Abstract
Background
Populations affected by humanitarian crises likely experience high burdens of pneumococcal disease. Streptococcus pneumoniae carriage estimates are essential to understand pneumococcal transmission dynamics and the potential impact of pneumococcal conjugate vaccines (PCV). Over 100 million people are forcibly displaced worldwide, yet here we present only the second pneumococcal carriage estimates for a displaced population.
Methods
In October 2019, we conducted a cross-sectional survey among internally displaced people (IDP) living in Digaale, a permanent IDP camp in Somaliland where PCV has not been implemented. We collected nasopharyngeal swab samples from 453 residents which were assessed for presence of pneumococci and serotyped using DNA microarray.
Results
We found that pneumococcal carriage prevalence was 36% (95%CI 31–40) in all ages, and 70% (95%CI 64–76) in children under 5. The three most common serotypes were vaccine serotypes 6B, 19F, and 23F. We estimated that the serotypes included in the 10-valent PNEUMOSIL vaccine were carried by 41% (95%CI 33–49) of all pneumococcal carriers and extrapolated that they caused 52% (95%CI 35–70) of invasive pneumococcal disease. We found some evidence that pneumococcal carriage was associated with recent respiratory symptoms, the total number of physical contacts made, and with malnutrition in children under 5. Through linking with a nested contact survey we projected that pneumococcal exposure of children under 2 was predominantly due to contact with children aged 2–5 (39%; 95%CI 31–48) and 6–14 (25%; 95%CI 17–34).
Conclusions
These findings suggest considerable potential for direct and indirect protection against pneumococcal disease in Digaale through PCV use in children and potentially adolescents.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41479-024-00148-6.
Keywords: Streptococcus pneumoniae, Displaced population, Humanitarian crisis, Pneumococcal conjugate vaccine, Somaliland
Introduction
The United Nations High Commissioner for Refugees estimates that over 100 million people were forcibly displaced worldwide in 2022, of whom over half are internally displaced and more than 40% are children [1]. These people typically live in overcrowded settings, have poor access to hygiene and healthcare, and thus experience high morbidity and mortality from respiratory diseases [2–4] including disease from Streptococcus pneumoniae (pneumococcus) [5]. There is a lack of health research in these populations in general [6], and for pneumococcal disease specifically [2, 4]. Pneumococcal prevalence estimates are only available for one other displaced population globally, living in Mae La refugee camp, Thailand [7].
Pneumococcal Conjugate Vaccines (PCV) are highly efficacious and have been used routinely for protecting children against pneumococcal colonization and disease in most countries worldwide. Five PCVs are currently used for childhood immunization: Prevenar 20 (20 valent, targeting 20 of the over 100 serotypes), Vaxneuvance (15 valent), Prevenar 13 (13 valent), Synflorix and PNEUMOSIL (both 10 valent) [8–10]. However, despite the high prevalence of risk factors for severe disease and intense transmission, they are rarely offered to populations that have become displaced as a result of food insecurity, conflict, natural disasters, or other emergencies [2].
Pneumococcal colonization is common and is a precursor to disease, thus providing an opportunity to assess the likely risk for disease, even in small populations, without costly disease surveillance programmes that would be difficult to establish in displaced populations [11]. Such information is crucial to inform the design of effective pneumococcal immunization strategies in displaced populations where routine immunization is rarely possible [2].
We conducted a cross-sectional survey to estimate nasopharyngeal carriage prevalence and related risk factors in Digaale, a camp for internally displaced people (IDPs) in Somaliland [2]. PCVs had not yet been introduced in Somaliland at the time of writing.
Methods
Study population and sampling method
We conducted a cross-sectional survey in October-November 2019. The Digaale IDP camp was established in 2014 and, at the time of our study, housed an estimated population of 3,000 people largely displaced due to drought and food insecurity during 2013 and 2014 [12]. The camp is located about 4 km south-east of Hargeisa, the capital of Somaliland, and consists of corrugated-steel shelters. The population is served by a school and primary health centre.
We visited all 894 shelters in Digaale and invited households to participate in our survey. We first administered a structured household survey among consenting households to establish their composition and collect household-level information on shelter conditions, pneumococcal risk factors, and retrospective mortality and demographic changes. We then selected and invited individual household members for participation in a survey on carriage, contact and individual-level risk factors. We aimed to sample 100 individuals in each of the following age groups: <1, 1, 2–5, 6–14, 15–29, 30–49, and ≥ 50 years (y) old to detect age-specific pneumococcal prevalence within 10% precision. We purposively oversampled young children who have the highest incidence of pneumococcal carriage and disease. Quota sampling by age was used to select individual household members.
We returned to individual participants two days after the household survey and study enrolment to conduct the contact and risk factor survey, in which we asked about individual-level risk factors including social contacts, and measured anthropometry for children aged 6–59 months. Further details about the design and sampling method, as well as detailed social contact and household-level findings, are described in Van Zandvoort et al. [12]. In the final week of data collection, one to four weeks after participation in the contact survey, we followed up participants to collect a nasopharyngeal swab sample. To compensate for loss-to-follow up, additional participants were sampled from household members of participating households.
All swabs were collected at a community hall in the centre of the camp. During swab collection, we asked participants whether they had experienced any respiratory symptoms (cough, sore throat, sneezing, wheezing, headache, or fever) or used any antibiotics in the two weeks prior. Responses for children under 10y were provided by an adult parent or caregiver. Participants with a contraindication for a nasopharyngeal swab such as facial trauma were excluded.
Sample collection, storage, and shipment
Trained nurses collected nasopharyngeal swabs from each participant using flexible paediatric- (Ultra Minitip) or adult-size (Flexible Minitip) flocked swabs (FLOQSwabs; Copan Diagnostics, USA), following WHO recommendations [13, 14]. Paediatric-sized swabs were used for children under 15y. The nasopharyngeal swabs were stored in screw-capped tubes containing 1 ml of skim milk-tryptone-glucose-glycerol (STGG) medium and kept on wet ice in cool boxes immediately after collection. Samples were transferred to a -20 °C freezer at the Ministry of Health Development national cold chain facility within eight hours of collection [15]. Within two weeks after collection, all samples were transferred to an ultra-low temperature (ULT) freezer at the culture laboratory of the Somaliland National Tuberculosis Hospital. Due to technical issues with the ULT freezer, samples were temporarily stored at -20 °C for 15 days in March 2020.
