Abstract
Background
Increased whole blood viscosity (WBV) was associated with impaired peripheral glucose metabolism, type 2 diabetes, and cardiovascular disease (CVD). Impaired myocardial glucose metabolism is a risk factor for CVD. Whether an increased WBV is associated with impaired myocardial glucose metabolism is still undefined.
Methods
To elucidate this issue, we evaluated the association between WBV and myocardial glucose metabolic rate (MRGlu) in 57 individuals with different glucose tolerance status. Myocardial MRGlu was assessed using dynamic cardiac 18F-FDG PET combined with euglycemic hyperinsulinemic clamp. WBV was calculated using a validated equation including hematocrit and plasma proteins: WBV = [0.12 × h] + [0.17 × (p − 2.07)], where h is the hematocrit (%) and p the plasma proteins (g/dl). The subjects were stratified into tertiles according to their myocardial MrGlu values.
Results
As compared with individuals in the highest myocardial MrGlu tertile, those in the lowest tertile showed an age-adjusted increase in WBV (5.54 ± 0.3 cP vs. 6.13 ± 0.4 cP respectively; P = 0.001), hematocrit (39.1 ± 3.1% vs. 43.2 ± 3.7% respectively; P = 0.004), and total proteins (7.06 ± 0.3 g/l vs. 7.60 ± 0.3 g/l respectively; P < 0.0001). WBV was negatively correlated with myocardial MRGlu (r = − 0.416, P = 0.001). In a stepwise multivariate regression analysis, including several cardiovascular risk factors, the only variables significantly associated with myocardial MrGlu were WBV (β − 0.505; P < 0.0001), fasting insulin (β − 0.346; P = 0.004), fasting plasma glucose (β − 0.287; P = 0.01), and sex (β 0.280; P = 0.003) explaining the 69.6% of its variation.
Conclusions
The current study showed a strongly association between an increase of WBV and an impaired myocardial glucose metabolism in individuals with a broad spectrum of glucose tolerance.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02513-7.
Keywords: Blood viscosity, Myocardial glucose metabolism, Cardiovascular disease, Type 2 diabetes, Cardiac 18F-FDG PET, Hematocrit, Insulin resistance
Introduction
Blood viscosity is a measure of the intrinsic resistance of blood to flow in vessels, and is produced by the frictional interactions between the main blood components including plasma, plasma proteins, and red blood cells [1]. There is evidence indicating that raised whole blood viscosity (WBV) is associated with cardio-metabolic risk factors including dyslipidemia, prediabetes/type 2 diabetes (T2DM), hypertension, obesity, and metabolic syndrome [2–5], and target organ damage such as sub-clinical carotid atherosclerosis, vascular stiffness, reduced myocardial mechano-energetic efficiency and left ventricular hypertrophy [6–16] leading to higher risk of incident cardiovascular (CV) events [1, 17–21].
Impaired myocardial glucose metabolism is an early alteration observed in both individuals at increased risk of T2DM and in patients with overt T2DM [22–26]. Furthermore, myocardial insulin resistance is an independent predictor of CV events in individuals with coronary heart disease (CHD), and has been associated with early carotid, aortic and coronary atherosclerosis [27–30]. Previous studies have shown an association between increased WBV and impaired whole-body insulin sensitivity, assessed by euglycemic-hyperinsulinemic clamp technique [31–34] likely due to a reduced glucose and insulin delivery to metabolically active tissues. Whether an increased WBV is associated with impaired myocardial glucose metabolism is still undefined. To this purpose, we evaluated the relationship between WBV and insulin-stimulated myocardial glucose metabolic rate (MRGlu) assessed using cardiac dynamic PET with 18F-Fluorodeoxyglucose (18F-FDG) combined with euglycemic-hyperinsulinemic clamp, in individuals with a broad spectrum of glucose tolerance.
Methods
Study participants
The study cohort comprised 57 subjects participating in the CATAnzaro MEtabolic RIsk factors (CATAMERI), an ongoing observational study recruiting adult individuals with one or more cardio-metabolic risk factors recruited at a referral hospital of the University “Magna Graecia” of Catanzaro [29, 35]. Eligible subjects were recruited according to the following inclusion criteria: age between 30 and 70 years, and positivity for one or more cardio-metabolic risk factors including family history of diabetes, impaired fasting glucose, hypertension, dyslipidemia, and overweight/obesity. Exclusion criteria were type 1 diabetes, end-stage renal disease, previous CVD on the basis of medical history, resting electrocardiogram and stress test or myocardial scintigraphy for individuals with T2DM, history of atrial fibrillation or other arrhythmias, right and left bundle branch block, dyssynchrony in ventricular contraction, valvular heart disease, liver cirrhosis, history of malignant or autoimmune diseases, acute or chronic infections, history of alcohol or drug abuse and treatment with drugs known to influence glucose tolerance such as steroids and estro-progestins and medicaments affecting heart function including beta blockers and antiarrhythmic drugs. All subjects underwent anthropometrical evaluation including measurements of body mass index (BMI), waist circumference and body composition by bioelectrical impedance, and assessment of whole-body and cardiac insulin sensitivity. Readings of clinic blood pressure (BP) were measured at 3-min intervals using a standard sphygmomanometer, and BP values were the average of 3 measurements after a 10-min period of rest in the supine position. After an overnight fasting, biochemical determinations and a 75 g OGTT were performed in individuals with FPG < 126 mg/dl, HbA1c < 6.5% and no history of T2DM. According to the ADA criteria [36], individuals were classified as having normal glucose tolerance (NGT) when fasting plasma glucose was < 100 mg/dl (5.5 mmol/l), 2-h postload glucose < 140 mg/dl (< 7.77 mmol/l) and HbA1c < 5.7%, prediabetes when fasting plasma glucose was 100–125 mg/dl (5.5–6.9 mmol/l), 2-h postload glucose 140–199 mg/dl (7.77–11.0 mmol/l) or HbA1c 5.7–6.4%, T2DM when fasting plasma glucose was ≥ 126 mg/dl (> 7 mmol/l), 2-h post-load glucose was ≥ 200 mg/dl (> 11.1 mmol/l), HbA1c ≥ 6.5% or in treatment with antidiabetic drugs.
On the second day, after 12-h fasting, all subjects underwent 18F-FDG PET scan combined with euglycemic hyperinsulinemic clamp.
The study was approved by the Ethics Committee (Comitato Etico Azienda Ospedaliera “Mater Domini”), and informed consent was obtained from each subject in accordance with principles of the Declaration of Helsinki.
18F-FDG PET scan combined with euglycemic hyperinsulinemic clamp
Myocardial glucose metabolic rate (MrGlu) was measured by 18F-FDG-PET acquired during an euglycemic hyperinsulinemic clamp as previously described [26, 37]. Subjects received a priming dose of insulin (100 UI/ml) (Humulin R; Eli Lilly) during the initial 10 min to raise the serum insulin concentration acutely (80 mU/m2 × min), and then it was maintained by continuous insulin infusion fixed at 40 mU/m2 × min [35]. The blood glucose level was maintained constant at 90 mg/dl for the next 120 min by infusing 20% glucose at varying rates according to blood glucose measurements performed at 5-min intervals (mean coefficient of variation of blood glucose was < 4%). Glucose metabolized by the whole body (M) was calculated as the mean rate of glucose infusion measured during the last 60 min of the clamp examination (steady state) and was expressed as milligrams per minute per kilogram fat-free mass (MFFM).
