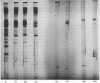
Abstract
A short procedure for the isolation of band-3 protein, the protein responsible for anion exchange in erythrocytes, in a reasonable degree of purity was developed. Using this protein preparation and a novel procedure for membrane-protein reconstitution, vesicles displaying the basic features of the anion-exchange system of the erythrocyte were obtained. The reconstitution procedure is based on slow direct removal of Triton X-100 from aqueous lipid/detergent solutions. According to the composition of the reconstitution medium, either small single-walled or large multi-walled vesicles are obtained. The procedure conserves protein properties well, as is revealed by the similarity of the rates of SO4(2-) exchange in erythrocytes and reconstituted vesicles when corrected for the relevant volumes. A number of functional features of the exchange system were studied and compared with those of the native membrane.
Full text
PDF

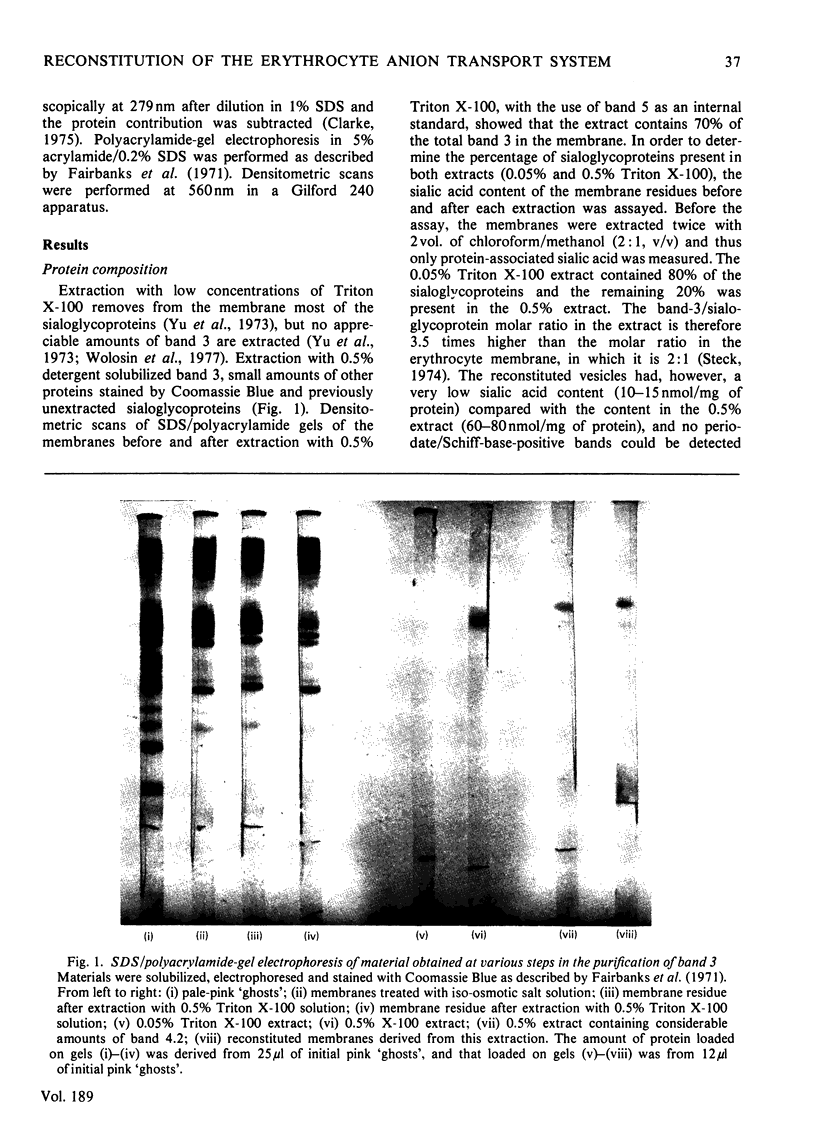
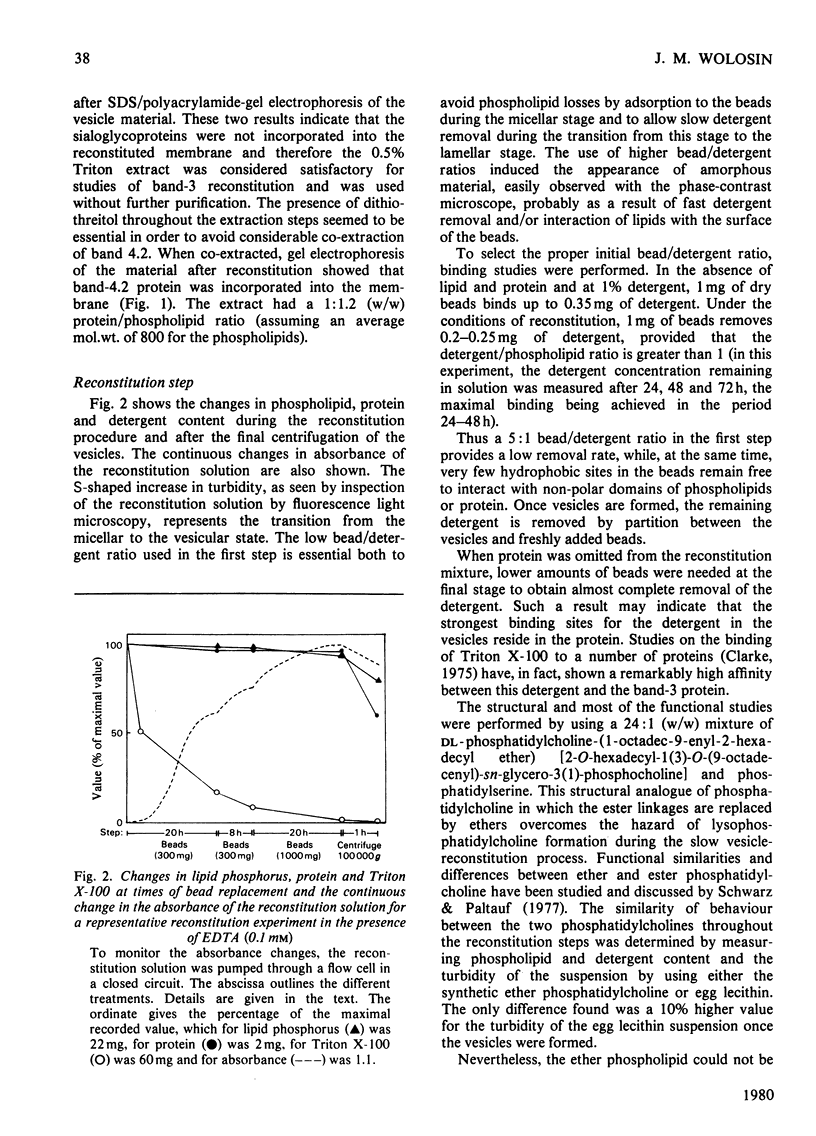
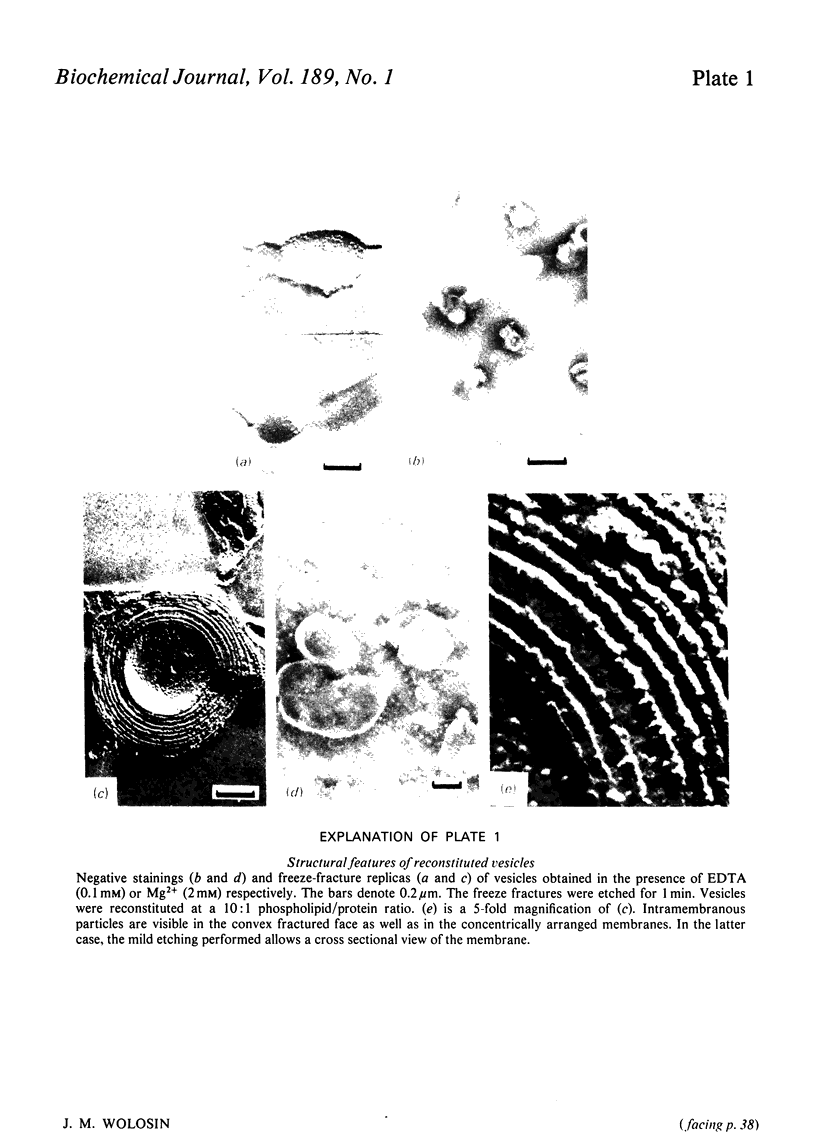
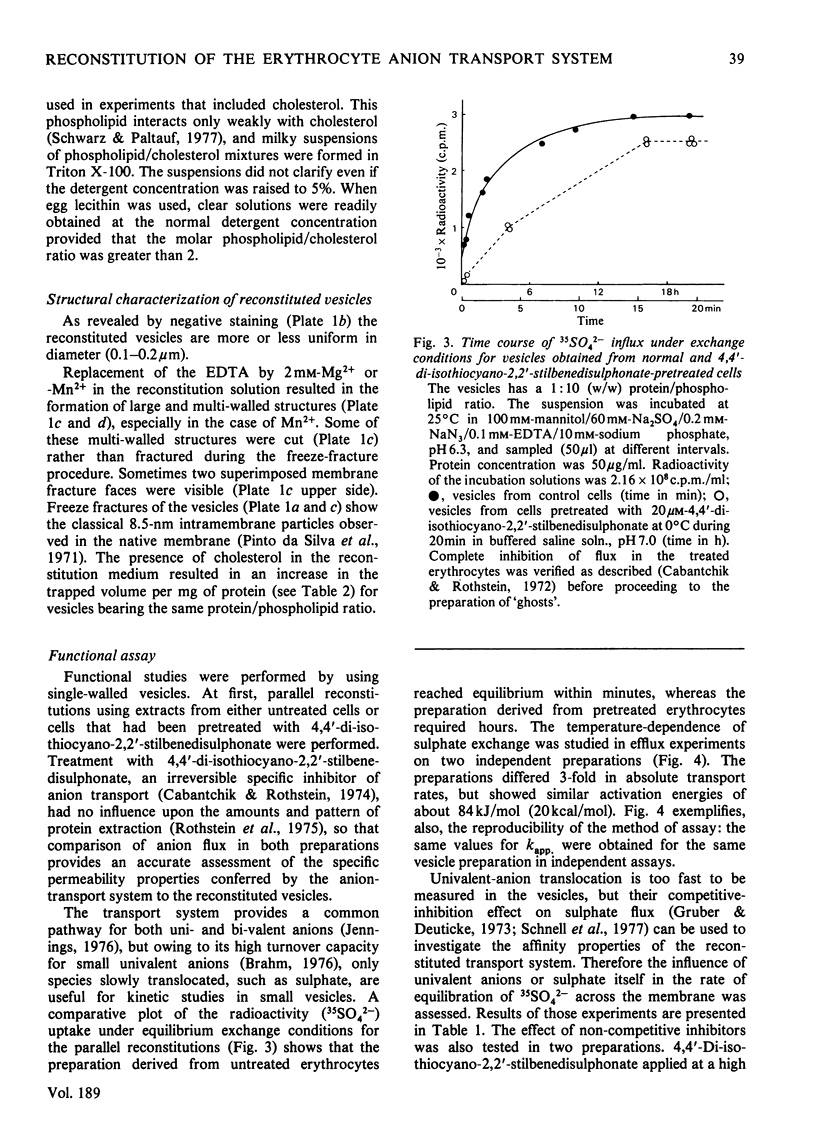
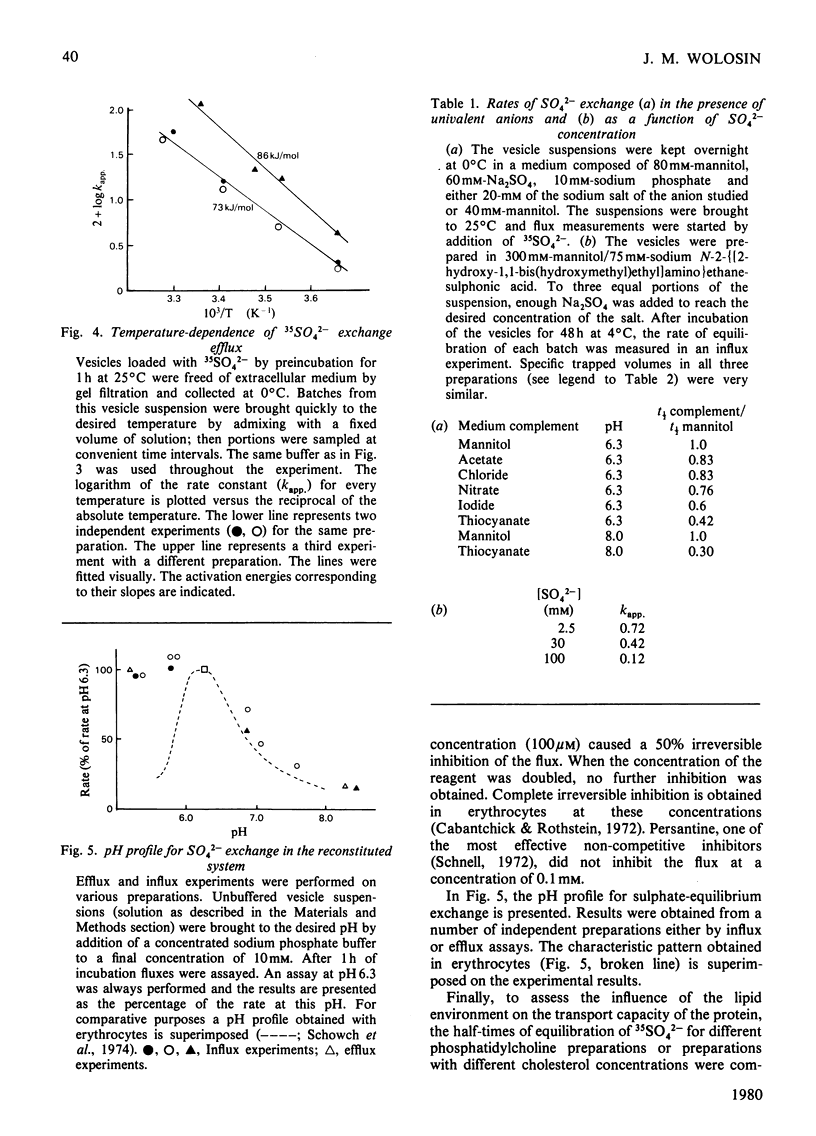




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Bürkli A., Busslinger M., Schneider G., Parish G. R. Rotational diffusion of band 3 proteins in the human erythrocyte membrane. Nature. 1976 Sep 30;263(5576):389–393. doi: 10.1038/263389a0. [DOI] [PubMed] [Google Scholar]
