Invest, 2024;134(4). [DOI] [PMC free article] [PubMed]
- 38.Rotterdam EA. .-S.P.c.w.g., revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. [DOI] [PubMed] [Google Scholar]
- 39.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiekkalinna T, et al. PSEUDOMARKER: a powerful program for joint linkage and/or linkage disequilibrium analysis on mixtures of singletons and related individuals. Hum Hered. 2011;71(4):256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar S, Ambrosini G, Bucher P. SNP2TFBS - a database of regulatory SNPs affecting predicted transcription factor binding site affinity. Nucleic Acids Res. 2017;45(D1):D139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z, Taylor JA. SNPinfo: Integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Research, 2009. 37(SUPPL. 2). [DOI] [PMC free article] [PubMed]
- 43.Liu C, et al. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genomics. 2012;2012 13(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyle AP, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22(9):1790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Switonski M, Mankowska M, Salamon S. Family of melanocortin receptor (MCR) genes in mammals-mutations, polymorphisms and phenotypic effects. J Appl Genet. 2013;54(4):461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu GS, et al. Sequence polymorphisms of MC1R gene and their association with depression and antidepressant response. Psychiatr Genet. 2010;21(1):14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yilmaz Z, et al. Association between MC4R rs17782313 polymorphism and overeating behaviors. Int J Obes. 2015;39(1):114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinzman JT, et al. GWAS and systems biology analysis of depressive symptoms among smokers from the COPDGene cohort. J Affect Disord. 2019;243:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chagnon YC, et al. Linkage and association studies between the melanocortin receptors 4 and 5 genes and obesity-related phenotypes in the Quebec Family Study. Mol Med. 1997;3(10):663–73. [PMC free article] [PubMed] [Google Scholar]
- 50.Wang N et al. PDX1 and MC4R genetic polymorphisms are associated with type 2 diabetes mellitus risk in the Chinese Han population. BMC Med Genom, 2021;14(1). [DOI] [PMC free article] [PubMed]
- 51.Batarfi AA, et al. MC4R variants rs12970134 and rs17782313 are associated with obese polycystic ovary syndrome patients in the western region of Saudi Arabia. BMC Med Genet. 2019;20(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet. 2019;12:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almawi WY, et al. Differential Association of FTO Gene variants and haplotypes with the susceptibility to polycystic ovary syndrome according to obesity in women with PCOS. Reprod Sci. 2023;30(7):2166–76. [DOI] [PubMed] [Google Scholar]
- 54.Sharma P, et al. Genetic variants of metabolism and inflammatory pathways, and PCOS risk -systematic review, meta-analysis, and in-silico analysis. Gene. 2023;888:147796. [DOI] [PubMed] [Google Scholar]
- 55.Turcu A, et al. Adrenal androgens and androgen precursors-definition, synthesis, regulation and physiologic actions. Compr Physiol. 2014;4(4):1369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fruzzetti F, et al. Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS). Gynecol Endocrinol. 2017;33(1):39–42. [DOI] [PubMed] [Google Scholar]
- 57.Parween S, Rihs S, Flück CE. Metformin inhibits the activation of melanocortin receptors 2 and 3 in vitro: a possible mechanism for its anti-androgenic and weight balancing effects in vivo? J Steroid Biochem Mol Biol. 2020;200:105684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data are available on reasonable request, and due to lacking specific patients’ consent and privacy restrictions, they are not publicly available.