The 18F-FDG-PET imaging procedure was performed on a hybrid PET/CT scanner (GE Discovery ST8-2D PET scanner), starting 60 min after the insulin infusion. A 60-min dynamic acquisition was started simultaneously with the intravenous injection of 370 MBq18F-FDG, according to the following time frame sampling: 8 × 15 s, 2 × 30 s, 2 × 120 s, 1 × 180 s, 6 × 300 s, 2 × 600 s [38]. PET images were reconstructed in a 128 × 128 matrix using a OSEM algorithm, and corrected for decay and attenuation based on co-registered CT. The insulin-glucose infusion continued during the entire PET acquisition. The estimation of myocardial MrGlu was performed by Patlak compartmental modelling [39], using the graphical tool specific for cardiac images analysis (PCARD) implemented in PMOD Software platform (Version 3.806) [39]. In PCARD, the full dynamic study is used for MRGlu calculation, and the arterial input function is extracted from a volume of interest (VOI) semi-automatically placed in the left ventricular cavity [40].
Whole blood viscosity
Whole blood viscosity (WBV) at 208 s − 1 of shear rate was calculated by a previously validated equation that takes into account hematocrit and plasma proteins [10]: WBV = [0.12 × h] + [0.17 × (p − 2.07)], where h is hematocrit (%) and p is plasma protein levels (g/dl).
Laboratory determinations
Plasma glucose, total and HDL cholesterol, and triglycerides were assayed using enzymatic methods (Roche Diagnostics, Mannheim, Germany). HbA1c was measured with high performance liquid chromatography using an NGSP-certified automated analyzer (Adams HA-8160 HbA1c analyzer, Menarini, Italy). Red blood cell count, haemoglobin, haematocrit and white blood cell count were analysed using an automated particle counter (Siemens Healthcare Diagnostics ADVIA® 120/2120 Haematology System). Serum insulin levels were determined by a chemiluminescence-based assay (Immulite®, Siemens Healthcare GmbH, Erlangen, Germany). Fibrinogen was measured by an automated nephelometric technology using the BNTMII System analyzer (Siemens Healthcare, Italy).
Statistical analyses
Triglycerides levels were natural log transformed for statistical analyses due to their skewed distribution. Continuous variables are expressed as means ± SD. Categorical variables were compared by χ2 test. Comparisons between women and men were performed using unpaired Student’s t test. A general linear model with post hoc Bonferroni correction for multiple comparisons was used to compare differences of continuous variables between groups. Relationships between variables were determined by Pearson’s correlation (r). Linear regression analysis was performed to determine the independent contributors to myocardial glucose metabolic rate. A stepwise multivariate regression analysis was performed to determine the independent contributors to myocardial glucose metabolic rate and whole blood viscosity. For all analyses a P value < 0.05 was considered to be statistically significant. All analyses were performed using SPSS software Version 29 for Mac.
Results
The subjects in study were stratified into tertiles according to their myocardial MrGlu values.
Clinical characteristics of the three groups of individuals stratified into tertiles according to their insulin-stimulated myocardial MrGlu values are shown in Table 1. Of the 57 recruited individuals, 20 (35.1%) had NGT, 11 (19.3%) had prediabetes, and 26 (45.6%) had T2DM. All the subjects with T2DM were treated with metformin.
Table 1.
Anthropometric and metabolic characteristics of study subjects stratified into tertiles according to myocardial MrGlu values
| Myocardial MrGlu tertile 1 Range 0.1–16.3 μmol/min/100 g (1) |
Myocardial MrGlu tertile 2 Range 16.4–26.29 μmol/min/100 g (2) |
Myocardial MrGlu tertile 3 Range 26.3–43 μmol/min/100 g (3) |
P (1 vs. 2)§ | P (1 vs. 3)§ | P (2 vs. 3)§ | |
|---|---|---|---|---|---|---|
| Sex (F/M) | 10/9 | 8/11 | 9/10 | 0.41 | 0.87 | 0.5 |
| Age (years) | 53 ± 10 | 50 ± 12 | 48 ± 11 | 0.9 | 0.08 | 0.09 |
| BMI (kg/m2) | 32.5 ± 5 | 28.1 ± 4 | 29.2 ± 4 | 0.01 | 0.02 | 0.6 |
| Waist circumference (cm) | 108 ± 12 | 98 ± 11 | 101 ± 10 | 0.5 | 0.4 | 0.7 |
| Systolic blood pressure (mmHg) | 130 ± 12 | 124 ± 16 | 115 ± 15 | 0.5 | 0.03 | 0.1 |
| Diastolic blood pressure (mmHg) | 79 ± 11 | 75 ± 11 | 75 ± 10 | 0.9 | 0.9 | 0.7 |
| Heart rate (bpm) | 78 ± 8 | 68 ± 7 | 68 ± 4 | < 0.0001 | < 0.0001 | 0.4 |
| Fasting plasma glucose (mg/dl) | 131 ± 45 | 114 ± 35 | 100 ± 27 | 0.1 | 0.054 | 0.3 |
| HbA1c (%) | 7.1 ± 1.2 | 6.5 ± 1.1 | 5.8 ± 1.1 | 0.2 | 0.01 | 0.1 |
| Total cholesterol (mg/dl) | 186 ± 37 | 196 ± 47 | 185 ± 29 | 0.2 | 0.7 | 0.3 |
| HDL cholesterol (mg/dl) | 45 ± 10 | 48 ± 8 | 49 ± 14 | 0.3 | 0.5 | 0.8 |
| LDL cholesterol (mg/dl) | 126 ± 36 | 127 ± 36 | 118 ± 28 | 0.6 | 0.9 | 0.3 |
| Triglycerides (mg/dl) | 164 ± 74 | 119 ± 64 | 115 ± 60 | 0.2 | 0.1 | 0.7 |
| Fasting insulin (mU/ml) | 18.8 ± 10 | 11.03 ± 6 | 12.2 ± 6.5 | 0.005 | 0.01 | 0.9 |
| NGT/prediabetes/T2DM (n) | 1/4/14 | 6/6/7 | 13/1/5 | 0.005 | 0.001 | 0.2 |
| Insulin-stimulated glucose disposal (mg/min × kg FFM) | 3.16 ± 1.8 | 4.8 ± 3.1 | 8.4 ± 7.7 | 0.1 | 0.02 | 0.2 |
| Fibrinogen (mg/dl) | 299 ± 65 | 280 ± 49 | 269 ± 82 | 0.4 | 0.2 | 0.9 |
| Glucose-lowering therapy (%) | ||||||
| Meftormin (%) | 70.6 | 33.3 | 26.3 | 0.02 | 0.01 | 0.8 |
| Antihypertensive therapy (%) | 58.3 | 35.3 | 16.7 | 0.4 | 0.06 | 0.1 |
| Lipid-lowering therapy (%) | 50 | 18.8 | 10.5 | 0.02 | 0.05 | 0.8 |
Data are means ± SD, unless otherwise indicated. Categorical variables were compared by χ2 test. Comparisons between the three groups were performed using a general linear model with post hoc Fisher's least significant difference correction for pairwise comparisons
BMI body mass index, NGT normal glucose tolerance, T2DM type 2 diabetes
§P values refer to results after analyses with adjustment for age
No differences were observed in sex distribution. Subjects in the lowest tertile of insulin-stimulated myocardial MrGlu were older and exhibited higher BMI than individuals in the highest tertile (32.5 ± 5 kg/m2 vs. 29.2 ± 4 kg/m2, P = 0.02) (Table 1).