- Clarke S. The size and detergent binding of membrane proteins. J Biol Chem. 1975 Jul 25;250(14):5459–5469. [PubMed] [Google Scholar]
- Dalmark M. Chloride transport in human red cells. J Physiol. 1975 Aug;250(1):39–64. doi: 10.1113/jphysiol.1975.sp011042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Deuticke B., Ruska C. Changes of nonelectrolyte permeability in cholesterol-loaded erythrocytes. Biochim Biophys Acta. 1976 May 21;433(3):638–653. doi: 10.1016/0005-2736(76)90287-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fisher K., Branton D. Application of the freeze-fracture technique to natural membranes. Methods Enzymol. 1974;32:35–44. doi: 10.1016/0076-6879(74)32006-x. [DOI] [PubMed] [Google Scholar]
- Gruber W., Deuticke B. Comparative aspects of phosphate transfer across mammalian erythrocyte membranes. J Membr Biol. 1973 Aug 30;13(1):19–36. doi: 10.1007/BF01868218. [DOI] [PubMed] [Google Scholar]
- Grunze M., Deuticke B. Changes of membrane permeability due to extensive cholesterol depletion in mammalian erythrocytes. Biochim Biophys Acta. 1974 Jul 12;356(1):125–130. doi: 10.1016/0005-2736(74)90300-9. [DOI] [PubMed] [Google Scholar]
