Abstract
Background
This study aims to evaluate the long-term outcomes of patients with large coronary arteries (LCA, reference vessel diameter more than 3.0 mm) de novo lesions treated by drug-coated balloon (DCB) versus second-generation drug-eluting stent (sDES) in real-world clinical practice.
Methods
Between January 2020 and June 2021, 2857 consecutive patients with equal number of LCA de novo lesions, including 708 lesions treated with paclitaxel DCB-only (DCB-only cohort) and 2149 lesions with sDES-only (sDES-only cohort), were enrolled in this retrospective study. The primary outcome was the clinically driven target lesion revascularization (CD-TLR) rate at two years. After propensity score matching, 708 patients treated with DCB-only and another 704 patients with sDES-only were successfully matched to study adjusted associations between treatment strategy and outcomes.
Results
CD-TLR rate was higher in the DCB-only group than sDES-only group (DCB: 5.5%, sDES: 3.1%, P = 0.028). However, lower major bleeding rate was observed in the DCB-only group compared to sDES-only group (0.8% vs. 3.0%, P = 0.003), which benefited from its short duration of antiplatelet therapy. Multivariate logistic regression analysis revealed that hypercholesteremia [odds ratio (OR), 2.516], diabetes (OR, 2.773), severe calcified lesions (OR, 5.184) and residual stenosis>30% (OR, 8.676) were risk predictors (P<0.01) of CD-TLR for DCB-only strategy; meanwhile, diabetes (OR, 3.255) and severe calcified lesions (OR, 2.152) were risk predictors (P<0.01) of CD-TLR for sDES strategy.
Conclusions
DCB-only strategy is feasible for LCA de novo lesions in patients with high bleeding risk, but not suitable in other patients, who should first choose intended stenting strategy especially with unmanageable hypercholesteremia, severe calcified lesions or non-ideal residual stenosis after preprocessing.
Keywords: Drug-coated balloon (DCB), Drug-eluting stent (DES), De novo lesions, Large coronary arteries, Real-world study
Introduction
In the 21st century, drug-eluting stents (DES) have become the main method of interventional treatment for coronary artery disease. Compared with the previous bare metal stents, the occurrence of in-stent restenosis has been significantly reduced which is due to the role of antiproliferative drugs on the surface of DES. However, with the implantation of DES, patients have a higher bleeding risk due to long-term use of antiplatelet drugs, and the risk of late stent thrombosis and in-stent restenosis is up to 2% per year [1, 2]. This clinical challenge promotes the application and development of drug-coated balloon (DCB) angioplasty. When DCB dilates coronary artery lesions, antiproliferative drugs are released to the coronary arteries, inhibiting the intima hyperplasia of the vessel wall to reduce the occurrence of target lesion restenosis, avoiding vascular inflammatory response caused by metal or polymer, which may reduce thrombotic events. At the same time, it preserves the normal anatomical structure and diastolic function of the coronary arteries and can also shorten the duration of dual antiplatelet therapy, thereby reducing the risk of bleeding in patients. The treatment effect of in-stent restenosis with DCB had long been clinically confirmed [3]. In recent years, the effect of DCB-only strategy in the treatment of small coronary arteries (SCA) de novo lesions was no inferior to that of DES strategy, which also had been verified [4–6]. However, its efficacy in the treatment of LCA de novo lesions and complex lesions is still controversial [7, 8]. With the popularity of the concept of “intervention without implantation”, numerous interventional physicians had the courage to challenge and had already accumulated certain experience in DCB-only strategy for LCA de novo lesions and complex lesions. They had also carried out single-arm studies and randomized controlled trials(RCT)on small samples to explore the efficacy and safety of DCB-only strategy in these lesions [9–14]. To further clarify this especially important and controversial clinical issue, this retrospective study intends to analyze the long-term prognosis difference between DCB and second-generation DES (sDES) in the treatment of LCA de novo lesions from the perspective of a large sample in the real world, which can better reflect the current clinical practice.
Methods
Study population
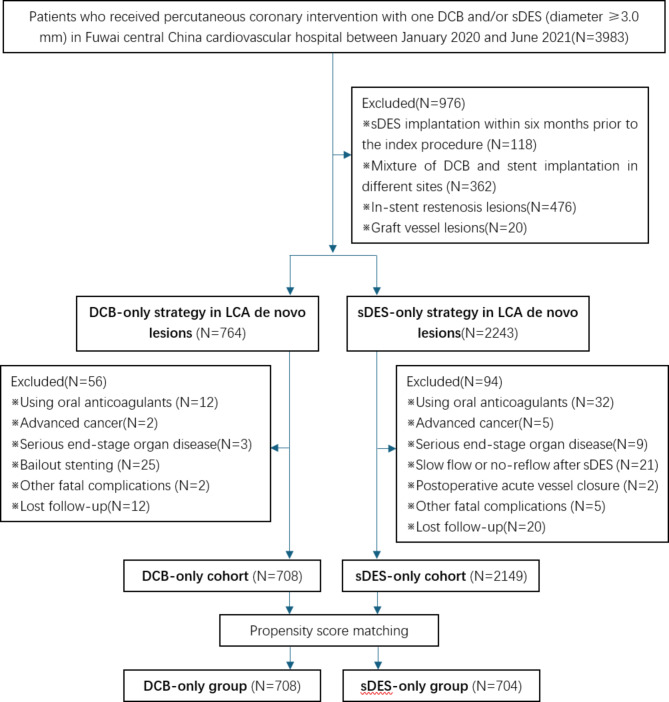
This retrospective study was conducted in Fuwai Central China Cardiovascular hospital from January 2020 to June 2021. Eligible patients were those with LCA de novo lesions (implanted DCB or sDES diameter ≥ 3.0 mm). Exclusion criteria were: (1) sDES implantation within six months prior to the index procedure, (2) mixture of DCB and stent implantation in different sites, (3) using oral anticoagulants, (4) advanced cancer, (5) serious end-stage organ diseases, (6) bailout stenting after DCB, (7) slow flow or no-reflow after sDES, (8) other fatal complications and (9) lost follow-up (shown in Fig. 1). The decision of initial treatment strategy selection (DCB or sDES) was based only on operator discretion, so this was a real-world study, which could better reflect the current clinical practice. All participants confirmed informed consent forms and follow-up agreements before enrolling. Propensity score matching was performed to study adjusted associations between therapeutic strategy and outcomes. The technical proposal of DCB-only strategy for de novo lesions in large coronary arteries was approved by the ethical committee of Fuwai Central China Cardiovascular Hospital and the study protocol was performed in accordance with the Declaration of Helsinki for humans. All patients’ general clinical condition, medication use, lesion characteristics and procedural characteristics were collected.