Cardiovascular risk factors and metabolic parameters in individuals stratified according to insulin-stimulated myocardial MrGlu values
As shown in Table 1, no differences between individuals in lowest myocardial MrGlu tertile as compared with those in the highest tertile were observed in waist circumference (108 ± 12 cm vs. 101 ± 10 cm; P = 0.4), total cholesterol (186 ± 37 mg/dl, vs. 185 ± 29 mg/dl; P = 0.7), HDL (45 ± 10 mg/dl vs. 49 ± 14 mg/dl; P = 0.5) and LDL cholesterol (126 ± 36 mg/dl vs. 118 ± 28 mg/dl; P = 0.9), triglycerides (164 ± 74 mg/dl vs. 115 ± 60 mg/dl; P = 0.1), fasting plasma glucose (131 ± 45 mg/dl vs. 100 ± 27 mg/dl; P = 0.054) diastolic blood pressure (79 ± 11 mmHg vs. 75 ± 10 mmHg; P = 0.9) and fibrinogen (299 ± 65 mg/dl vs. 269 ± 82 mg/dl; P = 0.2) (Table 1). Individuals in the lowest tertile showed an age-adjusted increase in systolic blood pressure (130 ± 12 mmHg vs. 115 ± 15 mmHg; P = 0.03), resting heart rate (78 ± 8 bpm vs. 68 ± 4 bpm; P < 0.0001), fasting insulin (18.8 ± 10 mU/ml vs. 12.2 ± 6.5 mU/ml; P = 0.01) and HbA1c (7.1 ± 1.2% vs. 5.8 ± 1.1%; P = 0.01), and a lower whole-body insulin-stimulated glucose disposal as compared with subjects in the highest tertile (3.16 ± 1.8 vs. 8.4 ± 7.7 mg/min × kg FFM; P = 0.02) as compared with individuals in the highest tertile (Table 1). Furthermore, as compared with individuals in the highest tertile, a higher proportion of individuals in the lowest tertile had prediabetes or T2DM (P = 0.001) and were treated with glucose-lowering (P = 0.01) and lipid-lowering therapy (P = 0.05) (Table 1).
Hemorheological parameters in individuals stratified according to insulin-stimulated myocardial MrGlu values
Hemorheological parameters of the study individuals stratified into tertiles according to insulin-stimulated myocardial MrGlu values are shown in Table 2. As compared with individuals in the highest tertile, those in the lowest tertile showed an age-adjusted increase in WBV (5.54 ± 0.3 cP vs. 6.13 ± 0.4 cP respectively; P = 0.001). These differences remained significant after further adjustment for glucose-lowering therapy and glucose tolerance status (P = 0.02).
Table 2.
Hemorheological parameters in subjects stratified according to insulin-stimulated myocardial MrGlu values
| Myocardial MrGlu tertile 1 Range 0.1–16.3 μmol/min/100 g (1) |
Myocardial MrGlu tertile 2 Range 16.4–26.29 μmol/min/100 g (2) |
Myocardial MrGlu tertile 3 Range 26.3–43 μmol/min/100 g (3) |
P (1 vs. 2)§ | P (1 vs. 3)§ | P (2 vs. 3)§ | |
|---|---|---|---|---|---|---|
| White blood cells count (× 109/l) | 7868 ± 1817 | 6072 ± 1510 | 6123 ± 917 | 0.01 | 0.006 | 0.02 |
| Red blood cells count (× 102/l) | 4.91 ± 0.5 | 4.84 ± 0.3 | 4.75 ± 0.3 | 0.5 | 0.2 | 0.6 |
| Hematocrit (%) | 43.2 ± 3.7 | 42.7 ± 4.2 | 39.1 ± 3.1 | 0.7 | 0.004 | 0.01 |
| Total proteins (g/l) | 7.6 ± 0.2 | 7 ± 0.2 | 7.06 ± 0.3 | < 0.0001 | < 0.0001 | 0.5 |
| Whole blood viscosity (cP) | 6.13 ± 0.4 | 5.97 ± 0.5 | 5.54 ± 0.3 | 0.2 | 0.001 | 0.8 |
Data are means ± SD, unless otherwise indicated. Categorical variables were compared by χ2 test. Comparisons between the three groups were performed using a general linear model with post hoc Fisher's least significant difference correction for pairwise comparisons.
§P values refer to results after analyses with adjustment for age
Moreover, as compared with individuals in the highest tertile, those in the lowest tertile showed an age-adjusted increase in hematocrit (39.1 ± 3.1% vs. 43.2 ± 3.7% respectively; P = 0.004), total proteins (7.60 ± 0.3 g/l vs. 7.06 ± 0.2 g/l respectively; P < 0.0001) and white blood cells (WBC) count (7868 ± 1817 × 109/l vs. 6123 ± 917 × 109/l respectively; P = 0.006) (Table 2). No difference between individuals in the lowest myocardial MrGlu tertile as compared with those in the highest tertile was observed in red blood cell (RBC) count (4.91 ± 0.5 × 102/l vs. 4.75 ± 0.3 × 102/l; P = 0.2) (Table 2).
Differences in cardiovascular risk factors and metabolic and hemorheological parameters of men and women stratified into tertiles according to myocardial MrGlu values
Anthropometric, cardiovascular and hemorheological features of individuals stratified into tertiles according to myocardial MrGlu values according to sex are shown in Supplemental Table S1.
No sex-related differences in age, anthropometric, glycemic and insulinemic parameters, insulin-stimulated glucose disposal, lipid profile, fibrinogen and WBC count were observed across the tertiles, except for levels of HDL cholesterol which were significantly lower in men as compared with women in all myocardial MrGlu tertiles. Moreover, in the highest tertile of insulin-stimulated myocardial MrGlu men were older, and more likely to be treated with antihypertensive drugs (P = 0.04) than women (Table S1).
In all myocardial MrGlu tertiles men exhibited significant higher values of WBV than women (Table S1). Additionally, in all myocardial MrGlu tertiles men showed significant higher values of red blood cell count and hematocrit as compared to women (Table S1).
Association between insulin-stimulated myocardial MrGlu, cardiovascular risk factors and hemorheological parameters
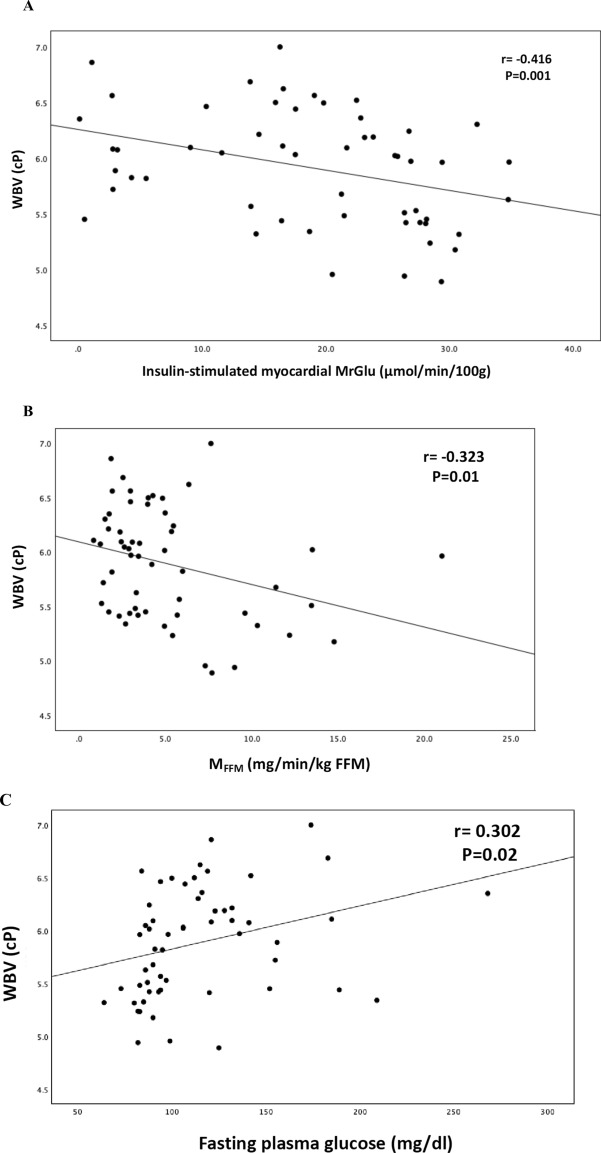
In a univariate analysis, WBV was negatively correlated with myocardial MRGlu (r = − 0.416, P = 0.001), whole-body insulin-stimulated glucose disposal (MFFM) (r = − 0.323, P = 0.01), and positively correlated with fasting plasma glucose (r = 0.302, P = 0.02), waist circumference (r = 0.414, P = 0.001), systolic (r = 0.406, P = 0.002), and diastolic blood pressure (r = 0.333, P = 0.01) (Fig. 1).