We used a prequalified shipping solution with phase change materials (PCMs) that provided passive cooling to keep contents below -15 °C for up to 96 h as per manufacturer specifications (Schaumaplast, DE) [16]. We found that it maintained temperatures below -15 °C for up to 160 h in an empty trial shipment from London to Hargeisa when conditioned at ULT (Supplemental Material Section B). Samples were first shipped to Nairobi, Kenya, where they were repackaged and placed on dry ice for further transport to the Murdoch Children’s Research Institute (MCRI) in Melbourne, Australia. We successfully completed a pilot shipment of 81 samples in May 2021, and shipped all remaining samples in December 2021. Transit delays during the second shipment extended the period during which only passive cooling was provided by PCMs to 11 days. There was no temperature monitoring during this second shipment, but we project that temperatures may have increased to > 0 °C for up to 2.5 days based on temperature measurements from the pilot shipment. We explored any difference in carriage estimates between the two shipments in a sensitivity analysis.
Microbiological analysis
Upon arrival at the MCRI laboratory, STGG swab samples were immediately stored at ULT until testing. Briefly, samples were thawed, vortexed, and DNA extracted from 100 µl using the QIAcube HT machine (Qiagen) following a protocol described previously [17]. Concurrently, each sample was cultured overnight on selective agar, and growth harvested if alpha-haemolytic colonies were present [18]. Real-time quantitative PCR, targeting the lytA gene (lytA qPCR) [19], was conducted as previously described, except for using 5 µl template DNA and the AriaMX PCR system (Agilent) [18]. For samples with presumptive pneumococci (lytA qPCR cycle threshold < 40 and alpha-haemolytic growth), DNA was extracted from harvested colonies using the QIAcube HT machine (Qiagen) and the resultant DNA serotyped using microarray (Senti-SP version 1.5, BUGS Bioscience) [18]. Pneumococcal density was calculated using a genomic DNA standard curve [18]. Serotype-specific density was calculated by multiplying the pneumococcal density (as determined by lytA qPCR) by the relative abundance of the serotype (as determined by microarray). Carriage density estimates were log10-transformed and reported as log10 genome equivalents/ml (GE/ml). Non-encapsulated serotypes (NESp) were categorized by their genetic lineage [20].
Statistical analysis
Serotypes were grouped as NESp, vaccine serotypes (VT) for each of PNEUMOSIL, Synflorix, Prevenar 13, Vaxneuvence, and Prevenar 20 vaccines, or non-vaccine serotypes (NVT). Unless stated otherwise, we used PNEUMOSIL targeted serotypes to define VT in our base case analysis, because PNEUMOSIL was specifically developed to provide a lower cost alternative that targets the main serotypes causing pneumococcal disease in low and middle income countries [21]. Analyses that defined VT using the other vaccines are presented in Section C in the Supplemental Material.
We estimated the population and age-specific prevalence of pneumococcal serotypes with 95% binomial confidence intervals. To account for multiple serotype carriage, serotypes were weighted by their relative abundance within a sample to calculate the serotype distribution including co-carried serotypes, so that the weights of all serotypes in a sample summed to one. Unweighted distributions of all and dominant-only serotypes were assessed in a sensitivity analysis. We define serotypes ranked with the highest relative abundance in a single sample as dominant serotypes, and used logistic regression to assess the odds that serotypes were dominant.
As we sampled a large proportion of households (65%) and individuals (17%) living in Digaale, we used finite population corrections (FPC) to calculate standard errors used in population-level estimates. To correct for imbalance resulting from quota sampling and thus improve the representativeness, we applied poststratification weights based on age group and gender [12, 22]. We assessed the sensitivity of our population level estimates to poststratification in additional analyses. We used the Survey package in R to perform poststratification weighting and to apply the FPC when estimating weighted means, proportions, and quantiles where applicable [23].
To project the proportion of current IPD cases caused by serotypes covered by PCVs, we combined the dataset with age- and serotype-specific estimates of invasiveness by Løchen et al. [24], and computed confidence intervals by bootstrapping our dataset and invasiveness estimates. More details are provided in Supplemental Material Section D.
To estimate the likely contribution of different age groups to pneumococcal exposure and transmission, we followed a method developed by Qian et al. [25] and calculated the proportion of colonisations attributable to contact with different age groups by linking the estimates of carriage prevalence with previously reported contact patterns [12] in those individuals and the likelihood of colonisation among those contacts. As the contact and carriage datasets featured different statistical error processes, we computed confidence intervals for this analysis through bootstrapping from each parameter’s uncertainty distribution.
We used logistic regression to test the univariate association of likely risk factors with the odds of pneumococcal carriage and used linear regression to test their association with mean logged overall carriage density in pneumococcal carriers. Age and sex were included as a priori defined confounding variables in both analyses, but no other multivariate analyses were conducted due to data sparsity. We also used logistic regression to test for any association between the odds of multiple carriage and age, and to assess whether the odds of carrying NESp serotypes differed by age. FPC and poststratification weights were not applied in regression analyses.
All analyses were conducted in R4.2.2.
Results
Participant sampling and enrolment
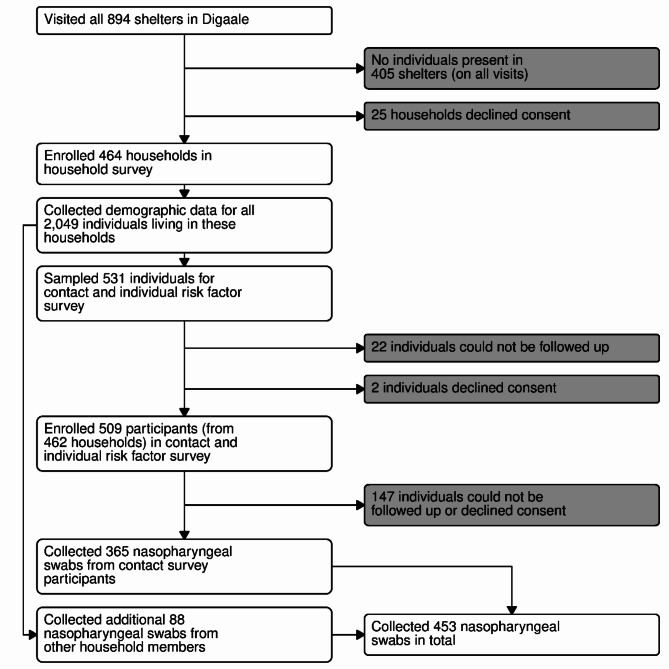
We enrolled 464 (65%) households and collected demographic data from 2,049 individuals living in these households. A contact and individual-level risk-factor survey was conducted among 509 participants. We collected a nasopharyngeal swab from 365 of these participants. An additional 88 nasopharyngeal swabs were collected from other individuals from consenting households. In total, 453 swabs were collected (Fig. 1).
Fig. 1.