Fig. 1.
DCB, Drug-coated balloon; sDES, second-generation drug-eluting stent; LCA, large coronary arteries
Lesions judgment and interventional procedure
All angiograms were analyzed using a quantitative coronary angiographic system (QCA, CASS system). Lesions type, Lesions length, reference vessel diameter (RVD), percent diameter stenosis (DS%) and residual DS% after procedure were judged and measured by two independent technicians who were not involved in the interventional procedures. In this study, left main coronary artery (LM) bifurcation lesion which involved (1, 1, 1), (1, 1, 0) and (1, 0, 1) based on the Medina classification system, was classified as LM lesion rather than left anterior descending artery (LAD) lesion or left circumflex artery (LCX) lesion. Based on American College of Cardiology/American Heart Association (ACC/AHA) morphological classification, lesions complexity was divided into Type A, B and C. Based on the NHLBI classification system, dissection after lesions procedure was divided into Type A, B, C, D, E and F. Long lesions were defined as lesion length>20 mm; severe calcified lesions defined as sharp and dense vessel shadows in coronary angiography seen in both beating and non-beating heart; angulation lesions defined as lesion angle> 45°. Ostial lesions defined as within 3 mm of the LM or right coronary artery (RCA) ostium. All DCB of the study were paclitaxel-coated balloons, including Sequent® Please (Braun, Germany), bingo® (YINYI BIOTECH, China), Vesselin® (Lepus Medical, China), RESTORE DEB® (Grand Pharmaceutical, China) and Swide® (Shenqi Medical, China). While all stents of the study were second-generation DES.
Study outcomes and follow-Up
The primary outcome was defined as the clinically driven target lesion revascularization (CD-TLR) at two years. Secondary outcomes were all-cause mortality, major bleeding (Bleeding Academic Research Consortium bleeding type 2–5, BARC 2–5 type bleeding) and major adverse cardiac events (MACE), defined as the composite of CD-TLR, cardiac death and myocardial infarction (MI) also after two years follow-up. When the cause of death was unknown or undeterminable, it was assumed to be cardiac death. Due to inclusion criteria, bifurcation lesions in our study were relatively simple (the side branch wasn’t very narrow or its diameter was smaller). After either one DCB or one sDES implanted, if the side branch was affected, sometimes it was dilated with a regular balloon. CD-TLR in our study also included side branch revascularization therapy. All events were adjudicated by independent clinical researchers who had no knowledge of the research purpose or the patient’s treatment status. All patients were followed-up through telephone interviews, outpatient visits, or hospital records.
Statistical analysis
For continuous variables, mean ± standard deviation was used for normally distributed data, while median with interquartile range (IQR) was calculated for abnormally distributed data. Student’s t-test or the Wilcoxon rank-sum test was used, as appropriate, for the analysis of intergroup differences. For categorical variables, the data were expressed as frequencies with percentages, and intergroup differences were compared by the Chi-square test or Fisher’s exact test. Binary logistic regression analysis was conducted to determine the association between pre-identified covariates of interest and CD-TLR. A 1:1 nearest neighbor propensity score matching by the logistic regression model with matching tolerance of 0.02 was also performed to minimize selection bias. Two-sided P values less than 0.05 were considered statistically significant. All analyses were processed with SPSS 27.0 (IBM, Munich, Germany).
Results
Baseline clinical and lesion characteristics
There were 3983 patients who received percutaneous coronary intervention (PCI) with one DCB and/or sDES (diameter ≥ 3.0 mm) from January 2020 to June 2021 in our hospital. Based on the exclusion criteria, a total of 2857 consecutive patients with equal number of LCA de novo lesions, including 708 lesions treated with DCB only (DCB cohort) and 2149 lesions with sDES only (sDES cohort), were finally recruited (shown in Fig. 1). Unstable angina (UA) was the most common diagnosis in both cohorts, followed by acute non-ST elevation myocardial infarction (NSTEMI), stable coronary artery disease (SCAD) and acute ST elevation myocardial infarction (STEMI) (shown in Table 1). The proportion of type A and type B/C lesions was similar in both cohorts, meanwhile type B/C lesions were significantly more than type A lesions (shown in Table 2).
Table 1.