- Gunn R. B., Dalmark M., Tosteson D. C., Wieth J. O. Characteristics of chloride transport in human red blood cells. J Gen Physiol. 1973 Feb;61(2):185–206. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hilden S., Rhee H. M., Hokin L. E. Sodium transport by phospholipid vesicles containing purified sodium and potassium ion-activated adenosine triphosphatase. J Biol Chem. 1974 Dec 10;249(23):7432–7440. [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Jenkins R. E., Tanner J. A. The structure of the major protein of the human erythrocyte membrane. Characterization of the intact protein and major fragments. Biochem J. 1977 Jan 1;161(1):139–147. doi: 10.1042/bj1610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. L. Proton fluxes associated with erythrocyte membrane anion exchange. J Membr Biol. 1976 Aug 26;28(2-3):187–205. doi: 10.1007/BF01869697. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- Munn E. A. The application of the negative staining technique to the study of membranes. Methods Enzymol. 1974;32:20–35. doi: 10.1016/0076-6879(74)32005-8. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Moscarello M. Interaction of a purified hydrophobic protein from myelin with phospholipid membranes: studies on ultrastructure, phase transitions and permeability. J Membr Biol. 1975;22(2):143–164. doi: 10.1007/BF01868168. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Douglas S. D., Branton D. Localization of A antigen sites on human erythrocyte ghosts. Nature. 1971 Jul 16;232(5307):194–196. doi: 10.1038/232194a0. [DOI] [PubMed] [Google Scholar]