Flowchart and sampling procedure
All households that were present were invited to participate in the survey. A structured household survey was conducted in consenting households. Individual household members were selected using quota sampling and invited for a contact and individual-level risk factor survey. Participants were followed up after one to four weeks to administer a nasopharyngeal swab. Additional household members were sampled from consenting households using quota sampling to compensate for loss to follow-up.
Sample characteristics
Two (0.4%) nasopharyngeal swab samples were excluded due to insufficient sample volume for laboratory testing. An additional two (0.4%) samples were lytA positive, indicating pneumococcal colonization, but with no growth prohibiting microarray serotyping; these were included only in non-serotype specific carriage prevalence analyses. Due to data entry errors, 53 swabs (12%) could not be fully linked to records in contact or household datasets. This included 16 swabs that could not be linked to their household information, and 6 samples without information on age and sex (Supplemental Material Section A). Data from these swabs are excluded in analyses that require linking to those data, but included otherwise.
67% of the study participants from whom swabs were collected were female (Table 1). Median age was 13y and median household size was 5 people. Pneumococci were detected in 39% (175/445) of samples, with at least one PNEUMOSIL VT present in 49% (85/175) of positive samples. We estimated the overall carriage prevalence in Digaale as 36% (95%CI 31–40), with 41% (95%CI 33–49) of carriers carrying at least one VT. Estimated prevalence was 70% (95%CI 64–76) in children under 5y, with VTs carried by 61% (95%CI 53–69) of carriers. Large proportions of participants reported respiratory symptoms (64%) and antibiotic use (36%) in the two weeks leading up to the sampling.
Table 1.
Sample characteristics, carriage prevalence, and invasive disease likely caused by vaccine serotypes
| Variable | Obsa | Sample value | Population estb | Population est (< 5y)b | ||
|---|---|---|---|---|---|---|
| Sample characteristics (unweighted) | ||||||
| Median age (y) | 447 | 13 (IQR 3–40) | . | . | ||
| Percentage female | 298/447 | 66.7% | . | . | ||
| Median household size (people) | 433 | 5 (IQR 3–6) | . | . | ||
| Median household members < 5y (people) | 433 | 0 (IQR 0–2) | . | . | ||
| Potential risk factors | ||||||
| Respiratory symptoms | 261/410 | 63.7% | 61.1% | 55.9–66.2 | 73.5% | 67.0–79.9 |
| Antibiotic use | 148/409 | 36.2% | 31.6% | 27.0–36.3 | 45.9% | 38.6–53.2 |
| Direct contacts | 364 | 9.6 | 9.6 | 9.1–10.0 | 9.9 | 9.4–10.3 |
| Carriage prevalence | ||||||
| Pneumococcal carriage | 175/445c | 39.3% | 35.8% | 31.1–40.4 | 70.0% | 63.7–76.4 |
| Non-encapsulated carriage | 19/175 | 10.9% | 13.2% | 6.9–19.5 | 6.6% | 2.8–10.5 |
| Proportion of carriers with VT | ||||||
| PNEUMOSILd | 85/175 | 48.6% | 40.8% | 32.9–48.7 | 60.9% | 52.7–69.1 |
| Synflorixe | 84/175 | 48.0% | 40.0% | 32.1–47.8 | 59.7% | 51.4–68.0 |
| Prevenar 13f | 98/175 | 56.0% | 51.0% | 43.1–58.9 | 62.9% | 54.8–71.0 |
| Vaxneuvanceg | 98/175 | 56.0% | 51.0% | 43.1–58.9 | 62.9% | 54.8–71.0 |
| Prevenar 20h | 110/175 | 62.9% | 58.0% | 50.1–65.9 | 68.3% | 60.4–76.3 |
| Projected proportion of IPD covered by PCVs i | ||||||
| PNEUMOSILd | . | 52.9% | 51.9% | 34.8–69.1 | 67.8% | 50.9–81.6 |
| Synflorixe | . | 60.8% | 60.1% | 41.8–75.0 | 74.1% | 57.1–85.6 |
| Prevenar 13f | . | 72.4% | 74.9% | 60.0–85.3 | 77.9% | 61.0–88.6 |
| Vaxneuvanceg | . | 73.0% | 75.5% | 60.8–85.8 | 78.9% | 61.9–89.0 |
| Prevenar 20h | . | 80.9% | 81.6% | 66.9–89.9 | 88.0% | 77.0–93.7 |
a. Total number of observations in the dataset. For proportions, both numerators and denominators are shown
b. Mean value and corresponding 95%CI. Post-stratification weights and finite population corrections have been used to calculate population estimates
c. Six observations excluded due to missing age or gender data prohibiting post-stratification weighting
d. Serotypes 1, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, and 23F
e. Serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F
f. Serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F
g. Serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F
h. Serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F
i. Estimated from serotype-specific invasiveness estimates. See Section D in the Supplemental Material for more details
Serotype distribution
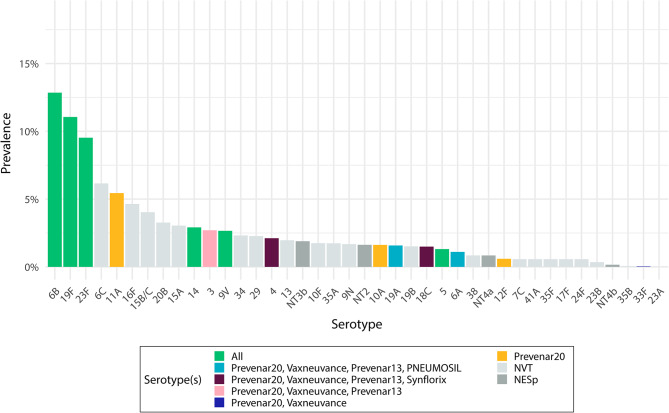
The three most prevalent pneumococcal serotypes overall (6B, 19F, and 23F) were VTs included in all PCVs, followed by three serotypes (6C, 11A, 16F) of which 11A is only included in Prevenar 20, and all others are NVTs (Fig. 2). A similar serotype distribution was observed in children under 5y, while 15B/C, 6B, and 6C were the most prevalent serotypes in people over 5y (Fig C2 in the Supplemental Material). There was little difference in the serotype distribution when restricting analysis to dominant serotypes alone, or without weighting for relative abundance in multiple serotype carriers (Fig C1 in the Supplemental Material). We extrapolate that 52% (95%CI 35–70) of all IPD cases and 68% (95%CI 51–82) of IPD cases in < 5y were caused by VT serotypes, with slightly increased proportions for higher valency vaccines (Table 1). Out of all 248 identified serotypes in all samples, 20 were identified as NESp. These were categorized as NT2 (8), NT3b (6), NT4a (4), and NT4b (2) genetic lineages.