Baseline clinical characteristics
| Before propensity matching | After propensity matching | |||||
|---|---|---|---|---|---|---|
| DCB | sDES | P-value | sDES | P-value | ||
| Number of patients | 708 | 2149 | 704 | |||
| Age [(years), median (IQR)] | 56(48,65) | 56(50,64) | 0.109 | 57(51,64) | 0.066 | |
| Age>65 years, n (%) | 185(26.1%) | 510(23.7%) | 0.197 | 177(25.1%) | 0.671 | |
| Male, n (%) | 568(80.2%) | 1563(72.7%) | <0.001 | 561(79.7%) | 0.800 | |
| CAD diagnosis | ||||||
| ACS | 662(93.5%) | 2022(94.1%) | 0.570 | 664(94.3%) | 0.522 | |
| UA, n (%) | 499(70.5%) | 1513(70.4%) | 0.970 | 478(67.9%) | 0.293 | |
| NSTEMI, n (%) | 121(17.1%) | 407(18.9%) | 0.272 | 141(20.0%) | 0.156 | |
| STEMI, n (%) | 42(5.9%) | 102(4.8%) | 0.211 | 45(6.4%) | 0.719 | |
| SCAD, n (%) | 46(6.5%) | 127(5.9%) | 0.570 | 40(5.7%) | 0.522 | |
| Previous MI, n (%) | 79(11.2%) | 333(15.5%) | 0.004 | 81(11.5%) | 0.837 | |
| Previous PCI, n (%) | 101(14.3%) | 259(12.1%) | 0.124 | 80(11.4%) | 0.103 | |
| Previous CABG, n (%) | 3(0.4%) | 18(0.8%) | 0.264 | 4(0.5%) | 0.725 | |
| Atrial flutter/Fibrillation, n (%) | 28(4.0%) | 77(3.6%) | 0.648 | 26(3.7%) | 0.798 | |
| Hypertension, n (%) | 411(58.1%) | 1334(62.1%) | 0.057 | 426(60.5%) | 0.347 | |
| Hypercholesteremia, n (%) | 256(36.2%) | 741(34.5%) | 0.417 | 259(36.8%) | 0.805 | |
| Diabetes, n (%) | 239(33.8%) | 734(34.2%) | 0.846 | 233(33.1%) | 0.793 | |
| Heart failure, n (%) | 30(4.2%) | 134(6.2%) | 0.047 | 31(4.4%) | 0.878 | |
| Renal insufficiency, n (%) | 17(2.4%) | 86(4.0%) | 0.048 | 19(2.7%) | 0.723 | |
| Anemia, n (%) | 110(15.5%) | 235(10.9%) | 0.001 | 113(16.1%) | 0.791 | |
| COPD, n (%) | 10(1.4%) | 53(2.5%) | 0.098 | 17(2.4%) | 0.169 | |
| History of smoking, n (%) | 358(50.6%) | 1001(46.6%) | 0.066 | 366(52.0%) | 0.593 | |
| Family history of CAD, n (%) | 21(3.0%) | 50(2.3%) | 0.343 | 13(1.8%) | 0.170 | |
| Examinations | ||||||
| LVEF (%) | 58.48 ± 7.68 | 57.91 ± 9.24 | 0.106 | 57.68 ± 8.87 | 0.071 | |
| Hb (g/L) | 132.11 ± 14.95 | 131.04 ± 17.11 | 0.110 | 130.48 ± 18.93 | 0.072 | |
| sCr (mmol/L) | 73(64,82) | 71(62,84) | 0.189 | 71(62,81) | 0.073 | |
| LDL-C (mmol/L) | 2.07 ± 0.79 | 2.16 ± 0.88 | 0.012 | 2.14 ± 0.86 | 0.111 | |
| Medication use | ||||||
| DAPT, n (%) | 708(100%) | 2149(100%) | 1.000 | 704(100%) | 1.000 | |
| Duration of DAPT (month) | 6(6,12) | 12(12,12) | <0.001 | 12(12,12) | <0.001 | |
| Statins, n (%) | 692(97.7%) | 2122(98.7%) | 0.057 | 694(98.6%) | 0.241 | |
| Beta-Blockers, n (%) | 635(89.7%) | 1899(88.4%) | 0.335 | 639(90.8%) | 0.495 | |
| ACEI/ARB, n (%) | 216(30.5%) | 638(29.7%) | 0.679 | 212(30.1%) | 0.872 | |
| PCSK9i, n (%) | 49(6.9%) | 160(7.4%) | 0.642 | 34(4.8%) | 0.095 | |
Notes: DCB, Drug-coated balloon; sDES, second-generation drug-eluting stent; CAD, coronary artery disease; ACS, acute coronary syndrome; UA, unstable angina; NSTEMI, acute non-ST-elevation myocardial infarction; STEMI, acute ST-elevation myocardial infarction; SCAD, stable coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; Hb, hemoglobin; sCr, serum creatinine; LDL -C, low-density lipoprotein cholesterol; DAPT, dual antiplatelet therapy; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; PCSK9i, the proprotein convertase subtilin Kexin-9 inhibitor; continuous variables were presented as mean ± standard deviation or median (interquartile range, IQR); categoric variables were shown as number (%)
Table 2.
Lesion characteristics
| Before propensity matching | After propensity matching | |||||
|---|---|---|---|---|---|---|
| DCB | sDES | P-value | sDES | P-value | ||
| Number of patients | 708 | 2149 | 704 | |||
| Lesion vessel | ||||||
| LM, n (%) | 7(1.0%) | 186(8.7%) | <0.001 | 13(1.8%) | 0.173 | |
| LAD, n (%) | 381(53.8%) | 1025(47.7%) | 0.005 | 364(51.7%) | 0.427 | |
| LCX, n (%) | 88(12.4%) | 238(11.1%) | 0.326 | 89(12.6%) | 0.904 | |
| RCA, n (%) | 232(32.8%) | 700(32.6%) | 0.924 | 238(33.8%) | 0.679 | |
| Lesion type | ||||||
| Type A | 138(19.5%) | 354(16.5%) | 0.065 | 148(21.0%) | 0.474 | |
| Type B/C | ||||||
| Long lesion, n (%) | 379(53.5%) | 1362(63.4%) | <0.001 | 378(53.7%) | 0.951 | |
| Severe calcified lesion, n (%) | 26(3.7%) | 218(10.1%) | <0.001 | 32(4.5%) | 0.408 | |
| Bifurcation lesion, n (%) | 265(37.4%) | 780(36.3%) | 0.587 | 299(42.5%) | 0.053 | |
| Ostial lesion, n (%) | 1(0.1%) | 14(0.7%) | 0.136 | 4(0.6%) | 0.217 | |
| Angulation lesion, n (%) | 111(15.7%) | 261(12.1%) | 0.015 | 108(15.3%) | 0.861 | |
| Thrombus lesion, n (%) | 16(2.3%) | 68(3.2%) | 0.217 | 24(3.4%) | 0.193 | |
| CTO lesion, n (%) | 30(4.2%) | 103(4.8%) | 0.543 | 27(3.8%) | 0.701 | |
| Lesion parameter | ||||||
| Reference vessel diameter (mm) | 3.4(3.3–3.6) | 3.5(3.2–3.7) | 0.444 | 3.5(3.2–3.7) | 0.404 | |
| Length (mm) | 21.08 ± 7.17 | 24.04 ± 7.79 | <0.001 | 23.07 ± 7.89 | <0.001 | |
| Diameter stenosis (%) | 81.76 ± 8.27 | 81.11 ± 9.23 | 0.077 | 81.58 ± 8.26 | 0.689 | |
Notes: DCB, Drug-coated balloon; sDES, second-generation drug-eluting stent; LM, left main coronary artery; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; CTO, chronic total occlusion; continuous variables were presented as mean ± standard deviation or median (interquartile range, IQR); categoric variables were shown as number (%)
Compared to patients treated with sDES, those with DCB had significantly higher proportion of male, LAD lesions, angulation lesion and history of anemia, while lower proportion of LM lesions, long lesions, severe calcified lesions, history of myocardial infarction, heart failure and renal insufficiency. After propensity score matching by the logistic regression model, 708 patients treated with DCB only and another 704 patients with sDES only were yielded (shown in Fig. 1). The two propensity matched groups were well-balanced in terms of baseline clinical and lesion characteristics except for “duration of DAPT” and “lesion length” variables, however, its difference could be eliminated by “DAPT” and “long lesions” variables (shown in Tables 1 and 2).