Fig. 1.
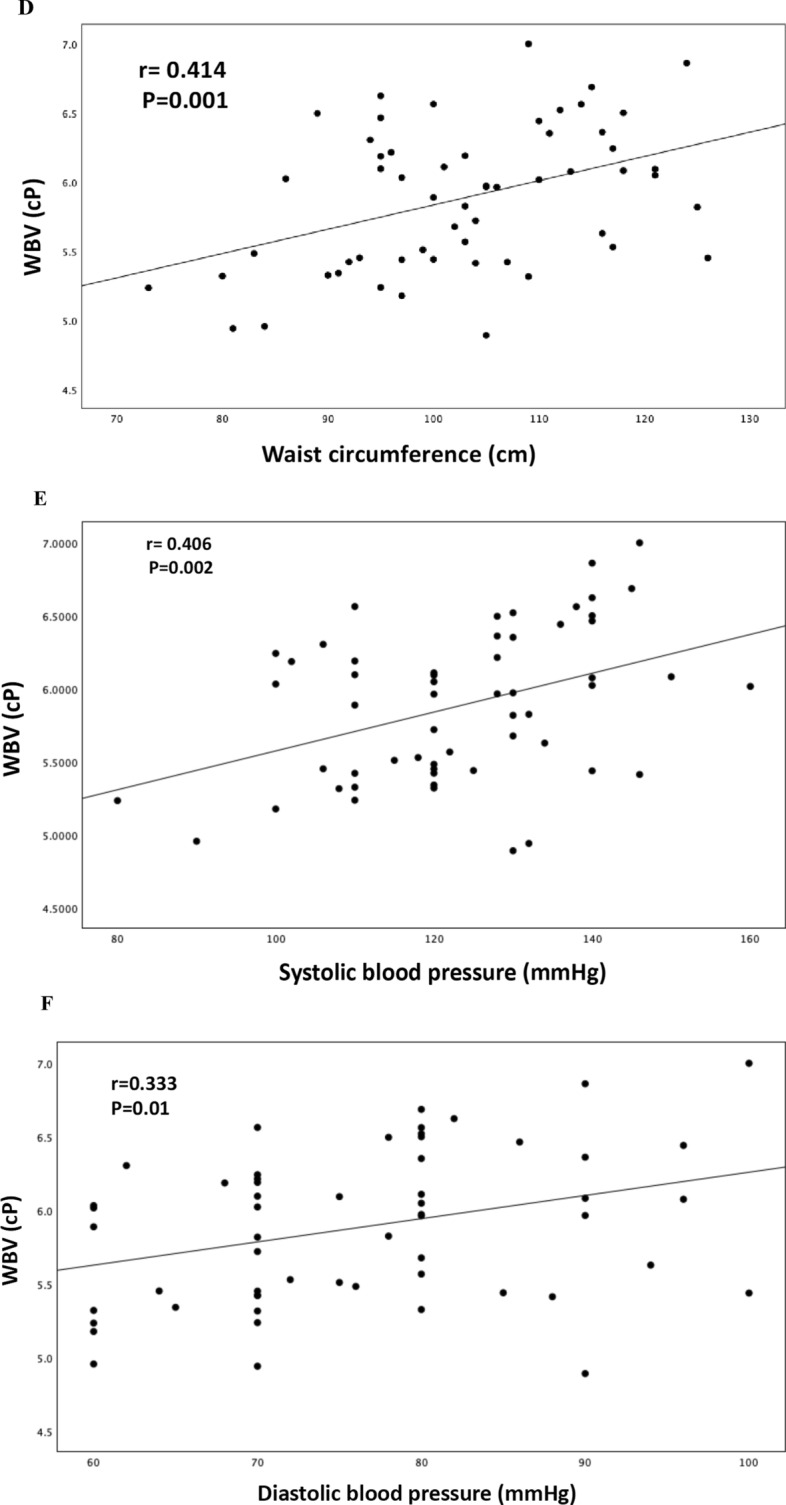
Relationship between WBV and insulin-stimulated myocardial MrGlu (A), MFFM (B), fasting plasma glucose (C), waist circumference (D), systolic blood pressure (E), diastolic blood pressure (F)
Furthermore, in a univariate analysis divided by sex, myocardial MRGlu was negatively correlated with WBV (r = − 0.416, P = 0.001), waist circumference (r = − 0.378, P = 0.004), fasting plasma glucose (r = − 0.354, P = 0.007), HbA1c (r = − 0.489, P < 0.0001), hematocrit (r = − 0.353, P = 0.007), fibrinogen (r = − 0.329, P = 0.01), fasting plasma insulin (r = − 0.353, P = 0.008), and positively correlated with whole-body insulin-stimulated glucose disposal (r = 0.441, P = 0.001) (Fig. 2).
Fig. 2.
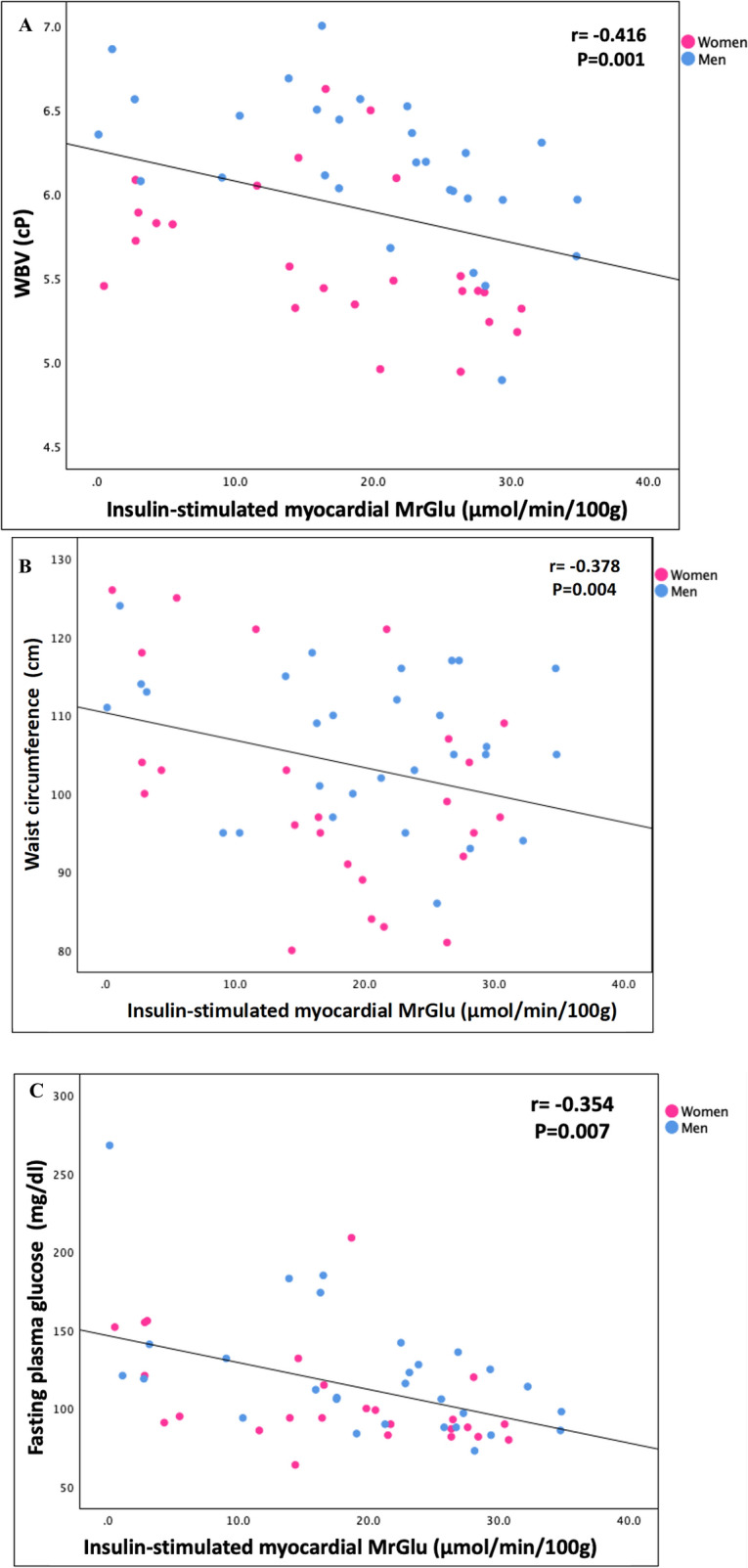
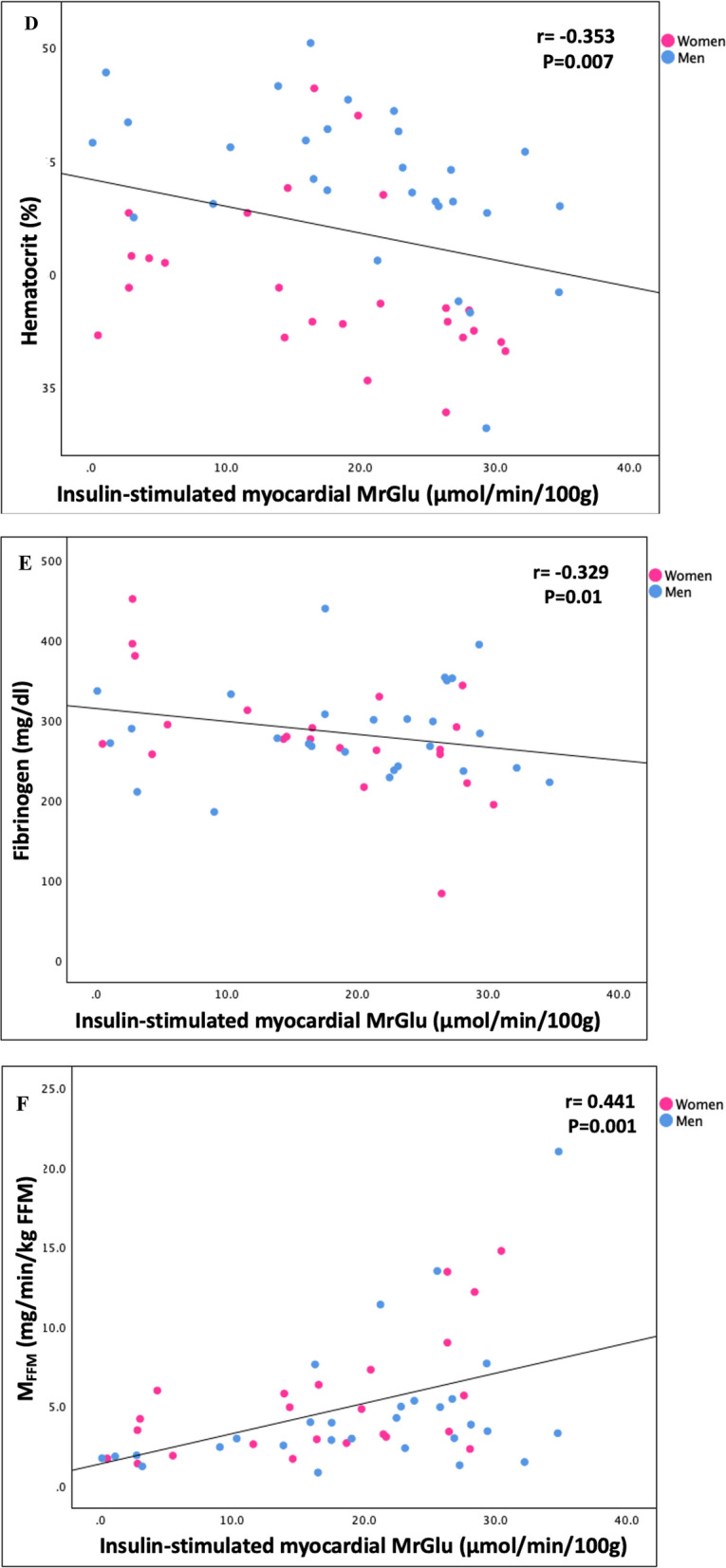
Relationship divided by sex between insulin-stimulated myocardial MrGlu and WBV (A), waist circumference (B), fasting plasma glucose (C), hematocrit (D), fibrinogen (E), MFFM (F)
In order to evaluate the independent contributors to myocardial MrGlu, we performed a linear regression analysis in a model having myocardial MrGlu as dependent variable and including age, sex, BMI, waist circumference, blood pressure, lipid profile, fasting plasma glucose, HbA1c, WBV, fasting insulin, and fibrinogen as independent variables. The only variable significantly associated with myocardial MrGlu was whole blood viscosity (β − 0.447; P = 0.01) (Table 3). Moreover, in a stepwise multivariate regression analysis in a model having myocardial MrGlu as dependent variable and including the same risk factors above reported, the only variables significantly associated with myocardial MrGlu were whole blood viscosity (β − 0.505; P < 0.0001), fasting insulin (β − 0.346; P = 0.004), fasting plasma glucose (β − 0.287; P = 0.01), and sex (male) (β 0.280; P = 0.003) explaining the 69.6% of its variation (Table 4).