Fig. 2.
Pneumococcal serotype distribution. Bars show the carriage prevalence of pneumococcal serotypes identified in Digaale IDP camp, weighted by their relative abundance. Coloured bars show the serotypes included in the five PCVs, dark grey bars non-encapsulated pneumococci, and light grey bars other serotypes not included in the PCVs. Error bars show 95% confidence intervals for each estimate
Multiple serotype carriage
Co-colonization with more than one serotype was detected in 30% (52/176) of samples with pneumococci, corresponding to a population-level prevalence of 39% (95%CI 35–44). There were 36 samples in which two serotypes were detected, 13 samples with three serotypes, and three samples with more than three serotypes. The odds that the serotype was dominant was 2.0 (95%CI 1.1–3.7) times higher for VTs than for NVTs in all carriers, and 2.8 (95%CI 1.0–8.0) times higher among carriers colonized with both VT and NVT (Table C2 in the Supplemental Material).
Sensitivity analyses
We found no evidence of a difference in pneumococcal carriage, density of pneumococcal serotypes, number of carried serotypes, or serotype distribution between our two sample shipments (Supplemental Material Section B). Post stratifying our estimates did not substantially affect pneumococcal carriage prevalence estimates (Table C5 in the Supplemental Material).
Antimicrobial resistance
Microarray assays detected the presence of select antimicrobial resistance genes in 30% (95%CI 21–41) of samples (Table C2 in the Supplemental Material). In those samples, the most common detected genes typically associated with antimicrobial resistance were tetM (28% [95%CI 19–39]) and ermB (9% [95%CI 4–16]). We restricted this analysis to samples in which no other species and only a single pneumococcal serotype were detected.
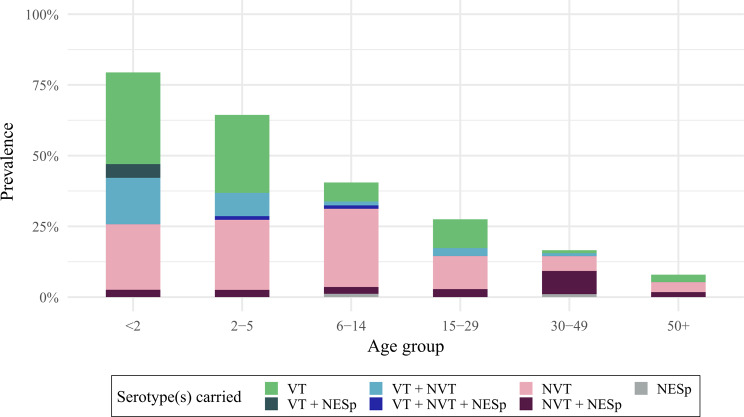
Carriage prevalence and serotype distribution by age
Overall carriage prevalence was 79% (95%CI 70–88) and 66% (95%CI 57–75) in children under 2 and 2-5y, respectively. Carriage prevalence was 41% (95%CI 31–51) in children aged 6-14y, 25% (95%CI 14–36) in people aged 15-29y, 17% (95%CI 9–26) in adults aged 30-49y and 10% (95%CI 4–15) in adults aged ≥ 50y (Fig. 3). Co-colonization rates decreased by age alongside reductions in overall prevalence, although this reduction was not statistically significant. The proportion of VT among all carriers was similar across age groups and robust to the definition of VT for different PCV products (Fig C3 in the Supplemental Material).
Fig. 3.
Prevalence and serotype distribution by age. Bars show the estimated prevalence of pneumococcal serotypes by age group, weighted for age and sex. Error bars show 95% confidence intervals around overall pneumococcal carriage prevalence. Colours show the prevalence of serotypes that are carried; VT: only vaccine type(s), NVT: only non-vaccine type(s); NESp: only non-encapsulated type(s); VT + NVT: both vaccine- and non-vaccine type(s). Multiple carriage with non-encapsulated type(s) is shown as a darker shading
We found weak evidence that the odds of carrying a NESp among carriers was 4.8 (95%CI 1.01–22.34) times higher in adults 30-49y compared to children under 2y, but no evidence for a difference in NESp carriage in other age groups.
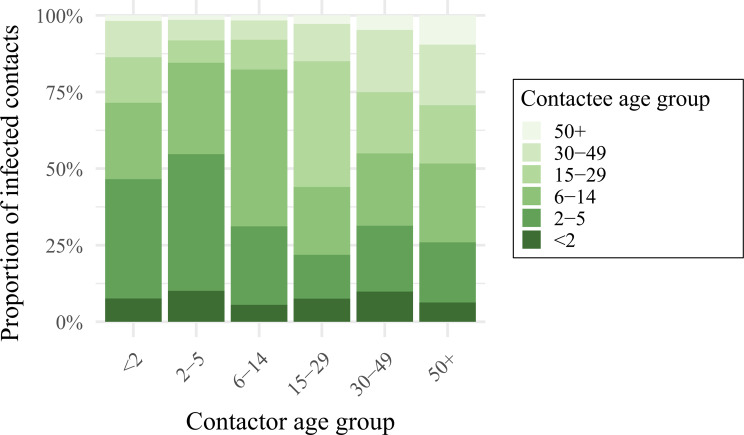
Contribution of different age groups to pneumococcal exposure
We projected that a large proportion (39% [95%CI 31–48]) of pneumococcal exposure of children < 2y may be attributed to contact with 2-5y children, followed by school age children aged 6-14y (25% [95%CI 17–34]) (Fig. 4 and Table C6 in the Supplemental Material). A similar contribution was made by carriers of these age groups to exposure of children aged 2-5y (45% [95%CI 37–52]; and 30% [95%CI 23–38]). Most of the exposure of school age children, however, was found to be attributable to other school age children (52% [95%CI 42–60]), followed by 2-5y olds (26% [95%CI 20–32]). While carriage prevalence was high in children < 2y, this age group was found to contribute relatively little to onward transmission to any age group.
Fig. 4.
The contribution of different age groups towards the age-specific exposure to pneumococcus. Bars show the average proportion of contacts made by a contactor of age i (x-axis), with pneumococci-carrying contactees of different age groups. Shades of green indicate the age group of the contactee, i.e. the person potentially transmitting to the contactor
Association of risk factors with pneumococcal carriage and carriage density
We found no evidence that the number of overall household members increased the odds of pneumococcal carriage, but weak evidence that living with one additional household member < 5y of age increased the odds of carriage by 1.3 (95%CI 1.0–1.8) (Table 2). The odds of carriage were 2.0 (95%CI 1.2–3.3) times higher in people with recent respiratory symptoms. Having a cough (2.0 [95%CI 1.2–3.3]) had the strongest association, followed by having a sore throat (1.7 [95%CI 1.0–3.0]). There was some evidence that the odds of carriage increased by 1.1 (95%CI 1.0–1.2) for every additional physical contact reported. We found good evidence for a reduction in the odds of carriage for improved scores of weight-for-age (0.6 [95%CI 0.4–0.9]) and height-for-age (0.6 [95%CI 0.4–0.8]) among children 6–59 months old, but no evidence for an association with weight-for-height or middle-upper arm circumference. Notably, we did not find any evidence of an association with self-reported antibiotic use.