Procedural characteristics
In two matched groups, the usage ratio of Rotablator and intravascular imaging (including intravascular ultrasound and optical coherence tomography) was similar. The diameter of the DCB finally implanted was significantly smaller than that of the sDES [3.0 (3.0-3.5) mm vs. 3.5 (3.0-3.5)mm, P<0.001], and the ratio of the implanted devices diameter to the RVD was also significantly different[0.94 (0.91–0.97) vs. 0.97 (0.92-1.0), P<0.001] despite the similar pre-procedural RVD [3.4 (3.3–3.6) mm vs. 3.5 (3.2–3.7)mm, P = 0.404] (shown in Tables 2 and 3). Meanwhile, the DCB length was shorter than sDES (24.85 ± 7.21 mm vs. 26.82 ± 8.01 mm, P<0.001). The mean DCB inflated time was around 70 s and the proportion of excessive inflated time (more than 60 s) was 65.4%. A certain more residual stenosis was allowed after DCB-only treatment (21.22 ± 5.43 vs. 12.27 ± 3.17, P<0.001), meanwhile, the proportion of residual stenosis>30% was certain higher than sDES-only group (1.4% vs. 0%, P = 0.002). Dissection after devices implantation occurred in 25.8% of DCB-only group, significantly higher than sDES-only group (1.2%). Most dissections were types A or B, and type C dissection was only observed in DCB-only group.
Table 3.
Procedural characteristics
| Before propensity matching | After propensity matching | |||||
|---|---|---|---|---|---|---|
| DCB | sDES | P-value | sDES | P-value | ||
| Number of patients | 708 | 2149 | 704 | |||
| Cutting or NSE balloon using, n (%) | 650(91.9%) | N/A | N/A | |||
| Rotablator | 22(3.1%) | 185(8.6%) | <0.001 | 30(4.3%) | 0.250 | |
| Intravascular imaging | 124(17.5%) | 447(20.8%) | 0.058 | 139(19.7%) | 0.282 | |
| Device (DCB or sDES) parameter | ||||||
| Diameter (mm) | 3.0(3.0-3.5) | 3.5(3.0-3.5) | <0.001 | 3.5(3.0-3.5) | <0.001 | |
| Length (mm) | 24.85 ± 7.21 | 27.74 ± 7.85 | <0.001 | 26.82 ± 8.01 | <0.001 | |
| inflated time (sec) | 70(60,80) | 10(10,10) | <0.001 | 10(10,10) | <0.001 | |
| Device/RVD ratio | 0.94(0.91–0.97) | 0.95(0.92-1.0) | <0.001 | 0.97(0.92-1.0) | <0.001 | |
| DCB excessive inflated time, n (%) | 463(65.4%) | N/A | N/A | |||
| Residual stenosis (%) | 21.22 ± 5.43 | 11.99 ± 3.17 | <0.001 | 12.27 ± 3.17 | <0.001 | |
| Residual stenosis>30%, n (%) | 10(1.4%) | 0(0%) | <0.001 | 0(0%) | 0.002 | |
| Dissection after procedure | ||||||
| Type A, n (%) | 121(17.1%) | 20(0.9%) | <0.001 | 8(1.1%) | <0.001 | |
| Type B, n (%) | 54(7.6%) | 4(0.2%) | <0.001 | 1(0.1%) | <0.001 | |
| Type C, n (%) | 8 (1.1%) | 0 | <0.001 | 0 | 0.008 | |
Notes: DCB, Drug-coated balloon; sDES, second-generation drug-eluting stent; NSE, Nitinol Spine Balloon; RVD, reference vessel diameter; continuous variables were presented as mean ± standard deviation or median (interquartile range, IQR); categoric variables were shown as number (%)
Clinical endpoints
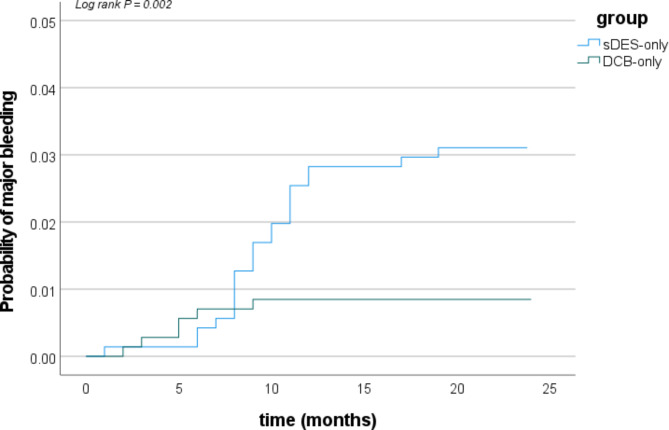
At two years follow-up, CD-TLR rate was higher in the DCB-only group than sDES-only group (DCB: 5.5%, sDES: 3.1%, P = 0.028). There were no significant difference in the incidences of MACE and all cause death between the two matched groups (MACE: 7.6% vs. 5.7%, P = 0.143; all cause death: 1.4% vs. 1.6%, P = 0.816) after propensity matching (shown in Table 4). However, lower major bleeding was observed in the DCB-only group compared to sDES-only group (0.8% vs. 3.0%, P = 0.003), maybe because the duration of DAPT in the DCB-only group was shorter than sDES-only group [6 (6–12) months vs. 12 (12–12) months, P<0.001] (shown in Table 1; Fig. 2).