Table 3.
Independent predictors of myocardial glucose metabolic rate after linear regression analysis
| Total r2 (%) | β | t | P | |
|---|---|---|---|---|
| Whole blood viscosity (cP) | 74.1 | − 0.447 | − 2.732 | 0.01 |
Model including age, sex, BMI, waist circumference, systolic and diastolic blood pressure, total cholesterol, HDL, triglycerides, fasting plasma glucose, HbA1c, whole blood viscosity, fasting plasma insulin, fibrinogen
Table 4.
Independent predictors of myocardial glucose metabolic rate after stepwise multiple regression analysis
| Total r2 (%) | β | t | P | |
|---|---|---|---|---|
| Whole blood viscosity (cP) | 48.7 | − 0.505 | − 3.690 | < 0.0001 |
| Fasting insulin (mU/ml) | 59.3 | − 0.346 | − 3.038 | 0.004 |
| Fasting plasma glucose (mg/dl) | 65.5 | − 0.287 | − 2.442 | 0.01 |
| Sex (male) | 69.6 | 0.280 | 2.159 | 0.03 |
Model including age, sex, BMI, waist circumference, systolic and diastolic blood pressure, total cholesterol, HDL, triglycerides, fasting plasma glucose, HbA1c, whole blood viscosity, fasting plasma insulin, fibrinogen
Moreover, in order to evaluate the independent contributors to WBV, we performed a stepwise multivariate regression analysis in a model having whole blood viscosity as dependent variable and including several risk factors, including age, sex, BMI, waist circumference, blood pressure, lipid profile, fasting plasma glucose, HbA1c, fasting insulin, whole-body insulin sensitivity, myocardial glucose metabolism, white blood cells and fibrinogen as independent variables. Results of the regression analysis showed that the only variables significantly associated with whole blood viscosity were waist circumference (β 0.229; P = 0.003), sex (male) (β 0.388; P = 0.004) and myocardial MrGlu (β − 0.400; P = 0.002) explaining the 72.7% of its variation (Table 5).
Table 5.