- Ross A. H., McConnell H. M. Reconstitution of the erythrocyte anion channel. J Biol Chem. 1978 Jul 10;253(13):4777–4782. [PubMed] [Google Scholar]
- Rothstein A., Cabantchik Z. I., Balshin M., Juliano R. Enhancement of anion permeability in lecithin vesicles by hydrophobic proteins extracted from red blood cell membranes. Biochem Biophys Res Commun. 1975 May 5;64(1):144–150. doi: 10.1016/0006-291x(75)90230-2. [DOI] [PubMed] [Google Scholar]
- Rottem S., Cirillo V. P., de Kruyff B., Shinitzky M., Razin S. Cholesterol in mycoplasma membranes. Correlation of enzymic and transport activities with physical state of lipids in membranes of Mycoplasma mycoides var. capri adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):509–519. doi: 10.1016/0005-2736(73)90159-4. [DOI] [PubMed] [Google Scholar]
- Schlieper P., De Robertis E. Triton X-100 as a channel-forming substance in artificial lipid bilayer membranes. Arch Biochem Biophys. 1977 Nov;184(1):204–208. doi: 10.1016/0003-9861(77)90343-5. [DOI] [PubMed] [Google Scholar]
- Schnell K. F., Gerhardt S., Schöppe-Fredenburg A. Kinetic characteristics of the sulfate self-exchange in human red blood cells and red blood cell ghosts. J Membr Biol. 1977 Jan 28;30(4):319–350. doi: 10.1007/BF01869675. [DOI] [PubMed] [Google Scholar]