Table 4.
Clinical endpoints
| Before propensity matching | After propensity matching | |||||
|---|---|---|---|---|---|---|
| DCB | sDES | P-value | sDES | P-value | ||
| Number of patients | 708 | 2149 | 704 | |||
| MACE, n (%) | 54(7.6%) | 140(6.5%) | 0.308 | 40(5.7%) | 0.143 | |
| CD-TLR, n (%) | 39(5.5%) | 101(4.7%) | 0.387 | 22(3.1%) | 0.028 | |
| MI, n (%) | 11(1.6%) | 32(1.5%) | 0.903 | 12(1.8%) | 0.670 | |
| Cardiac death, n (%) | 7(1.0%) | 17(0.8%) | 0.617 | 8(1.3%) | 0.607 | |
| All cause death, n (%) | 10(1.4%) | 28(1.3%) | 0.825 | 11(1.6%) | 0.816 | |
| Major bleeding, n (%) | 6(0.8%) | 36(1.7%) | 0.112 | 21(3.0%) | 0.003 | |
Notes: DCB, Drug-coated balloon; sDES, second-generation drug-eluting stent; MACE, major adverse cardiac events; CD-TLR, clinically driven target lesion revascularization; MI, myocardial infarction; continuous variables were presented as mean ± standard deviation or median (interquartile range, IQR); categoric variables were shown as number (%)
Fig. 2.
Cumulative risks of major bleeding at 2 years follow-up in the matched group (Kaplan–Meier time-to-first event curves). sDES, second-generation drug-eluting stent; DCB, Drug-coated balloon
Prognostic factors analysis
The prognostic factors analysis of two treatment strategy (DCB-only cohort and sDES-only cohort) involved 20 clinical variants, 7 lesion variants and 4 procedural variants. Table 5 displayed the rough result of logistic regression analysis for these variants. According to the introduced criteria (P<0.01) and exclusive criteria (P>0.10), the statistically significant variants were introduced successfully and retained in forward conditional stepwise multivariate regression equation (shown in Table 6). In the DCB-only cohort, the multivariate logistic regression analysis revealed that Hypercholesteremia [odds ratio (OR): 2.516; 95% confidence interval (CI): 1.250, 5.063; P = 0.010], diabetes (OR: 2.773; CI: 1.379, 5.577; P = 0.004), severe calcified lesions (OR: 5.184; CI: 1.664, 16.150; P = 0.005) and residual stenosis>30% (OR: 8.676; CI: 1.823, 41.296; P = 0.007) were risk predictors of CD-TLR; meanwhile, in the sDES-only cohort, diabetes (OR: 3.255; CI: 2.156, 4.914; P<0.001) and severe calcified lesions (OR: 2.152; CI: 1.271, 3.645; P = 0.004) were risk predictors of CD-TLR.
Table 5.
Predictive factors for CD-TLR using univariate logistic regression analysis
| Variants | OR (95% CI) for DCB cohort | P | OR (95% CI) for sDES cohort | P |
|---|---|---|---|---|
| Clinical variants | ||||
| Age>65 years | 0.780 (0.317–1.921) | 0.589 | 1.091 (0.678–1.755) | 0.719 |
| Male | 0.792 (0.255–2.456) | 0.686 | 0.849 (0.464–1.556) | 0.597 |
| ACS | 1.069 (0.216–5.302) | 0.935 | 1.155 (0.480–2.777) | 0.747 |
| Previous MI | 1.461 (0.420–5.081) | 0.551 | 1.411 (0.790–2.518) | 0.244 |
| Previous PCI | 2.008 (0.798–5.050) | 0.138 | 1.502 (0.858–2.627) | 0.154 |
| Previous CABG | 2.671 (0.068-105.043) | 0.600 | 1.855 (0.379–9.086) | 0.446 |
| Atrial flutter/Fibrillation | 0.868 (0.096–7.883) | 0.900 | 1.153 (0.393–3.382) | 0.796 |
| Hypertension | 1.258 (0.585–2.709) | 0.557 | 1.264 (0.803–1.989) | 0.311 |
| Hypercholesteremia | 2.877 (1.316–6.286) | 0.008 | 1.781 (1.168–2.715) | 0.007 |
| Diabetes | 2.560 (1.196–5.481) | 0.015 | 3.021 (1.974–4.622) | <0.001 |
| Heart failure | 1.363 (0.280–6.637) | 0.702 | 1.206 (0.582–2.498) | 0.614 |
| Renal insufficiency | 2.335 (0.349–15.612) | 0.382 | 1.048 (0.411–2.675) | 0.922 |
| Anemia | 1.287 (0.436–3.799) | 0.647 | 1.198 (0.616–2.329 | 0.595 |
| COPD | 2.637 (0.268–25.955) | 0.406 | 1.771 (0.646–4.858) | 0.267 |
| History of smoking | 1.125 (0.477–2.657) | 0.787 | 1.504 (0.883–2.559) | 0.133 |
| Family history of CAD | 2.187 (0.334–14.297) | 0.414 | 1.996 (0.675–5.904) | 0.211 |
| Statins | 0.640 (0.067–6.138) | 0.699 | 0.744 (0.160–3.470) | 0.707 |
| Beta-Blockers | 0.834 (0.260–2.677) | 0.761 | 0.804 (0.439–1.473) | 0.480 |
| ACEI/ARB | 1.111 (0.470–2.623) | 0.811 | 1.069(0.643–1.777) | 0.796 |
| PCSK9i | 0.636 (0.143–2.835) | 0.553 | 0.623 (0.243–1.596) | 0.324 |
| Lesion variants | ||||
| Long lesion | 1.462 (0.679–3.146) | 0.331 | 0.989 (0.637–1.535) | 0.960 |
| Severe calcified lesion | 36.319 (3.506-376.245) | 0.003 | 2.963 (1.284–6.838) | 0.011 |
| Bifurcation lesion | 0.767 (0.336–1.748) | 0.528 | 1.423 (0.927–2.185) | 0.107 |
| Ostial lesion | <0.001 | 1.000 | 2.158 (0.249–18.671) | 0.485 |
| Angulation lesion | 2.129 (0.886–5.116) | 0.091 | 1.761 (1.016–3.052) | 0.044 |
| Thrombus lesion | 3.572 (0.591–21.595) | 0.165 | 1.866 (0.682–5.106) | 0.224 |