Independent predictors of whole blood viscosity after stepwise multiple regression analysis
| Total r2 (%) | β | t | P | |
|---|---|---|---|---|
| Waist circumference (cm) | 50.7 | 0.229 | 3.171 | 0.003 |
| Sex (male) | 62.9 | 0.461 | 4.010 | < 0.0001 |
| Myocardial MrGlu (μmol/min/100 g) | 72.7 | − 0.400 | − 3.350 | 0.002 |
Model including age, sex, BMI, waist circumference, systolic and diastolic blood pressure, total cholesterol, HDL, triglycerides, fasting plasma glucose, HbA1c, fasting plasma insulin, Insulin-stimulated glucose disposal, myocardial glucose metabolism, white blood cells and fibrinogen
Discussion
In this cross-sectional study, we showed that whole blood viscosity was negatively associated with a myocardial glucose metabolism, measured using cardiac dynamic 18F-FDG-PET combined with euglycemic-hyperinsulinemic clamp, in individuals with different degrees of glucose tolerance and no history of coronary heart disease (r = − 0.416, P = 0.001). We found that individuals with lower myocardial glucose metabolic rate showed an age-adjusted increase in WBV as compared with individuals with higher myocardial glucose metabolism (6.13 ± 0.4 cP vs. 5.54 ± 0.3 cP respectively; P = 0.001) and these differences remained significant also after further adjustment for glucose-lowering therapy and glucose tolerance status (P = 0.02). These findings were strengthened by results of a stepwise multivariate regression analysis performed in order to investigate whether whole blood viscosity was associated with myocardial MrGlu independently of well-established cardio-metabolic risk factors including age, sex, BMI, waist circumference, blood pressure, lipid profile, fasting plasma glucose, HbA1c, WBV, fasting plasma insulin, and fibrinogen. We found that WBV was a major determinant of myocardial MrGlu independently of known cardiovascular risk factors explaining the 69.6% of its variation. In addition, a linear regression analysis confirmed that, among well-established cardio-metabolic risk factors, the only variable significantly associated with myocardial MrGlu was whole blood viscosity (β − 0.447; P = 0.01).
Our study extends previous findings showing an association between WBV and whole-body insulin resistance measured by either euglycemic-hyperinsulinemic clamp in small numbers of subjects [31–33] or proxy indices of insulin resistance in larger samples [3, 4, 41, 42]. However, to the best of our knowledge, the current study was the first to show an association between WBV and myocardial glucose metabolism in individuals with a broad spectrum of glucose tolerance.
There is evidence that increased blood viscosity is an independent predictor of ischemic heart disease and stroke in the general population [1]. Blood viscosity has been shown to be associated with target organ damage such as subclinical atherosclerosis, vascular stiffness, decrease of myocardial mechano-energetic efficiency, and left ventricular hypertrophy [6–16].
Myocardial insulin resistance is a condition related to an unfavorable cardiometabolic risk profile and early carotid, aortic and coronary atherosclerosis, and has been shown to be a predictor of CV events [27–30, 43]. Previous studies have shown that impaired myocardial glucose metabolism is associated with a reduced ejection fraction, a depressed myocardial mechano-energetic efficiency, and an increased cardiac workload, all strong predictors of heart failure [29, 36, 44, 45]. The relative contribution of free fatty acids (FFA) and glucose to energy provision for the human heart is 70% and 30%, respectively, but this proportion varies with the physiological state. Under normal state, cardiac ATP is predominantly derived from fatty acid oxidation, with glucose metabolism contributing less. Conversely, under stress conditions, fatty acid oxidation decreases, while glucose utilization increases [46]. These changes lead to mitochondrial dysfunction with a low energy production and, consequently, death of cardiomyocytes, alterations of mechano-energetic performance, mitochondrial dysfunction, left ventricular maladaptive changes, cardiac dysfunction, and therefore contribute to the development of heart failure and coronary heart disease [25–27, 29, 30, 47–50]. Taken together, these data support the idea that reduced myocardial glucose metabolism may represent one of the pathophysiologic mechanisms contributing to the increased risk of CV disease observed in individuals with increased WBV. The mechanism by which WBV negatively affect myocardial glucose uptake remains to be fully established. Elevated whole blood viscosity and high red blood cells and hematocrit, its main determinant, are associated to peripheral insulin resistance and impaired blood flow [4, 42, 51–54]. Decreased blood flow might affect myocardial glucose metabolism by limiting delivery of glucose and, consequently, myocardial glucose uptake [4, 42, 51].
On the other hand, a reduction of myocardial glucose metabolism could have an impact on whole blood viscosity. In a stepwise multivariate regression analysis, we found that myocardial glucose metabolism was an independent contributor of whole blood viscosity along with waist circumference and male sex explaining the 72.7% of its variation. The study of the pathophysiological mechanism linking impaired myocardial glucose uptake with increased whole blood viscosity is beyond the scope of this investigation. However, there is evidence that impaired insulin-stimulated myocardial glucose metabolism is strongly correlated with whole-body insulin resistance, which has been repeatedly reported to be associated with increased whole blood viscosity. Indeed, higher blood viscosity is associated with decreased flow, which, in turn, counteracts the transport of glucose to tissue [22, 27, 29]. A reduction in whole-body glucose uptake causes an increase in circulating glucose levels leading to increased insulin secretion and compensatory hyperinsulinemia. Hyperinsulinemia may cause vasoconstriction via sympathetic neural activation, which, in turn, would lead to hemoconcentration by increasing hematopoiesis, and hematocrit and, thereby, increased whole blood viscosity [42, 51].
Additionally, we found that men exhibited significantly higher values of hemorheological parameters, including WBV, hematocrit and RBC count as compared with women in all myocardial MrGlu tertiles. Accordingly, in the multivariate regression analysis male sex was an independent contributor of whole blood viscosity. Our results were in agreement with those of a previous study showing that male sex was the demographic variable most related to WBV, probably due to the higher hematocrit levels in men, but also influenced by sex-related differences in plasma volume regulation [10].
We also found an age-adjusted increase in hemorheological parameters, including hematocrit, total proteins and white blood cells count in subjects with low myocardial MrGlu. These findings are in line with results from prior studies showing a remarkable role of hemorheological parameters, including white blood cells count, in the development of insulin resistance, and type 2 diabetes [42, 51, 55]. White blood cells are independently associated with blood viscosity [3] and are an independent predictor of ischemic heart disease, both alone and along with viscosity [56].
This study has several strengths that merit considerations. A main strength is the use of gold standard methods to assess myocardial glucose metabolism by cardiac FDG PET combined with the euglycemic-hyperinsulinemic clamp technique, which allows the valuation of insulin-stimulated myocardial glucose uptake under uniform experimental conditions of euglycemia and physiological hyperinsulinemia [27, 57]. Moreover, glucose tolerance was accurately assessed using FPG, 2 h post-load glucose levels during an OGTT, and HbA1c according to ADA criteria thus excluding any potential misclassification of participants [36]. Additionally, all tests including anthropometric measures, OGTT, and 18F-FGD PET scan combined with euglycemic hyperinsulinemic clamp were collected by skilled examiners after a standardized training, who were blinded to the clinical data of the study participants.
Nevertheless, some limitations should be taken into account. First, whole blood viscosity has not been directly measured by capillary viscometry. However, we estimated whole blood viscosity using an indirect measure that has been previously validated, and is suitable in clinical practice and large observational studies [10]. Furthermore, the consistency of the observed associations using routine haematological parameters may have useful implications for the clinical practice. Second, we have measured red blood cell count only before euglycemic hyperinsulinemic clamp, and therefore, we cannot account for possible variations induced by insulin infusion during clamp procedure. Moreover, this analysis includes only White individuals aging between 30 and 70 years with at least one cardiovascular risk factors attending a referral university hospital, thus limiting the generalizability of the present results to other ethnicities or to the general population. Furthermore, the cross-sectional design of the study precludes causal inferences, and, therefore, no conclusions regarding cause-effect relationships can be made. Additionally, the present findings were observed in an observational study, rather than in a randomized controlled trial thus the results may be subject to residual unknown confounding factors.