- Schnell K. F. On the mechanism of inhibition of the sulfate transfer across the human erythrocyte membrane. Biochim Biophys Acta. 1972 Sep 1;282(1):265–276. doi: 10.1016/0005-2736(72)90333-1. [DOI] [PubMed] [Google Scholar]
- Schwarz F. T., Paltauf F. Influence of the ester carbonyl oxygens of lecithin on the permeability properties of mixed lecithin-cholesterol bilayers. Biochemistry. 1977 Oct 4;16(20):4335–4339. doi: 10.1021/bi00639a001. [DOI] [PubMed] [Google Scholar]
- Schwoch G., Rudloff V., Wood-Guth I., Passow H. Effect of temperature on sulfate movements across chemically or enzymatically modified membranes of human red blood cells. Biochim Biophys Acta. 1974 Feb 26;339(1):126–138. doi: 10.1016/0005-2736(74)90338-1. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Gulik-Krzywicki T., Sardet C. Association of the membrane-penetrating polypeptide segment of the human erythrocyte MN-glycoprotein with phospholipid bilayers. I. Formation of freeze-etch intramembranous particles. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3294–3298. doi: 10.1073/pnas.71.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Robards A. W. Plastic deformation during freeze-cleavage: a review. J Microsc. 1977 May;110(1):1–25. doi: 10.1111/j.1365-2818.1977.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Bertaud W. S. Temperature and contamination dependent freeze-etch images of frozen water and glycerol solutions. J Ultrastruct Res. 1971 Oct;37(1):146–168. doi: 10.1016/s0022-5320(71)80047-3. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trissl H. W., Darszon A., Montal M. Rhodopsin in model membranes: charge displacements in interfacial layers. Proc Natl Acad Sci U S A. 1977 Jan;74(1):207–210. doi: 10.1073/pnas.74.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reconstitution of a calcium pump using defined membrane components. Proc Natl Acad Sci U S A. 1974 Mar;71(3):622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin J. M., Ginsburg H., Cabantchik Z. I. Functional characterization of anion transport system isolated from human erythrocyte membranes. J Biol Chem. 1977 Apr 10;252(7):2419–2427. [PubMed] [Google Scholar]
- Yu J., Branton D. Reconstitution of intramembrane particles in recombinants of erythrocyte protein band 3 and lipid: effects of spectrin-actin association. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3891–3895. doi: 10.1073/pnas.73.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]
- Yu J., Steck T. L. Isolation and characterization of band 3, the predominant polypeptide of the human erythrocyte membrane. J Biol Chem. 1975 Dec 10;250(23):9170–9175. [PubMed] [Google Scholar]