| CTO lesion | 1.941 (0.430–8.764) | 0.389 | 1.721 (0.750–3.947) | 0.200 |
| Procedural variants | ||||
| DCB excessive inflated time | 1.052 (0.477–2.318) | 0.900 | N/A | N/A |
| Residual stenosis>30% | 8.356(1.578–44.256) | 0.013 | N/A | N/A |
| Type C dissection | 3.562 (0.372–34.107) | 0.270 | N/A | N/A |
| Intravascular imaging | 0.145 (0.018–1.163) | 0.069 | 0.663 (0.312–1.410) | 0.286 |
Notes: DCB, Drug-coated balloon; sDES, second-generation drug-eluting stent; OR, odds ratio; CI, confidence interval; other abbreviations see table above
Table 6.
Predictive factors for CD-TLR using multivariate logistic regression analysis
| Variants | OR (95% CI) in DCB cohort | P | OR (95% CI) in sDES cohort | P |
|---|---|---|---|---|
| Clinical variants | ||||
| Hypercholesteremia | 2.516 (1.250–5.063) | 0.010 | ||
| Diabetes | 2.773 (1.379–5.577) | 0.004 | 3.255 (2.156–4.914) | <0.001 |
| Lesion variants | ||||
| Severe calcified lesions | 5.184 (1.664–16.150) | 0.005 | 2.152 (1.271–3.645) | 0.004 |
| Procedural variants | ||||
| Residual stenosis>30% | 8.676 (1.823–41.296) | 0.007 |
Notes: DCB, Drug-coated balloon; sDES, second-generation drug-eluting stent; OR, odds ratio; CI, confidence interval
Discussion
The rate of CD-TLR after DCB implantation was 5.5% at two years in this study. Similar results had also been reported in previous observational studies [10, 15, 16]. But the incidence of CD-TLR was lower in the sDES-only group. We didn’t find that DCB-only strategy for LCA de novo lesions was non-inferior to sDES-only strategy, similar to the REC-CAGEFREE I clinical trial [17]. However, patients treated with DCB-only strategy had lower incidence of major bleeding which benefited from its short duration of antiplatelet therapy in this study (shown in Fig. 2). Therefore, this study provided evidence regarding the feasibility and safety of DCB-only strategy for LCA de novo lesions to some extent. But the feasibility of reducing the duration of DAPT was also gradually demonstrated in patients with high bleeding risk after sDES implantation in previous studies [18]. So, we look forward to better technological breakthroughs in the future of DCB strategy.
The application of DCB for LCA de novo lesions was gradually developed based on the accumulative experience in the treatment of SCA de novo lesions. Since LCA de novo lesions are often located in the main branch or big branch of the coronary arteries and involve a wider range of blood supply, more caution is required during DCB procedures. In addition, the smooth muscle layer, and elastic fibers of LCA are more abundant than those of SCA and are more prone to elastic retraction, so more adequate preparation for lesions prior to DCB angioplasty is required. To achieve desirable pre-dilation effect and avoid severe dissection, special balloons (such as cutting balloons, scoring balloons and NSE balloons) are often used more frequently, especially in significant calcified lesions and heavy plaque load lesions. In this study, the usage rate of cutting or NSE balloons was up to 91.9% in the DCB-only group.
Once lesion preparation has been performed, an optimal balloon angioplasty result should be confirmed prior to DCB delivery, which consists of the following factors [8]: (1) a fully inflated balloon of the correct size for the vessel; (2) residual stenosis ≤ 30%; (3) TIMI (Thrombolysis In Myocardial Infarction) flow grade 3; and (4) the absence of a flow-limiting dissection. The main function of DCB is to deliver the drug to the lesion and transfer it into the vessel wall, rather than expanding the lumen. To avoid technical failure, an excessively large DCB or release at a pressure significantly above the nominal pressure should be avoided. In addition, logistic regression analysis in this study showed that excessive inflated time of DCB (more than 60 s) could not reduce the rate of CD-TLR. DCB-only strategy for de novo lesions has several advantages; however, acute vessel closure due to elastic recoil and flow-limiting dissections can restrict DCB. In our study, 25 patients after DCB implantation encountered bailout stenting due to intraoperative slow blood flow, severe residual stenosis, flow-limiting dissections, and postoperative acute vessel closure; 2 patients died because of cardiac rupture and no-reflow. Above patients were excluded in this study (shown in Fig. 1). So, it had been calculated that operation success rate of DCB-only strategy was 96.5% (737 divided by 764).