Conclusions
In conclusion, to the best of our knowledge, the current study showed an association between an increase of WBV and an impaired myocardial glucose metabolism in individuals with a broad spectrum of glucose tolerance. Indeed, whole blood viscosity was the main independent contributor of myocardial glucose metabolism. On the other hand, myocardial glucose metabolism was an independent contributor of whole blood viscosity along with waist circumference and male sex. These data support the idea that reduced myocardial glucose metabolism may represent one of the pathophysiologic mechanisms which could at least in part contribute to the increased risk of CV disease observed in individuals with increased WBV. On the other hand, a reduction of myocardial glucose metabolism could have an impact on whole blood viscosity. Clearly, our findings need to be confirmed by future prospective studies and should presently be considered as hypothesis generating.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- WBV
Whole blood viscosity
- T2DM
Type 2 diabetes mellitus
- CV
Cardiovascular
- CHD
Coronary heart disease
- MRGlu
Myocardial glucose metabolic rate
- PET
Positron emission tomography
- 18F-FDG
18F-Fluorodeoxyglucose
- BMI
Body mass index
- BP
Blood pressure
- OGTT
Oral glucose tolerance test
- FPG
Fasting plasma glucose
- ADA
American Diabetes Association
- NGT
Normal glucose tolerance
- MFFM
Insulin-stimulated glucose disposal corrected for fat-free mass
- FFM
Fat-free mass
- PCARD
Tool specific for cardiac images analysis
- VOI
Volume of interest
- WBC
White blood cells
Author contributions
E.S. designed the study, researched and analyzed data and wrote and edited the manuscript. P.Vi. and P.H.G. analyzed the data from the cardiac PET scans, F.C. performed cardiac PET scans. M.R., T.V.F., M.P., G.C.M., and A.S. researched data and reviewed the manuscript. P.Ve., G.L.C. and F.A. contributed to the discussion and reviewed the manuscript. G.S. designed the study, analyzed the data, and wrote and reviewed the manuscript. All authors reviewed the manuscript.
Funding
This research received no external funding.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee (Comitato Etico Azienda Ospedaliera “Mater Domini”), and informed consent was obtained from each subject in accordance with principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lowe G, Lee A, Rumley A, Price J, Fowkes F. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery study. Br J Haematol. 1997;96:168–73. [DOI] [PubMed] [Google Scholar]
- 2.Irace C, Carallo C, Scavelli F, De Franceschi MS, Esposito T, Gnasso A. Blood viscosity in subjects with normoglycemia and prediabetes. Diabetes Care. 2014;37:488–92. [DOI] [PubMed] [Google Scholar]
- 3.Marini MA, Fiorentino TV, Andreozzi F, Mannino GC, Perticone M, Sciacqua A, et al. Elevated 1-h post-challenge plasma glucose levels in subjects with normal glucose tolerance or impaired glucose tolerance are associated with whole blood viscosity. Acta Diabetol. 2017;54:775–84. [DOI] [PubMed] [Google Scholar]
- 4.Høieggen A, Fossum E, Moan A, Enger E, Kjeldsen SE. Whole-blood viscosity and the insulin-resistance syndrome. J Hypertens. 1998;16:203–10. [DOI] [PubMed] [Google Scholar]
- 5.Marini MA, Fiorentino TV, Andreozzi F, Mannino GC, Succurro E, Sciacqua A, Mannino GC, Succurro E, Sciacqua A, et al. Hemorheological alterations in adults with prediabetes identified by hemoglobin A1c levels. Nutr Metab Cardiovasc Dis. 2017;27:601–8. [DOI] [PubMed] [Google Scholar]
- 6.Sloop GD, Garber DW. The effects of low-density lipoprotein and high-density lipoprotein on blood viscosity correlate with their association with risk of atherosclerosis in humans. Clin Sci (Lond). 1997;92:473–9. [DOI] [PubMed] [Google Scholar]
- 7.Letcher RL, Chien S, Pickering TG, Laragh JH. Elevated blood viscosity in patients with borderline essential hypertension. Hypertension. 1983;5:757–62. [DOI] [PubMed] [Google Scholar]
- 8.Ciuffetti G, Schillaci G, Lombardini R, Pirro M, Vaudo G, Mannarino E. Prognostic impact of low-shear whole blood viscosity in hypertensive men. Eur J Clin Investig. 2005;35:93–8. [DOI] [PubMed] [Google Scholar]
- 9.Rillaerts E, van Gaal L, Xiang DZ, Vansant G, De Leeuw I. Blood viscosity in human obesity: relation to glucose tolerance and insulin status. Int J Obes (Lond). 1989;13:739–45. [PubMed] [Google Scholar]
- 10.de Simone G, Devereux RB, Chien S, Alderman MH, Atlas SA, Laragh JH. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990;81:107–17. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Drayer JI, Chien S, Pickering TG, Letcher RL, DeYoung JL, et al. Whole blood viscosity as a determinant of cardiac hypertrophy in systemic hypertension. Am J Cardiol. 1984;54:592–5. [DOI] [PubMed] [Google Scholar]
- 12.Zannad F, Voisin P, Brunotte F, Bruntz JF, Stoltz JF, Gilgenkrantz JM. Haemorheological abnormalities in arterial hypertension and their relation to cardiac hypertrophy. J Hypertens. 1988;6:293–7. [PubMed] [Google Scholar]
- 13.Lee AJ, Mowbray PI, Lowe GD, Rumley A, Fowkes FG, Allan PL. Blood viscosity and elevated carotid intima-media thickness in men and women: the Edinburgh artery study. Circulation. 1998;97:1467–73. [DOI] [PubMed] [Google Scholar]
- 14.Tripolino C, Irace C, Carallo C, De Franceschi MS, Scavelli F, Della Valle E, et al. Association between blood viscosity and common carotid artery elasticity. Clin Hemorheol Microcirc. 2016;62:55–62. [DOI] [PubMed] [Google Scholar]
- 15.Riccio A, Cefalo CMA, Mazzanti C, Vero L, Fiorentino TV, Massimino M, et al. Whole blood viscosity is associated with reduced myocardial mechano-energetic efficiency in nondiabetic individuals. Eur J Clin Investig. 2024;54(3): e14127. [DOI] [PubMed] [Google Scholar]
- 16.Lowe G, Rumley A, Norrie J, Ford I, Shepherd J, Cobbe S, et al. Blood rheology, cardiovascular risk factors, and cardiovascular disease: the west of Scotland coronary prevention study. Thromb Haemost. 2000;84:553–8. [PubMed] [Google Scholar]
- 17.Tohgi H, Yamanouchi H, Murakami M, Kameyama M. Importance of the hematocrit as a risk factor in cerebral infarction. Stroke. 1978;9:369–74. [DOI] [PubMed] [Google Scholar]
- 18.Woodward M, Lowe GD, Campbell DJ, Colman S, Rumley A, Chalmers J, et al. Associations of inflammatory and hemostatic variables with the risk of recurrent stroke. Stroke. 2005;36:2143–7. [DOI] [PubMed] [Google Scholar]
- 19.Neumann FJ, Katus HA, Hoberg E, Roebruck P, Braun M, Haupt HM, et al. Increased plasma viscosity and erythrocyte aggregation: indicators of an unfavourable clinical outcome in patients with unstable angina pectoris. Br Heart J. 1991;66:425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erikssen G, Thaulow E, Sandvik L, Stormorken H, Erikssen J. Haematocrit: A predictor of cardiovascular mortality? J Intern Med. 1993;234:493–9. [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21:515–20. [DOI] [PubMed] [Google Scholar]
- 22.Succurro E, Pedace E, Andreozzi F, Papa A, Vizza P, Fiorentino TV, et al. Reduction in global myocardial glucose metabolism in subjects with 1-hour postload hyperglycemia and impaired glucose tolerance. Diabetes Care. 2020;43:669–76. [DOI] [PubMed] [Google Scholar]
- 23.Ohtake T, Yokoyama I, Watanabe T, Momose T, Serezawa T, Nishikawa J, et al. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36:456–63. [PubMed] [Google Scholar]