Table 2.
Association between risk factors and pneumococcal carriage
| Variable | ORa | 95%CI | p-value | N b |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Household size | 1.05 | 0.95–1.17 | 0.336 | 431 |
| Household members < 5y | 1.32 | 0.99–1.76 | 0.054 | 431 |
| Shelter quality | ||||
| House leakage | 1.15 | 0.69–1.95 | 0.591 | 431 |
| House draught | 0.68 | 0.41–1.13 | 0.140 | 431 |
| Indoor air pollution | ||||
| Use firewood as fuel | 1.03 | 0.55–1.92 | 0.938 | 434 |
| Use charcoal as fuel | 0.75 | 0.46–1.19 | 0.223 | 434 |
| Ventilation | 431 | |||
| yes | 0.49 | 0.20–1.16 | ||
| cook outside | 0.56 | 0.25–1.24 | 0.348 | |
| Current health c | ||||
| Antibiotic use | 1.28 | 0.78–2.10 | 0.324 | 407 |
| Respiratory symptoms | 1.99 | 1.20–3.32 | 0.008 | 408 |
| Cough | 2.00 | 1.23–3.25 | 0.005 | 408 |
| Sore throat | 1.69 | 0.95–3.00 | 0.072 | 408 |
| Headache | 0.74 | 0.41–1.32 | 0.309 | 408 |
| Fever | 1.17 | 0.70–1.94 | 0.545 | 408 |
| Diarrhoea | 1.62 | 0.73–3.73 | 0.244 | 408 |
| Morbidities d | ||||
| Pneumonia 6me | 1.28 | 0.68–2.41 | 0.451 | 363 |
| Sickle Cell | 1.25 | 0.38–3.77 | 0.697 | 363 |
| Asthma | 0.96 | 0.11–5.73 | 0.968 | 363 |
| Diabetes | 1.09 | 0.05–8.57 | 0.939 | 363 |
| Individual substance use | ||||
| Tobacco | 0.50 | 0.03–2.92 | 0.524 | 363 |
| Khat | 0.63 | 0.03–3.93 | 0.681 | 363 |
| Household substance use f | ||||
| Smoking | 1.42 | 0.84–2.39 | 0.186 | 434 |
| Snuff | 1.64 | 0.66–4.17 | 0.287 | 434 |
| Khat | 1.31 | 0.81–2.12 | 0.265 | 434 |
| Contact behaviour | ||||
| Total number of direct contacts | 1.04 | 0.97–1.12 | 0.278 | 362 |
| Total number of physical contacts | 1.08 | 1.01–1.15 | 0.034 | 362 |
| Malnutrition in < 5y olds | ||||
| Weight-for-age z-score | 0.59 | 0.37–0.90 | 0.018 | 112 |
| Weight-for-height z-score | 1.16 | 0.79–1.70 | 0.440 | 112 |
| Height-for-age z-score | 0.61 | 0.43–0.82 | 0.003 | 112 |
| MUACg (in cm) | 0.80 | 0.53–1.21 | 0.229 | 112 |
a. Estimates are adjusted for age and gender
b. Total number of records used in regression
c. Self-reported antibiotic use and symptoms in 2 weeks preceding the survey
d. Self-reported diagnosed morbidities
e. Pneumonia diagnosis in the 6 m preceding the survey
f. Substance use by at least one household member
g. Middle-Upper-Arm-Circumference
We also tested the association between these risk factors and the density of pneumococcal carriage (Table C3 in the Supplemental Material) and found weak evidence that living with one additional household member < 5y was associated with a small 0.2 (95%CI 0.0–0.4) increase in mean log10 GE/ml, while associations with respiratory symptoms were either non-significant or negative: the mean log10 density was 0.40 (95%CI -0.8–0.0) lower in participants reporting a sore throat in the two weeks preceding the survey. Again, we did not find any significant association with self-reported antibiotic use. There was very weak evidence for an increase in children’s mean log10 density with a one-unit increase in weight-for-height z-score (0.2 [95%CI 0.0–0.4]).
Discussion
This is the first study to have estimated pneumococcal serotype prevalence in Somaliland and in an IDP camp. We find high carriage prevalence of 36% in all age groups, and 70% in children under 5y. Between 40 and 58% of pneumococcal carriers carried serotypes included in PCVs, depending on the PCV product, and the three most prevalent serotypes were covered by all PCVs. The majority of exposure to pneumococcal carriers in children younger than 15y may have been attributable to carriers aged 2-5y and 6-14y, with little exposure from carriers aged younger than two years of age. We found that pneumococcal carriage was associated with the number of household members aged < 5y, a recent cough, the total number of physical contacts in all age groups, and with stunting in children aged < 5y. We estimate that all PCVs cover a substantial proportion of serotypes likely causing IPD in this population.
While we did not find local evidence of a significant association for all, many risk factors previously found to be associated with pneumococcal carriage are present in this population [26]. Residents in Digaale live in overcrowded conditions and likely experience high levels of indoor air pollution. On average, one in five children are malnourished, and residents report a high frequency of direct contacts involving physical touch [12, 27, 28]. While carriage prevalence was high, it is similar to that observed in non-displaced populations in other high-transmission settings in East Africa, and not as high as prevalence observed in rural Gambia where high carriage prevalence is sustained into older adulthood [29–32]. Despite a high disease burden, displaced populations are understudied, and we are only aware of one other published carriage survey conducted in Mae La, a long-term camp for displaced people in Thailand, where carriage prevalence was estimated at a similar 80% in children < 2y [7].
The most prevalent serotypes in Digaale (6B, 19F, and 23F) have often been observed to dominate carriage in other PCV-naïve populations, although the relatively high prevalence of 6C and low prevalence of 6A and 19A in our study is unusual [29–31, 33]. Around 50% of serotypes we detected were VTs, and the prevalence of observed serotypes included in the 10-valent Synflorix and PNEUMOSIL PCVs were similar. The proportion of serotypes that were VTs increased with valency of the vaccine. However, due to our relatively small sample size, serotype-specific confidence intervals are very wide. We estimate that any of the five PCVs are likely to cover the serotypes causing the majority of the pneumococcal disease burden. While serotype replacement would mitigate the overall PCV impact, substantial reductions in pneumococcal disease have been observed where PCVs have been introduced with sufficient coverage [34, 35]. While we did not collect data on the pneumococcal disease burden in Digaale, 43% of children under two years of age were reported to have been diagnosed with pneumonia in the six months preceding the survey [12], and pneumococci were one of the leading causes of childhood pneumonia globally in the pre-PCV era [36].