In this study, there were 10 patients whose lesions residual stenosis was greater than 30% measured by QCA in DCB-only cohort. Among them, 6 patients occurred CD-TLR events. Multivariate logistic regression analysis also confirmed that residual stenosis>30% (OR, 8.676) was a most risk predictor of CD-TLR for DCB-only strategy. Therefore, after DCB implantation, the degree of residual stenosis measured by QCA should be paid attention to, rather than just visually looking at angiography images.
Type A and B dissection after successful DCB deployment in DCB-only cohort was observed in 175 (24.7%) lesions in our study, and only 8 lesions (including 4 type A dissection and 4 type B dissection) occurred CD-TLR. The CD-TLR rate was 4.6% (8 divided 175) in patients with type A and B dissection and 5.5% (29 divided 525) in those without dissection, that is to say, aforementioned dissection did not increase the incidence of CD-TLR (P = 0.626), similar to previous reports [10, 19]. However, whether type C dissection after DCB implantation requires bailout stenting is controversial in clinical practice [8]. The CD-TLR rate was 25% (2 divided 8) in patients with type C dissection after DCB implantation in our study. Logistic regression analysis (univariate analysis) showed that type C dissection (OR, 3.562) might be a risk predictor of CD-TLR for DCB-only strategy, although the difference was not statistically significant (P = 0.270) in our study which need to be confirmed by larger samples study in the future.
Additionally, this study showed that diabetes and severe calcified lesions were risk predictors of CD-TLR for both DCB-only strategy and sDES-only strategy. Previous studies had demonstrated that diabetes and severe calcified lesions were predictors of in-stent restenosis [20, 21]. Regarding to severe calcified lesions, previous studies also demonstrated that DCB-only strategy had significant poor prognosis for LCA de novo lesions [22–24]. In our study, multivariate logistic regression analysis showed that severe calcified lesions were more dangerous predictor of CD-TLR for DCB-only strategy compared to sDES strategy. Therefore, for severely calcified lesions, if DCB-only strategy is planned, cutting balloons, NSE balloons, Rotablator, intravascular lithotripsy or rotational atherectomy must be used to obtain adequate preconditioning.
Furthermore, multivariate logistic regression analysis also showed that hypercholesteremia was a risk predictor of CD-TLR for DCB-only strategy. The reason might be that hypercholesteremia could lead to the formation of neoatherosclerosis which further causes restenosis and CD-TLR. This result suggested that DCB-only strategy for LCA de novo lesions had higher requirements for lipid-lowering therapy. Therefore, for hypercholesteremia, if DCB-only strategy is planned, LDL-C (low-density lipoprotein cholesterol) must reach the target(≤ 1.4mmol/L) or even be lower.
Finally, univariate logistic regression analysis showed that intravascular imaging guided-PCI might have a potential benefit in reducing CD-TLR compared to angiography-guided PCI in either group, due to the small sample size, no statistical significance was found.
Study limitations
There are several limitations in this study. First, this is a retrospective study, its non-randomized nature may introduce unavoidable bias. We conducted propensity score matching to minimize the difference in patient characteristics. However, there may still be residual selection bias and confounding. Second, we did not restrict and specify the brand of DCB and sDES, in view of that the efficacy of paclitaxel-coated balloons or sDES used at our institution seemed equivalent. However, differences may exist. Not all paclitaxel DCB have the same uniform efficacy. As such, the final conclusion cannot be generalized for each of the different DCB. Third, this was a single center study, a relatively small sample size of the patients was yielded by propensity score matching, larger population RCT study is needed to definitively confirm the efficacy and safety of DCB-only strategy for LCA de novo lesions or complex lesions.
Conclusions
After adjustment for baseline differences, DCB-only strategy was associated with higher risk of CD-TLR and significantly lower risk of bleeding compared with sDES-only strategy. Therefore, DCB-only strategy for LCA de novo lesions may be useful in patients with high bleeding risk, but not suitable for other patients, especially with unmanageable hypercholesteremia whose LDL-C do not reach the target (maybe because they cannot use PCSK9 inhibitors for a long time or some other reason), and patients with severe calcified lesions or non-ideal residual stenosis, who should first choose intended stenting strategy.
Acknowledgements
We would like to thank all clinical professors and radiology technicians in our institution and study students involved in this study for their continuous contribution.
Abbreviations
- LCA
Reference vessel diameter more than 3.0 mm
- SCA
Mall coronary arteries
- DCB
Drug-coated balloon
- sDES
Second-generation drug-eluting stent
- DES
Drug-eluting stent
- CD-TLR
Clinically driven target lesion revascularization
- RCT
Randomized controlled trials
- QCA
Quantitative coronary angiographic system
- DS%
Percent diameter stenosis
- CAD
Coronary artery disease
- ACS
Acute coronary syndrome
- UA
Unstable angina
- NSTEMI
Acute non-ST-elevation myocardial infarction
- STEMI
Acute ST-elevation myocardial infarction
- SCAD
Stable coronary artery disease
- MI
Myocardial infarction
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass grafting
- COPD
Chronic obstructive pulmonary disease
- LVEF
Left ventricular ejection fraction
- Hb
Hemoglobin
- sCr
Serum creatinine
- LDL -C
Low-density lipoprotein cholesterol
- DAPT
Dual antiplatelet therapy
- ACEI
Angiotensin-converting enzyme inhibitors
- ARB
Angiotensin receptor blockers
- PCSK9i
The proprotein convertase subtilin Kexin-9 inhibitor
- IQR
Interquartile range
- LM
Left main coronary artery
- LAD
Left anterior descending artery
- LCX
Left circumflex artery
- RCA
Right coronary artery
- CTO
Chronic total occlusion
- NSE
Nitinol Spine Balloon
- RVD
Reference vessel diameter
- MACE
Major adverse cardiac events
- OR
Odds ratio
- CI
Confidence interval
- TIMI
Thrombolysis In Myocardial Infarction
Author contributions
Kang Zhao, Lixin Rao, Muwei Li designed the study; Kang Zhao, Quan Guo, Zhenzhou Zhao, Haiyu Tang, Ran You, Liang Peng collected and analyzed the data; Kang Zhao drafted the article; and All authors reviewed and approved the final manuscript.