- 24.Hu L, Qiu C, Wang X, Shao X, Wang Y. The association between diabetes mellitus and reduction in myocardial glucose uptake: a population-based 18F-FDG PET/CT study. BMC Cardiovasc Disord. 2018;18:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen R, Jorsal A, Iversen P, Tolbod L, Bouchelouche K, Sørensen J, et al. Heart failure patients with prediabetes and newly diagnosed diabetes display abnormalities in myocardial metabolism. J Nucl Cardiol. 2018;25:169–76. [DOI] [PubMed] [Google Scholar]
- 26.Succurro E, Vizza P, Papa A, Cicone F, Monea G, Tradigo G, et al. Metabolic syndrome is associated with impaired insulin-stimulated myocardial glucose metabolic rate in individuals with type 2 diabetes: a cardiac dynamic 18 F-FDG-PET study. Front Cardiovasc Med. 2022;9: 924787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iozzo P, Chareonthaitawee P, Dutka D, Betteridge DJ, Ferrannini E, Camici PG. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51:3020–4. [DOI] [PubMed] [Google Scholar]
- 28.Devesa A, Fuster V, Vazirani R, García-Lunar I, Oliva B, España S, et al. Cardiac insulin resistance in subjects with metabolic syndrome traits and early subclinical atherosclerosis. Diabetes Care. 2023;46:2050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Succurro E, Cicone F, Papa A, Miceli S, Vizza P, Fiorentino TV, et al. Impaired insulin-stimulated myocardial glucose metabolic rate is associated with reduced estimated myocardial energetic efficiency in subjects with different degrees of glucose tolerance. Cardiovasc Diabetol. 2023;22:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang K, Lin J, Ji X, Lin T, Sun D, Zheng X, et al. Non-alcoholic fatty liver disease with reduced myocardial FDG uptake is associated with coronary atherosclerosis. J Nucl Cardiol. 2021;28:610–20. [DOI] [PubMed] [Google Scholar]
- 31.Moan A, Nordby G, Os I, Birkeland KI, Kjeldsen SE. Relationship between hemorrheologic factors and insulin sensitivity in healthy young men. Metabolism. 1994;43:423–7. [DOI] [PubMed] [Google Scholar]
- 32.Nordby G, Moan A, Kjeldsen SE, Os I. Relationship between hemorheological factors and insulin sensitivity in normotensive and hypertensive premenopausal women. Am J Hypertens. 1995;8:439–44. [DOI] [PubMed] [Google Scholar]
- 33.Catalano C, Muscelli E, Natali A, Mazzoni A, Masoni A, Bernardini B, et al. Reciprocal association between insulin sensitivity and the haematocrit in man. Eur J Clin Investig. 1997;27:634–7. [DOI] [PubMed] [Google Scholar]
- 34.Wannamethee SG, Perry IJ, Shaper AG. Hematocrit and risk of NIDDM. Diabetes. 1996;45:576–9. [DOI] [PubMed] [Google Scholar]
- 35.Succurro E, Vizza P, Cicone F, Cassano V, Massimino M, Giofrè F, et al. Sex-specific differences in myocardial glucose metabolic rate in non-diabetic, pre-diabetic and type 2 diabetic subjects. Cardiovasc Diabetol. 2024;23:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Sayed NA, Aleppo G, Bannuru RR, Bruemmer D, Collins BS, Ekhlaspour L, on behalf of the American Diabetes Association Professional Practice Committee, et al. Diagnosis and classification of diabetes: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S20–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Succurro E, Vizza P, Papa A, Miceli S, Cicone F, Fiorentino TV, et al. Effects of 26 weeks of treatment with empagliflozin versus glimepiride on the myocardial glucose metabolic rate in patients with type 2 diabetes: the randomized, open-label, crossover, active-comparator FIORE trial. Diabetes Obes Metab. 2022;24:2319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. [DOI] [PubMed] [Google Scholar]
- 39.Carson RE. Tracer kinetic modeling in PET. In Positron emission tomography. London: Springer; 2005, p. 127–59
- 40.Vizza P, Guzzi PH, Veltri P, Papa A, Cascini GL, Sesti G, Succurro E. Experiences on quantitative cardiac pet analysis. In 2016 IEEE international conference on bioinformatics and biomedicine (BIBM). Shenzen, China. 2016, p. 1148–53.
- 41.Facchini FS, Carantoni M, Jeppesen J, Reaven GM. Hematocrit and hemoglobin are independently related to insulin resistance and compensatory hyperinsulinemia in healthy, non-obese men and women. Metabolism. 1998;47:831–5. [DOI] [PubMed] [Google Scholar]
- 42.Hanley AJ, Retnakaran R, Qi Y, Gerstein HC, Perkins B, Raboud J, et al. Association of hematological parameters with insulin resistance and beta-cell dysfunction in nondiabetic subjects. J Clin Endocrinol Metab. 2009;94:3824–32. [DOI] [PubMed] [Google Scholar]
- 43.Kofoed KF, Carstensen S, Hove JD, Freiberg J, Bangsgaard R, Holm S, et al. Low whole-body insulin sensitivity in patients with ischaemic heart disease is associated with impaired myocardial glucose uptake predictive of poor outcome after revascularisation. Eur J Nucl Med Mol Imaging. 2002;29:991–8. [DOI] [PubMed] [Google Scholar]
- 44.Losi MA, Izzo R, Mancusi C, Wang W, Roman MJ, Lee ET, et al. Depressed myocardial energetic efficiency increases risk of incident heart failure: the strong heart study. J Clin Med. 2019;8:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Simone G, Izzo R, Losi MA, Stabile E, Rozza F, Canciello G, et al. Depressed myocardial energetic efficiency is associated with increased cardiovascular risk in hypertensive left ventricular hypertrophy. J Hypertens. 2016;34:1846–53. [DOI] [PubMed] [Google Scholar]
- 46.Wende AR, Brahma MK, McGinnis GR, Young ME. Metabolic origins of heart failure. JACC Basic Transl Sci. 2017;2:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17:585–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:665–79. [DOI] [PubMed] [Google Scholar]
- 50.Zweck E, Scheiber D, Jelenik T, Bönner F, Horn P, Pesta D, et al. Exposure to type 2 diabetes provokes mitochondrial impairment in apparently healthy human hearts. Diabetes Care. 2021;44:e82–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Investig. 2006;116:1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen A, Khafagy R, Hashemy H, Kuo KHM, Roshandel D, Paterson AD, et al. Investigating the association between fasting insulin, erythrocytosis and HbA1c through Mendelian randomization and observational analyses. Front Endocrinol (Lausanne). 2023;14:1146099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nah EH, Cho S, Park H, Kim S, Cho HI. Associations of complete blood count parameters with pancreatic beta-cell function and insulin resistance in prediabetes and type 2 diabetes mellitus. J Clin Lab Anal. 2022;36: e24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krisnamurti DGB, Purwaningsih EH, Tarigan TJE, Soetikno V, Louisa M. Hematological indices and their correlation with glucose control parameters in a prediabetic rat model. Vet World. 2022;15:672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–61. [DOI] [PubMed] [Google Scholar]
- 56.Yarnell JW, Baker IA, Sweetnam PM, Ainton D, O’Brien JR, Whitehead PJ, et al. Fibrinogen, viscosity, and white blood cell count are major risk factors for ischemic heart disease. The caerphilly and speed- well collaborative heart disease studies. Circulation. 1991;83:836–44. [DOI] [PubMed] [Google Scholar]
- 57.Gerber BL, Ordoubadi FF, Wijns W, Vanoverschelde JL, Knuuti MJ, Janier M, et al. Positron emission tomography using (18)F-fluoro-deoxyglucose and euglycaemic hyperinsulinaemic glucose clamp: optimal criteria for the prediction of recovery of post-ischaemic left ventricular dysfunction: results from the European Community Concerted Action multicenter study on use of (18)F-fluoro-deoxyglucose positron emission tomography for the detection of myocardial viability. Eur Heart J. 2001;22:1691–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.