The combined contact and prevalence estimates showed that pneumococcal transmission in the < 2y in Digaale was mostly driven by children aged 2-5y (39%) and 6-14y (25%), with little contribution to transmission from children younger than 2y old who have fewer social contacts. This could be important when designing vaccine strategies, especially those that partially rely on controlling pneumococcal transmission by indirect effects or need to prolong campaign effects in settings where continued vaccination through a routine EPI schedule is not possible, as this requires extending the age range of the eligible population [2]. While Digaale is an established camp that is safe and easy to access, this is not the case for many other displaced populations. In conflict settings, it is often not feasible to introduce routine immunization, and policy makers may choose alternative strategies that aim to immunize the subgroups that drive transmission, thereby indirectly protecting other subgroups at highest risk of severe disease.
Participants reported high rates of antibiotic usage in the two weeks preceding sample collection. This may be associated with the high proportion of participants with respiratory symptoms in the same period. However, we cannot rule out reporting bias. We found no association of antibiotic use with reduced carriage contrary to findings in other settings [30, 37, 38]. Although in this study we do not have estimates of phenotypic pneumococcal resistance, microarray testing identified genes typically associated with pneumococcal resistance in a third of all samples, which may be consistent with high antibiotic pressure. The tetM gene, known to encode tetracycline resistance, was identified in 28% of pneumococci, mirroring its high prevalence in other studies [39, 40]. The ermB gene was found in 9% of pneumococci, and is associated with macrolide resistance [41]. Future improved understanding of antimicrobial resistance in pneumococci would be useful to better understand the impact of more clinically-relevant antibiotics for standard care as well as the potential impact of mass-drug administration campaigns, a potential alternative intervention to reduce the pneumococcal disease burden proposed for crisis settings [42].
We assessed the relationship between a number of known risk factors with the odds of pneumococcal carriage and the mean pneumococcal carriage density. We found relationships in the expected direction for some risk-factors, such as an increased odds of carriage for participants with a higher number of household members under 5y of age, those with more direct contacts, and those with recent respiratory symptoms. Pneumococcal carriage has previously been found to be associated with rhinitis, and may be affected by other respiratory infections [43]. It should be noted that confidence intervals were very wide due to a relatively low sample size and low variability within many risk factors. Moreover, we only adjusted our estimates for age and sex, and they are likely affected by residual confounding, while the large number of significance tests means that some spurious associations may have been estimated.
There are several limitations to our study. The study population was substantially smaller than expected as many shelters were uninhabited at the time of the study. Thus, we only reached 65% of our target sample size of 700 participants, particularly in young children, where we only reached 24% and 43% of our target sample size of 100 each in children aged < 1 and 1y [12]. We therefore pooled the < 1 and 1y age groups in a single < 2y age group, which allowed us to estimate age-specific prevalence with sufficient precision, but a larger sample would have resulted in more detailed estimates. We could only conduct data collection during daylight hours and may have missed older individuals who work outside Digaale, as many leave the camp very early in the morning and return late at night. This is likely reflected in the gender distribution of the recruited sample, but unlikely to have affected our carriage estimates, as prevalence is low in these older age groups and unlikely to differ substantially from those who were present in Digaale. We post-stratified our estimates to adjust for any imbalances in our sample and did not detect differences. Although pneumococcal carriage is generally consistent across seasons [44], we only conducted a single cross-sectional survey and do not know how estimates may change throughout the year. Carriage prevalence was similar to that in general populations in East Africa, but no other prevalence estimates exist for Somaliland, and we cannot infer how results may differ from the general Somaliland population. Our study was not powered to detect relationships between carriage and risk factors, which may explain why we did not find statistically significant effects in most univariate analyses, and a prospective cohort design would be more suitable to infer causality. Finally, many risk factors were self-reported, and their accuracy may be affected by reporting bias.
Ideally, pneumococci are stored at ULT to maintain long-time viability, but we experienced several challenges related to sample storage and shipment. Sample shipment was substantially delayed, partly due to the COVID-19 pandemic, and after several months of storage at ULT, swabs had to be temporarily transferred to a -20 °C freezer to allow for ULT freezer repairs. We were not able to transport samples at ULT as local airlines did not accept shipments of dry ice. However, we have shown separately that effect on pneumococcal viability is limited if stored at -20 °C for up to three weeks [15], which was maintained in our study in the periods that ULT storage was not feasible. We further monitored sample viability by incorporation of lytA qPCR, a molecular screening assay that is not expected to be affected by culture viability, and did not observe a large number of non-culturable samples that were lytA positive. Despite transit delays during the second shipment of most of our samples during which temperatures may have exceeded 20 °C for up to 2.5 days, we found no difference between carriage and VT prevalence estimates between these samples and those transported during the first shipment. Hence, we believe any effect on sample viability was limited and did not greatly affected our results, supported by the ability to culture at high prevalence and with detection of serotypes carried at low abundance.
We collected nasopharyngeal swabs and assessed these using lytA qPCR and microarray consistent with the WHO guideline [14]. We did not examine oropharyngeal carriage which may have underestimated prevalence in adults [45]. Future studies could consider collecting oropharyngeal swab or saliva samples in addition to nasopharyngeal swabs for increased sensitivity in adults.
Conclusion
We found high pneumococcal carriage prevalence in a PCV-naïve population living in an IDP camp in Somaliland, consistent with carriage rates in non-displaced populations in other high transmission settings. About half of all circulating pneumococci were included in currently available PCVs. We estimate that at least half of all resulting IPD cases in this population were caused by serotypes included in PCVs, indicating the potential for substantial vaccine effects. Transmission was primarily driven by children 2–5 years and 6–14 years old, partially exceeding the proposed age eligibility for PCV campaigns that aim to temporarily reduce transmission in crisis-affected populations. These findings advance our understanding of pneumococcal carriage in crisis-affected populations and provide important evidence for the design of future vaccination strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank the Digaale community who participated in this study, as well as Hamse Shaban Ahmed, Ayaan Ismail Adan, Khadar Abiib Ahmed, Ayaan Mohamoud Ali, Hamda Awil Garaad, Ahmed Yusuf Mohamed, Suaad Abdi Osman, Amiin Abdi Ismail, Nimco Abdilahi Ismail, AbdiFatah Mohamed Ahmed, Mustafe Shugri Mohamed, and Nimco Mohamed Abdi from the Republic of Somaliland Ministry of Health Development for their data collection efforts. We also thank the Somaliland National TB Reference Laboratory in Hargeisa for the storage of samples. Electronic data solutions were provided by LSHTM Open Data Kit (odk.lshtm.ac.uk).