Funding
Medical Science and Technique Research Plan of Henan Province (Joint Co-construction Project) (Grant No. LHGJ20200095) financially supported this work.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of Fuwai Central China Cardiovascular Hospital, Zhengzhou University (Zhengzhou, China) (No. 2021 [13]). All participants confirmed informed consent forms and follow-up agreements before enrolling.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119(25):3198–206. [DOI] [PubMed] [Google Scholar]
- 2.Erdogan E, Bajaj R, Lansky A, Mathur A, Baumbach A, Bourantas CV. Intravascular imaging for Guiding In-Stent restenosis and stent thrombosis therapy. J Am Heart Assoc. 2022;11(22):e026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. [DOI] [PubMed] [Google Scholar]
- 4.Jeger RV, Farah A, Ohlow MA, et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet. 2020;396(10261):1504–10. [DOI] [PubMed] [Google Scholar]
- 5.Tang Y, Qiao S, Su X, et al. Drug-coated balloon Versus Drug-Eluting Stent for Small-Vessel Disease: the RESTORE SVD China Randomized Trial. JACC Cardiovasc Interv. 2018;11(23):2381–92. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed M, Saleem M, Kheiri B, Osman M. Meta-analysis of drug-coated balloons Versus Drug-Eluting stents for small Vessel De-novo Coronary Artery Disease. Am J Cardiol. 2021;142:157–8. [DOI] [PubMed] [Google Scholar]
- 7.Yerasi C, Case BC, Forrestal BJ, et al. Drug-coated balloon for De Novo Coronary Artery Disease: JACC State-of-the-art review. J Am Coll Cardiol. 2020;75(9):1061–73. [DOI] [PubMed] [Google Scholar]
- 8.Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-coated balloons for coronary artery disease: third report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13(12):1391–402. [DOI] [PubMed] [Google Scholar]
- 9.Yu X, Ji F, Xu F, et al. Treatment of large de novo coronary lesions with paclitaxel-coated balloon only: results from a Chinese institute. Clin Res Cardiol. 2019;108(3):234–43. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Zhang YJ, Deng LX, et al. 12-Month clinical results of drug-coated balloons for de novo coronary lesion in vessels exceeding 3.0 mm. Int J Cardiovasc Imaging. 2019;35(4):579–86. [DOI] [PubMed] [Google Scholar]
- 11.Vos NS, Fagel ND, Amoroso G, et al. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute myocardial infarction: the REVELATION Randomized Trial. JACC Cardiovasc Interv. 2019;12(17):1691–9. [DOI] [PubMed] [Google Scholar]
- 12.Lu W, Zhu Y, Han Z, et al. Short-term outcomes from drug-coated balloon for coronary de novo lesions in large vessels. J Cardiol. 2019;73(2):151–5. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg M, Waliszewski M, Krackhardt F et al. Drug Coated Balloon-Only Strategy in De Novo Lesions of Large Coronary Vessels. J Interv Cardiol. 2019. 2019: 6548696. [DOI] [PMC free article] [PubMed]
- 14.Nishiyama N, Komatsu T, Kuroyanagi T, et al. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int J Cardiol. 2016;222:113–8. [DOI] [PubMed] [Google Scholar]
- 15.Hu FW, Chang S, Li Q, et al. Long-term clinical outcomes after percutaneous coronary intervention with drug-coated balloon-only strategy in de novo lesions of large coronary arteries. Front Cardiovasc Med. 2022;9:882303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Ding X, Wang L, et al. Feasibility and safety of drug-coated balloon-only angioplasty for De Novo Ostial lesions of the Left Anterior descending artery: two-Center Retrospective Study. Front Cardiovasc Med. 2022;9:874394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao C, He X, Ouyang F, et al. Drug-coated balloon angioplasty with rescue stenting versus intended stenting for the treatment of patients with de novo coronary artery lesions (REC-CAGEFREE I): an open-label, randomised, non-inferiority trial. Lancet. 2024;404(10457):1040–50. [DOI] [PubMed] [Google Scholar]
- 18.O’Donoghue ML, Murphy SA, Sabatine MS. The safety and efficacy of aspirin discontinuation on a background of a P2Y(12) inhibitor in patients after percutaneous coronary intervention: a systematic review and Meta-analysis. Circulation. 2020;142(6):538–45. [DOI] [PubMed] [Google Scholar]
- 19.Cortese B, Silva Orrego P, Agostoni P, et al. Effect of drug-coated balloons in native coronary artery Disease Left with a dissection. JACC Cardiovasc Interv. 2015;8(15):2003–9. [DOI] [PubMed] [Google Scholar]
- 20.Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig lecture ESC 2014. Eur Heart J. 2015;36(47):3320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed MO, Polad J, Hildick-Smith D, et al. Impact of coronary lesion complexity in percutaneous coronary intervention: one-year outcomes from the large, multicentre e-Ultimaster registry. EuroIntervention. 2020;16(7):603–12. [DOI] [PubMed] [Google Scholar]
- 22.Ito R, Ueno K, Yoshida T, et al. Outcomes after drug-coated balloon treatment for patients with calcified coronary lesions. J Interv Cardiol. 2018;31(4):436–41. [DOI] [PubMed] [Google Scholar]
- 23.Nagai T, Mizobuchi M, Funatsu A, Kobayashi T, Nakamura S. Acute and mid-term outcomes of drug-coated balloon following rotational atherectomy. Cardiovasc Interv Ther. 2020;35(3):242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki Y, Koike J, Ko T, et al. Comparison of drug-eluting stents vs. drug-coated balloon after rotational atherectomy for severely calcified lesions of nonsmall vessels. Heart Vessels. 2021;36(2):189–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.