Abbreviations
- FPC
Finite population correction
- GE/ml
Genome equivalents/ml
- IDP
Internally displaced people
- IPD
Invasive pneumococcal disease
- MCRI
Murdoch Children’s Research Institute
- NESp
Non-encapsulated serotype
- NVT
Non-vaccine serotype
- PCM
Phase-change material
- PCV
Pneumococcal conjugate vaccine
- STGG
Skim milk-tryptone-glucose-glycerol
- ULT
Ultra-low temperature
- VT
Vaccine serotype
Author contributions
Conceptualization and funding acquisition: FC, SF, EKM, and CS; methodology: FC, SF, CS, and KvZ; data collection implementation: KvZ, AIH, MOB, MSA, and SY; supervision: KvZ, AIH, MOB, SMS, RC, and MH; statistical analysis: KvZ; microbiological analysis: CLP, JH, CS, and BDO; writing-original draft: KvZ; writing-review and editing: KvZ, CLP, RME, JH, RC, CRM, EKM, FC, and SF. All authors read and approved the final manuscript.
Funding
This work was supported by Elrha’s Research for Health in Humanitarian Crises (R2HC) Programme, which aims to improve health outcomes by strengthening the evidence base for public health interventions in humanitarian crises. The R2HC programme is funded by the UK Government (DFID), the Wellcome Trust, and the UK National Institute for Health Research (NIHR). SF acknowledges a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant: 208812/Z/17/Z). In addition, RME acknowledges an NIHR (grant: NIHR200908) for the Health Protection Research Unit in Modelling and Economics at LSHTM. MCRI was supported by the Victorian Government’s Operational Infrastructure Support Program. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK Department of Health and Social Care. The funding sources had no role in the study design; collection, analysis, and interpretation of data; or in writing the report.
Data availability
The anonymised aggregated data generated and analysed during the current study, including all analysis scripts, are available in the Zenodo repository 10.5281/zenodo.11493116.
Declarations
Ethics approval and consent to participate
Ethical approval for the study was granted by the Research Ethical Committee of the London School of Hygiene and Tropical Medicine (16577) and the Republic of Somaliland Ministry of Health Development (2/13075/2019).
Consent for publication
Not applicable.
Competing interests
EKM and CS are investigators on a clinical research collaboration with Pfizer on PCV vaccination in Mongolia and are investigators on a Merck Investigator Studies Program grant funded by MSD on pneumococcal serotype epidemiology in children. All other authors report no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations High Commissioner for Refugees. UNHCR - Refugee Statistics. https://www.unhcr.org/refugee-statistics/. Accessed 2 Mar 2023.
- 2.van Zandvoort K, Checchi F, Diggle E, Eggo RM, Gadroen K, Mulholland K, et al. Pneumococcal conjugate vaccine use during humanitarian crises. Vaccine. 2019;37:6787–92. [DOI] [PubMed] [Google Scholar]
- 3.Heudtlass P, Speybroeck N, Guha-Sapir D. Excess mortality in refugees, internally displaced persons and resident populations in complex humanitarian emergencies (1998–2012) – insights from operational data. Confl Health. 2016;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellos A, Mulholland K, O’Brien KL, Qazi SA, Gayer M, Checchi F. The burden of acute respiratory infections in crisis-affected populations: a systematic review. Confl Health. 2010;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien KL, Baggett HC, Brooks WA, Feikin DR, Hammitt LL, Higdon MM, et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394:757–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohrt BA, Mistry AS, Anand N, Beecroft B, Nuwayhid I. Health research in humanitarian crises: an urgent global imperative. BMJ Global Health. 2019;4:e001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner P, Turner C, Jankhot A, Helen N, Lee SJ, Day NP, et al. A longitudinal study of Streptococcus pneumoniae carriage in a cohort of infants and their mothers on the Thailand-Myanmar Border. PLoS ONE. 2012;7:e38271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Prequalified vaccines. Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control). 2019. https://extranet.who.int/pqweb/vaccines/prequalified-vaccines. Accessed 2 Mar 2023.
- 9.Pfizer. US, FDA Approves. PREVNAR 20™, Pfizer’s Pneumococcal 20-valent Conjugate Vaccine for Adults Ages 18 Years or Older. 2021. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-prevnar-20tm-pfizers-pneumococcal-20-valent. Accessed 14 Sep 2021.
- 10.Merck. Merck Announces US. FDA Approval of VAXNEUVANCE™ (Pneumococcal 15-valent Conjugate Vaccine) for the Prevention of Invasive Pneumococcal Disease in Adults 18 Years and Older Caused by 15 Serotypes. 2021. https://www.merck.com/news/merck-announces-u-s-fda-approval-of-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-for-the-prevention-of-invasive-pneumococcal-disease-in-adults-18-years-and-older-caused-by-15-serot/. Accessed 14 Sep 2021.
- 11.Bogaert D, De Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–54. [DOI] [PubMed] [Google Scholar]
- 12.van Zandvoort K, Bobe MO, Hassan AI, Abdi MI, Ahmed MS, Soleman SM, et al. Social contacts and other risk factors for respiratory infections among internally displaced people in Somaliland. Epidemics. 2022;41:100625. [DOI] [PubMed] [Google Scholar]
- 13.COPAN Diagnostics Inc. FLOQSwabs. https://www.copanusa.com/sample-collection-transport-processing/floqswabs/. Accessed 2 Mar 2023.
- 14.Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine. 2013;32:165–79. [DOI] [PubMed] [Google Scholar]
- 15.Pell CL, Ortika BD, Van Zandvoort K, Wee-Hee A, Mulholland EK, Checchi F et al. Effect of transport conditions on recovery of Streptococcus pneumoniae. 2022.
- 16.THERMOCON. THERMOCON CLASSIC 15.5. https://thermocon-coldchain.com/en/produkt/thermocon-classic-15-5/. Accessed 2 Mar 2023.
- 17.Dunne EM, Murad C, Sudigdoadi S, Fadlyana E, Tarigan R, Indriyani SAK et al. Carriage of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus in Indonesian children: A cross-sectional study. PLOS ONE. 2018;13:e0195098. [DOI] [PMC free article] [PubMed]
- 18.Satzke C, Dunne EM, Choummanivong M, Ortika BD, Neal EFG, Pell CL, et al. Pneumococcal carriage in vaccine-eligible children and unvaccinated infants in Lao PDR two years following the introduction of the 13-valent pneumococcal conjugate vaccine. Vaccine. 2019;37:296–305. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho Mda, GS, Tondella ML, McCaustland K, Weidlich L, McGee L, Mayer LW, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol. 2007;45:2460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salter SJ, Hinds J, Gould KA, Lambertsen L, Hanage WP, Antonio M, et al. Variation at the capsule locus, cps, of mistyped and non-typable Streptococcus pneumoniae isolates. Microbiol (Reading). 2012;158:1560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke E, Bashorun A, Adigweme I, Hydara MB, Umesi A, Futa A, Ochoge M, Obayemi D, Edem B, Saidy-Jah E, Onwuchekwa C. Immunogenicity and safety of a novel ten-valent pneumococcal conjugate vaccine in healthy infants in The Gambia: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2021;21(6):834–46. [DOI] [PubMed]
- 22.Lumley T. Complex surveys: a guide to analysis using R. Wiley; 2011.
- 23.Lumley T, Gao P, Schneider B. survey: Analysis of Complex Survey Samples [R package]. Version 4.2. 2023. https://CRAN.R-project.org/package=survey
- 24.Løchen A, Truscott JE, Croucher NJ. Analysing pneumococcal invasiveness using bayesian models of pathogen progression rates. PLoS Comput Biol. 2022;18:e1009389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian G, Toizumi M, Clifford S, Le LT, Papastylianou T, Satzke C, et al. Association of pneumococcal carriage in infants with the risk of carriage among their contacts in Nha Trang, Vietnam: a nested cross-sectional survey. PLoS Med. 2022;19:e1004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neal EFG, Chan J, Nguyen CD, Russell FM. Factors associated with pneumococcal nasopharyngeal carriage: a systematic review. PLOS Glob Public Health. 2022;2:e0000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.le Polain de Waroux O, Flasche S, Kucharski AJ, Langendorf C, Ndazima D, Mwanga-Amumpaire J, et al. Identifying human encounters that shape the transmission of Streptococcus pneumoniae and other acute respiratory infections. Epidemics. 2018;25:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal EFG, Flasche S, Nguyen CD, Ratu FT, Dunne EM, Koyamaibole L, et al. Associations between ethnicity, social contact, and pneumococcal carriage three years post-PCV10 in Fiji. Vaccine. 2020;38:202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill PC, Akisanya A, Sankareh K, Cheung YB, Saaka M, Lahai G, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin Infect Dis. 2006;43:673–9. [DOI] [PubMed] [Google Scholar]
- 30.Nackers F, Cohuet S, le, Polain de Waroux O, Langendorf C, Nyehangane D, Ndazima D et al. Carriage prevalence and serotype distribution of Streptococcus pneumoniae prior to 10-valent pneumococcal vaccine introduction: A population-based cross-sectional study in South Western Uganda, 2014. Vaccine. 2017;35:5271–7. [DOI] [PMC free article] [PubMed]
- 31.Hammitt LL, Akech DO, Morpeth SC, Karani A, Kihuha N, Nyongesa S, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Global Health. 2014;2:e397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinsbroek E, Tafatatha T, Phiri A, Swarthout TD, Alaerts M, Crampin AC, et al. Pneumococcal carriage in households in Karonga District, Malawi, before and after introduction of 13-valent pneumococcal conjugate vaccination. Vaccine. 2018;36:7369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clifford S, Knoll MD, O’Brien KL, Pollington TM, Moodley R, Prieto-Merino D et al. Global landscape of Streptococcus pneumoniae serotypes colonising healthy individuals worldwide before vaccine introduction; a systematic review and meta-analysis. 2023;:2023.03.09.23287027.
- 34.Cohen R, Cohen JF, Chalumeau M, Levy C. Impact of pneumococcal conjugate vaccines for children in high- and non–high-income countries. Expert Rev Vaccines. 2017;16:625–40. [DOI] [PubMed] [Google Scholar]
- 35.Flasche S, Hoek AJV, Sheasby E, Waight P, Andrews N, Sheppard C, et al. Effect of Pneumococcal Conjugate Vaccination on Serotype-Specific Carriage and Invasive Disease in England: a cross-sectional study. PLoS Med. 2011;8:e1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murad C, Dunne EM, Sudigdoadi S, Fadlyana E, Tarigan R, Pell CL, et al. Pneumococcal carriage, density, and co-colonization dynamics: a longitudinal study in Indonesian infants. Int J Infect Dis. 2019;86:73–81. [DOI] [PubMed] [Google Scholar]
- 38.Abdullahi O, Karani A, Tigoi CC, Mugo D, Kungu S, Wanjiru E, et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS ONE. 2012;7:e30787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupien A, Gingras H, Bergeron MG, Leprohon P, Ouellette M. Multiple mutations and increased RNA expression in tetracycline-resistant Streptococcus pneumoniae as determined by genome-wide DNA and mRNA sequencing. J Antimicrob Chemother. 2015;70:1946–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ousmane S, Diallo BA, Ouedraogo R. Genetic determinants of Tetracycline Resistance in Clinical Streptococcus pneumoniae serotype 1 isolates from Niger. Antibiotics. 2018;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okitsu N, Kaieda S, Yano H, Nakano R, Hosaka Y, Okamoto R, et al. Characterization of ermB Gene Transposition by Tn1545 and Tn917 in Macrolide-resistant Streptococcus pneumoniae isolates. J Clin Microbiol. 2005;43:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolfe RJ, Shaikh H, Tillekeratne LG. Mass drug administration of antibacterials: weighing the evidence regarding benefits and risks. Infect Dis Poverty. 2022;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigues F, Foster D, Nicoli E, Trotter C, Vipond B, Muir P, et al. Relationships between rhinitis symptoms, respiratory viral infections and nasopharyngeal colonization with Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in children attending daycare. Pediatr Infect Dis J. 2013;32:227–32. [DOI] [PubMed] [Google Scholar]
- 44.Bojang A, Jafali J, Egere UE, Hill PC, Antonio M, Jeffries D, et al. Seasonality of pneumococcal nasopharyngeal carriage in Rural Gambia determined within the context of a Cluster Randomized Pneumococcal Vaccine Trial. PLoS ONE. 2015;10:e0129649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miellet WR, Almeida ST, Trzciński K, Sá-Leão R. Streptococcus pneumoniae carriage studies in adimportancertance, challenges, and key issues to consider when using quantitative PCR-based approaches. Front Microbiol. 2023;14:1122276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymised aggregated data generated and analysed during the current study, including all analysis scripts, are available in the Zenodo repository 10.5281/zenodo.11493116.